Immobilization of Exfoliated g-C3N4 for Photocatalytical Removal of Organic Pollutants from Water
Abstract
1. Introduction
2. Results and Discussion
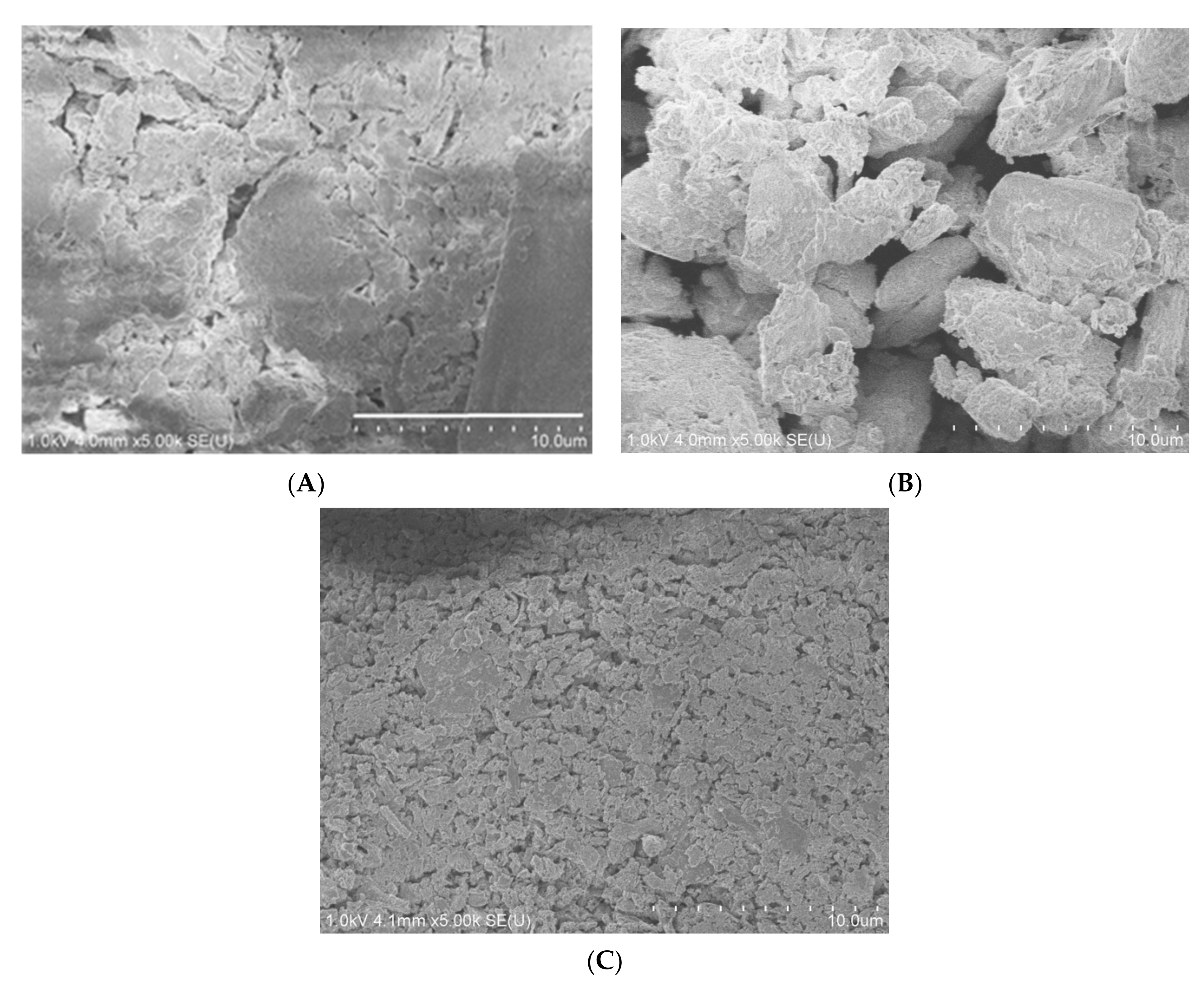
2.1. Material Characterization
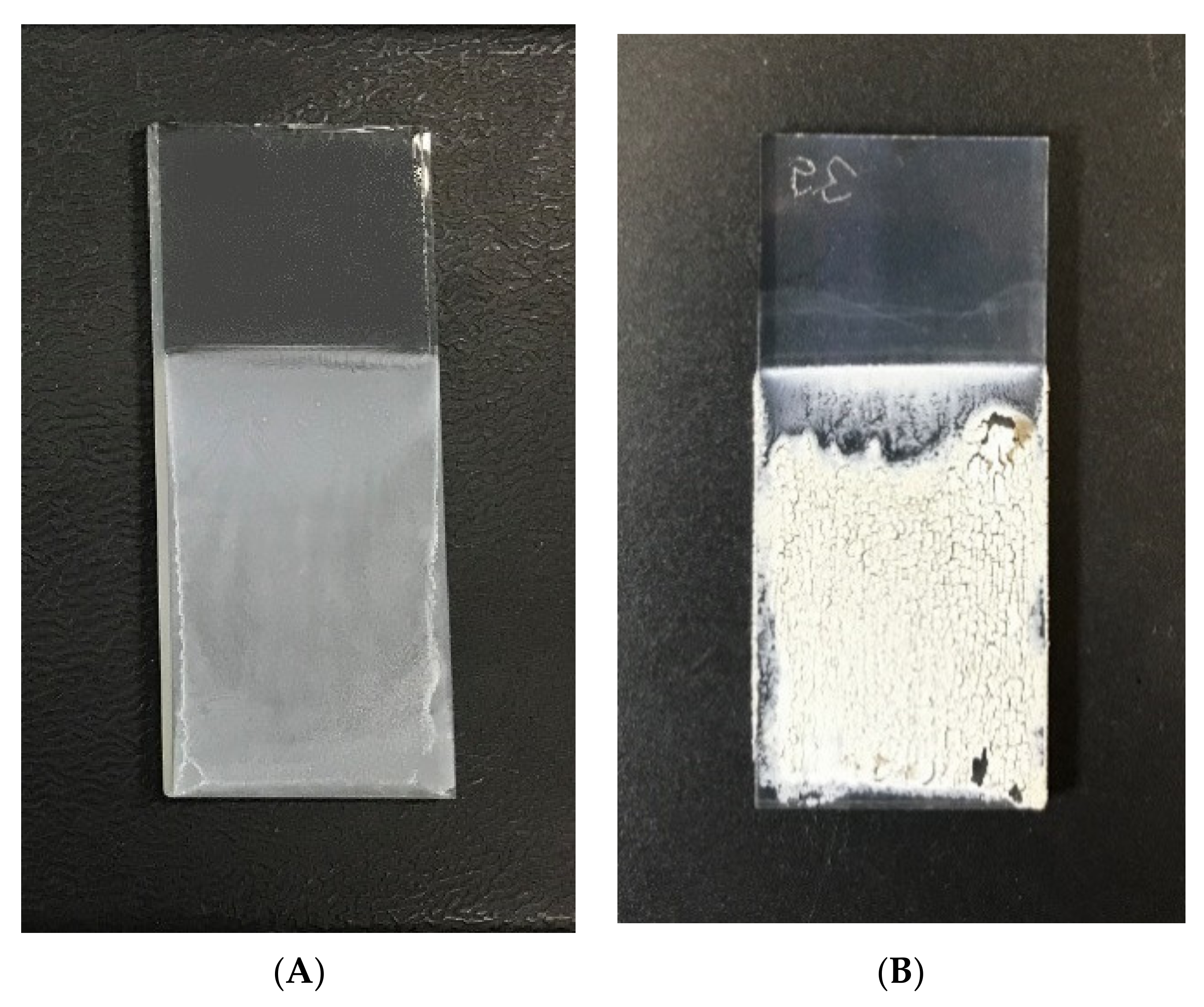
2.2. Electrophoretic Deposition
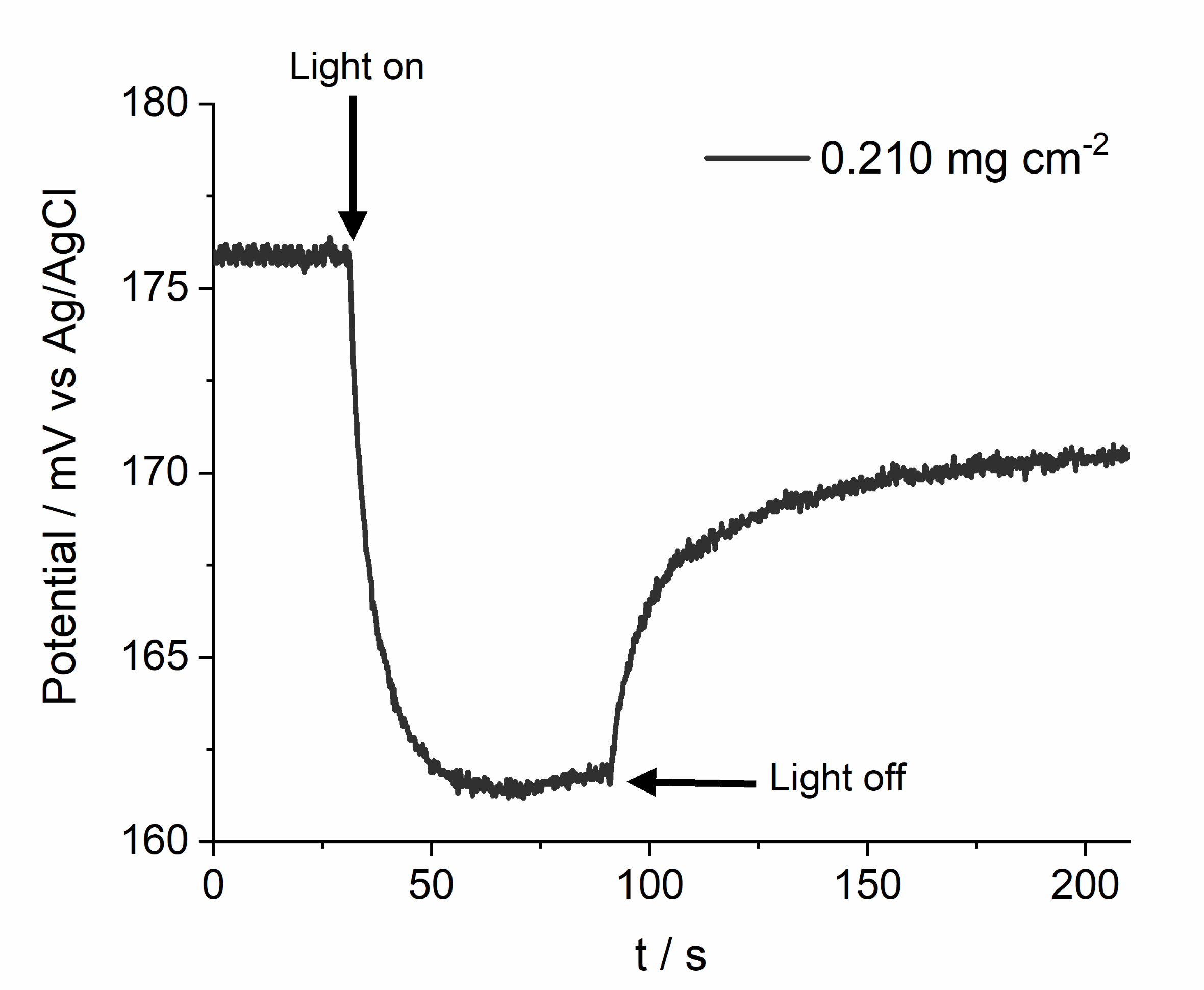
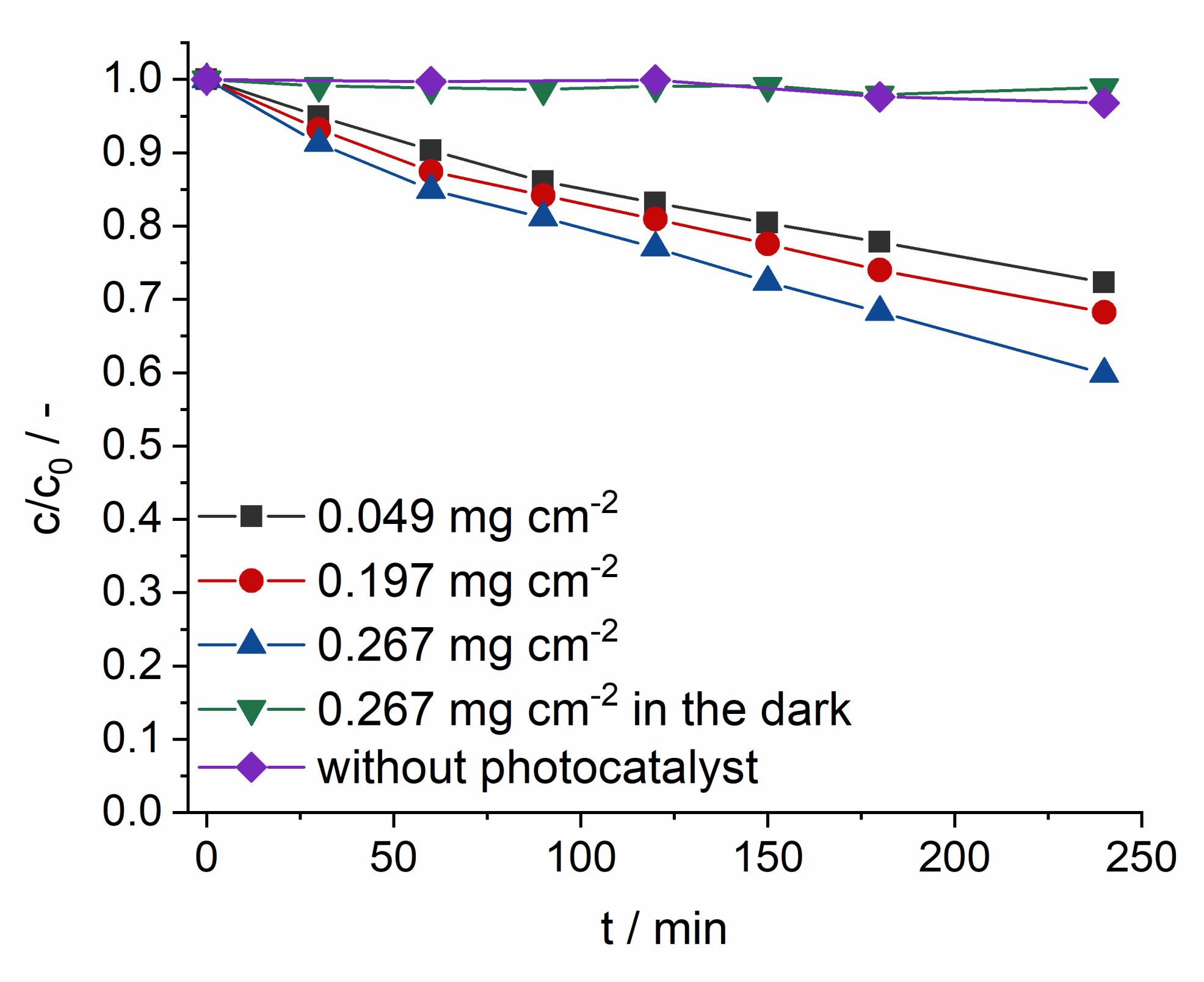
2.3. Photocatalytic Activity
3. Experimental
3.1. Materials, Chemicals, Synthesis of Catalyst
3.2. Preparation of Layers
3.3. Characterization and Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Bard, A.J. Design of semiconductor photoelectrochemical systems for solar energy conversion. J. Phys. Chem. 1982, 86, 172–177. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic Carbon Nitride: Synthesis, Properties, and Applications in Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Sun, X.; Liu, Y.; Ji, H.; Liu, W.; Wang, C.-C.; Ma, W.; Cai, Z. A carbon-rich g-C3N4 with promoted charge separation for highly efficient photocatalytic degradation of amoxicillin. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Ivanov, B.L.; Zambov, L.M.; Georgiev, G.T.; Popov, C.; Plass, M.F.; Kulisch, W. Low-Pressure CVD of Carbon Nitride Using Triazine-Containing Precursors. Chem. Vap. Depos. 1999, 5, 265–273. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.; Zou, Z.G. Photodegradation Performance of g-C3N4Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Fang, H.-B.; Luo, Y.; Zheng, Y.-Z.; Ma, W.; Tao, X. Facile Large-Scale Synthesis of Urea-Derived Porous Graphitic Carbon Nitride with Extraordinary Visible-Light Spectrum Photodegradation. Ind. Eng. Chem. Res. 2016, 55, 4506–4514. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Zong, R.; Zhu, Y. Enhancement of visible light photocatalytic activities via porous structure of g-C3N4. Appl. Catal. B Environ. 2014, 147, 229–235. [Google Scholar] [CrossRef]
- Dong, X.; Cheng, F. Recent development in exfoliated two-dimensional g-C3N4 nanosheets for photocatalytic applications. J. Mater. Chem. A 2015, 3, 23642–23652. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Dong, X.; Wang, H.; Dong, X. The amphoteric properties of g-C3N4 nanosheets and fabrication of their relevant heterostructure photocatalysts by an electrostatic re-assembly route. Chem. Commun. 2015, 51, 7176–7179. [Google Scholar] [CrossRef]
- Tong, J.; Cao, S.; Zhang, L.; Li, F. An efficient top-down approach for the fabrication of large-aspect-ratio g-C3N4 nanosheets with enhanced photocatalytic activities. Phys. Chem. Chem. Phys. 2015, 17, 23532–23537. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, H.; O’Donnell, K.M.; Ang, H.; Tadé, M.O.; Wang, S. Metal-free melem/g-C3N4 hybrid photocatalysts for water treatment. J. Colloid Interface Sci. 2016, 464, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, Z.; Li, Y.; Ho, W.-K.; Lee, S.C. Immobilization of Polymeric g-C3N4on Structured Ceramic Foam for Efficient Visible Light Photocatalytic Air Purification with Real Indoor Illumination. Environ. Sci. Technol. 2014, 48, 10345–10353. [Google Scholar] [CrossRef] [PubMed]
- Baudys, M.; Paušová, Š.; Praus, P.; Brezova, V.; Dvoranová, D.; Barbieriková, Z.; Krýsa, J. Graphitic Carbon Nitride for Photocatalytic Air Treatment. Materials 2020, 13, 3038. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, Q.; Bahadur, I.; Karlapudi, S.; Jiang, Y. A latest overview on photocatalytic application of g-C3N4 based nanostructured materials for hydrogen production. Int. J. Hydrog. Energy 2020, 45, 337–379. [Google Scholar] [CrossRef]
- Zou, X.; Sun, Z.; Hu, Y.H. g-C3N4-based photoelectrodes for photoelectrochemical water splitting: A review. J. Mater. Chem. A 2020, 8, 21474–21502. [Google Scholar] [CrossRef]
- Wena, X.; Wangb, W.; Yeb, Q.; Zhoub, Y.; Yangb, J.; Sunb, N.; Tanb, Y.; Wangb, W.; Houa, Y.; Yanb, C. One-step synthesis of rice husk carbon with dangling CC bonds loaded g-C3N4 for enhanced photocatalytic degradation. J. Clean. Prod. 2020, 272, 122625. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Lu, Y.; Liu, S.; Chen, X.; Wei, G.; Ye, G.; Chen, J. Microplasma electrochemistry (MIPEC) methods for improving the photocatalytic performance of g-C3N4 in degradation of RhB. Appl. Surf. Sci. 2020, 531, 147307. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.; Vázquez, A.; Sanchez-Martinez, D.; Obregón, S. Performance of the polymeric g-C3N4 photocatalyst through the degradation of pharmaceutical pollutants under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2016, 324, 47–52. [Google Scholar] [CrossRef]
- Senthil, R.; Theerthagiri, J.; Selvi, A.; Madhavan, J. Synthesis and characterization of low-cost g-C3N4/TiO2 composite with enhanced photocatalytic performance under visible-light irradiation. Opt. Mater. 2017, 64, 533–539. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Fang, J.; Gao, L.; Cai, W.; Li, X.; Xu, A.; Ruan, X. The photocatalytic performance of g-C3N4 from melamine hydrochloride for dyes degradation with peroxymonosulfate. J. Photochem. Photobiol. A Chem. 2017, 336, 54–62. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, J.; Fu, X.; Wang, X. Metal-free photocatalytic degradation of 4-chlorophenol in water by mesoporous carbon nitride semiconductors. Catal. Sci. Technol. 2012, 2, 1396–1402. [Google Scholar] [CrossRef]
- Xu, J.; Shalom, M. Electrophoretic Deposition of Carbon Nitride Layers for Photoelectrochemical Applications. ACS Appl. Mater. Interfaces 2016, 8, 13058–13063. [Google Scholar] [CrossRef]
- Seo, Y.J.; Das, P.K.; Arunachalam, M.; Ahn, K.-S.; Ha, J.-S.; Kang, S.H. Drawing the distinguished graphite carbon nitride (g-C3N4) on SnO2 nanoflake film for solar water oxidation. Int. J. Hydrog. Energy 2020, 45, 22567–22575. [Google Scholar] [CrossRef]
- Praus, P.; Smýkalová, A.; Foniok, K.; Velíšek, P.; Cvejn, D.; Zadny, J.; Storch, J. Post-Synthetic Derivatization of Graphitic Carbon Nitride with Methanesulfonyl Chloride: Synthesis, Characterization and Photocatalysis. Nanomaterials 2020, 10, 193. [Google Scholar] [CrossRef]
- Wang, L.; Tong, Y.; Feng, J.; Hou, J.; Li, J.; Hou, X.; Liang, J. g-C3N4-based films: A rising star for photoelectrochemical water splitting. Sustain. Mater. Technol. 2019, 19, e00089. [Google Scholar] [CrossRef]
- Krýsa, J.; Baudys, M.; Zlámal, M.; Krysova, H.; Morozová, M.; Klusoň, P. Photocatalytic and photoelectrochemical properties of sol–gel TiO2 films of controlled thickness and porosity. Catal. Today 2014, 230, 2–7. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Momeni, S.; Nematollahi, D. New insights into the electrochemical behavior of acid orange 7: Convergent paired electrochemical synthesis of new aminonaphthol derivatives. Sci. Rep. 2017, 7, srep41963. [Google Scholar] [CrossRef]
- Troupis, A.; Triantis, T.; Gkika, E.; Hiskia, A.; Papaconstantinou, E. Photocatalytic reductive–oxidative degradation of Acid Orange 7 by polyoxometalates. Appl. Catal. B Environ. 2009, 86, 98–107. [Google Scholar] [CrossRef]
- Waldner, G.; Krýsa, J. Photocurrents and degradation rates on particulate TiO2 layers. Electrochim. Acta 2005, 50, 4498–4504. [Google Scholar] [CrossRef]
- Kotrla, T.; Paušová, Š.; Zlámal, M.; Neumann-Spallart, M.; Krýsa, J. Preparation of Sn-doped semiconducting Fe2O3 (hematite) layers by aerosol pyrolysis. Catal. Today 2018, 313, 2–5. [Google Scholar] [CrossRef]


| Sample | Dx(50) [µm] | Particle Size Range [µm] |
|---|---|---|
| Ex-CN as prepared | 93 | 5–700 |
| Ex-CN settled particles from aq. susp. 3 h US and dried * | 14 | 0.5–90 |
| Ex-CN settled particles from eth. susp 3 h US and dried * | 12 | 0.3–80 |
| Ex-CN stable part of aq. susp. 3 h US * | 2 | 0.2–20 |
| Ex-CN stable part of eth. susp 3 h US * | 2 | 0.1–10 |
| Age of Electrolyte | Deposition Time/s | Deposited Amount/mg cm−2 |
|---|---|---|
| 1 h | 2 | 0.040 ± 1 * |
| 5 | 0.089 ± 1 * | |
| 30 | 1.356 ± 30 * | |
| One week | 5 | 0.050 ± 1 |
| 30 | 0.201 ± 4 | |
| 50 | 0.265 ± 3 | |
| Twelve weeks | 5 | 0.049 ± 1 |
| 30 | 0.206 ± 4 | |
| 50 | 0.264 ± 3 | |
| 70 | 0.305 ± 4 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusek, J.; Paušová, Š.; Praus, P.; Krýsa, J. Immobilization of Exfoliated g-C3N4 for Photocatalytical Removal of Organic Pollutants from Water. Catalysts 2021, 11, 203. https://doi.org/10.3390/catal11020203
Rusek J, Paušová Š, Praus P, Krýsa J. Immobilization of Exfoliated g-C3N4 for Photocatalytical Removal of Organic Pollutants from Water. Catalysts. 2021; 11(2):203. https://doi.org/10.3390/catal11020203
Chicago/Turabian StyleRusek, Jakub, Šárka Paušová, Petr Praus, and Josef Krýsa. 2021. "Immobilization of Exfoliated g-C3N4 for Photocatalytical Removal of Organic Pollutants from Water" Catalysts 11, no. 2: 203. https://doi.org/10.3390/catal11020203
APA StyleRusek, J., Paušová, Š., Praus, P., & Krýsa, J. (2021). Immobilization of Exfoliated g-C3N4 for Photocatalytical Removal of Organic Pollutants from Water. Catalysts, 11(2), 203. https://doi.org/10.3390/catal11020203







