Characteristics of High Surface Area Molybdenum Nitride and Its Activity for the Catalytic Decomposition of Ammonia
Abstract
1. Introduction
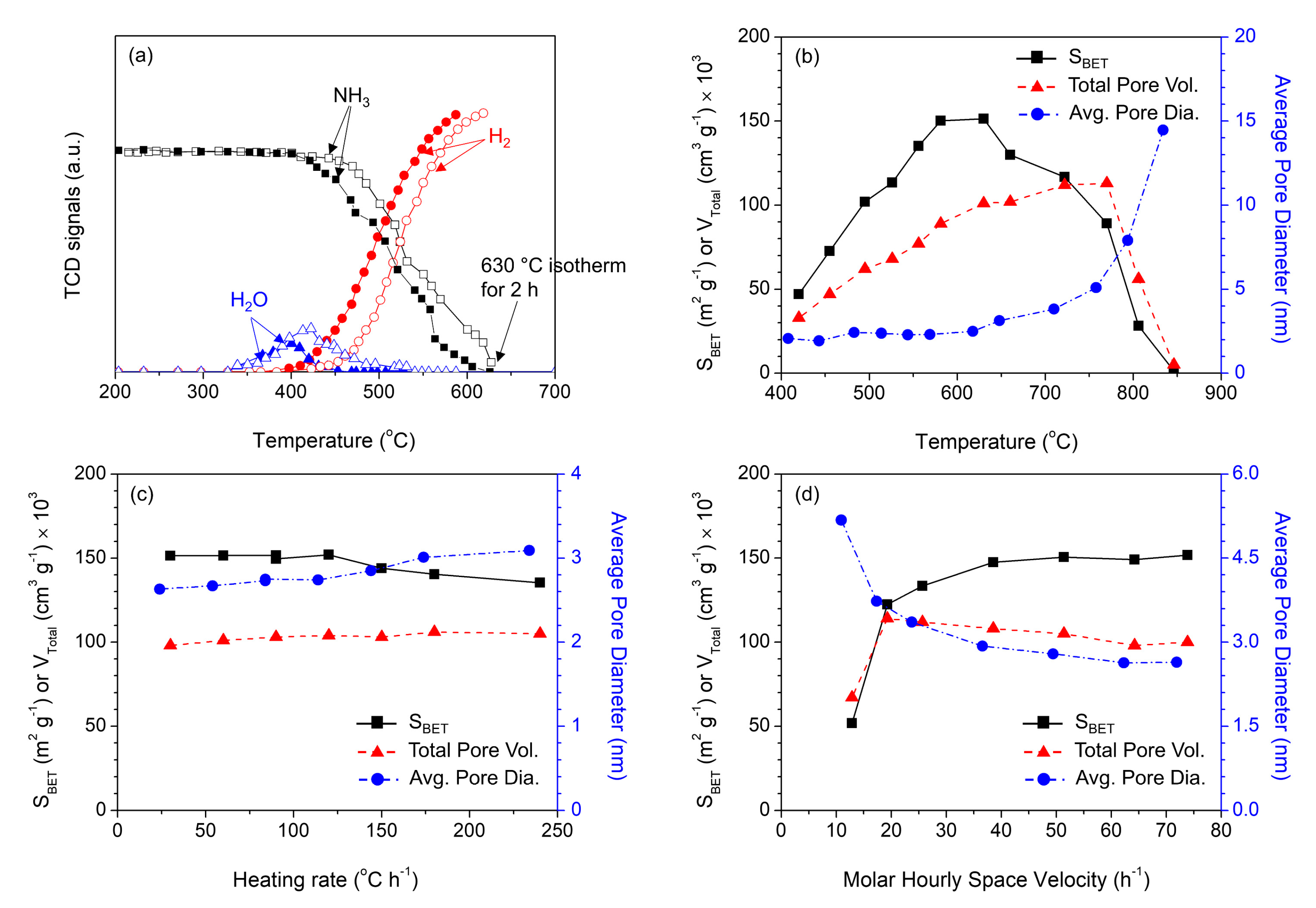
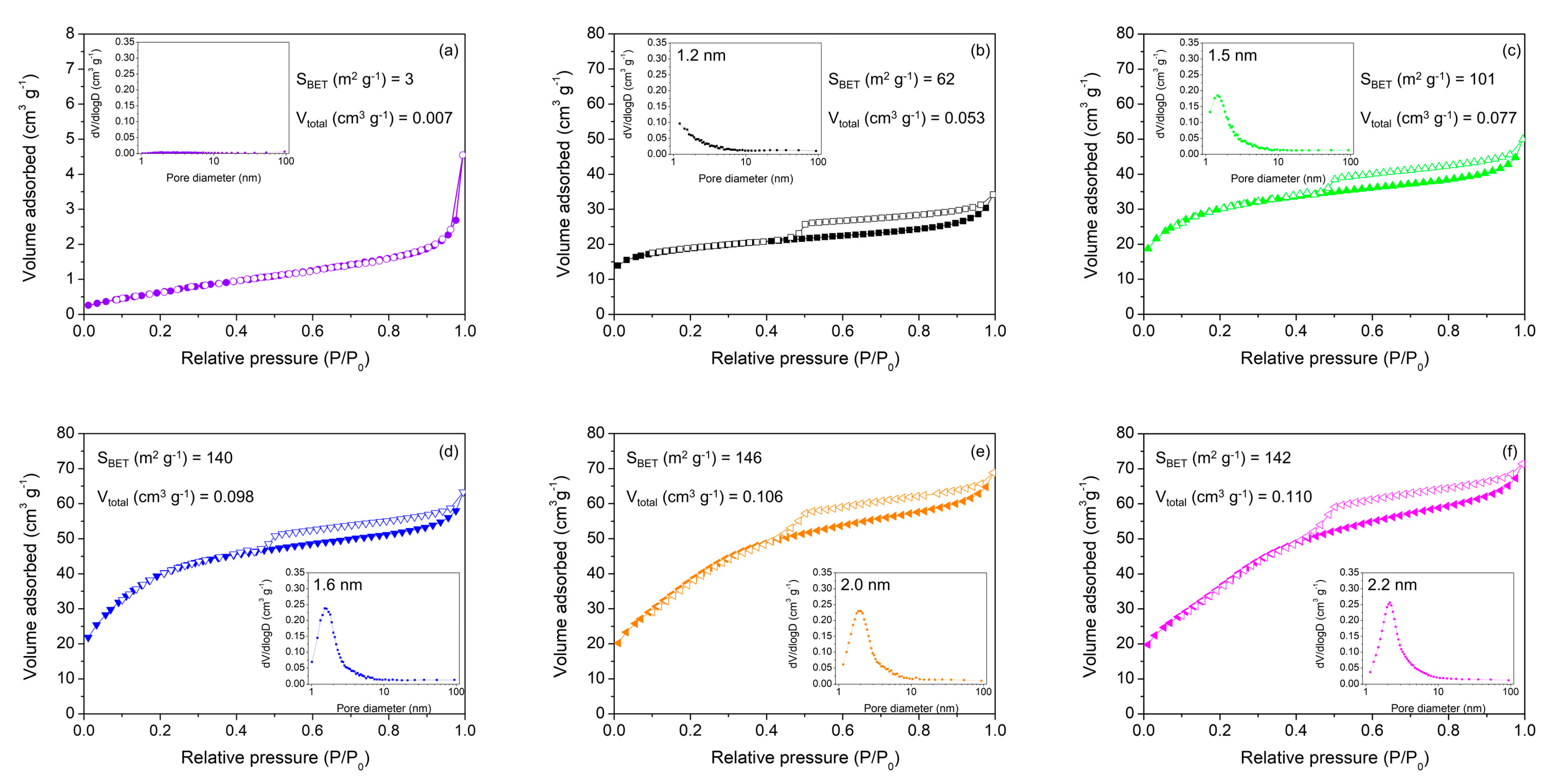
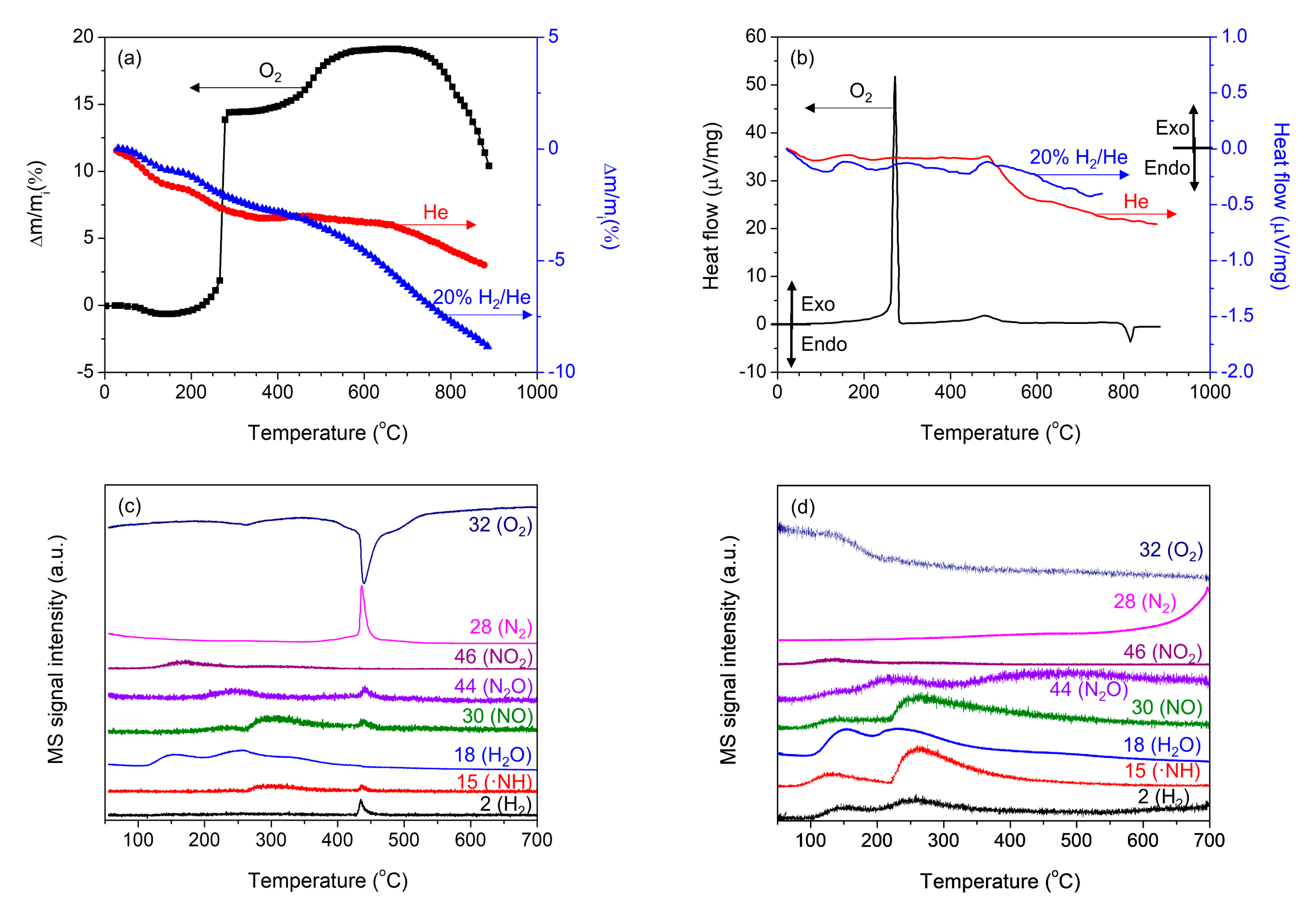
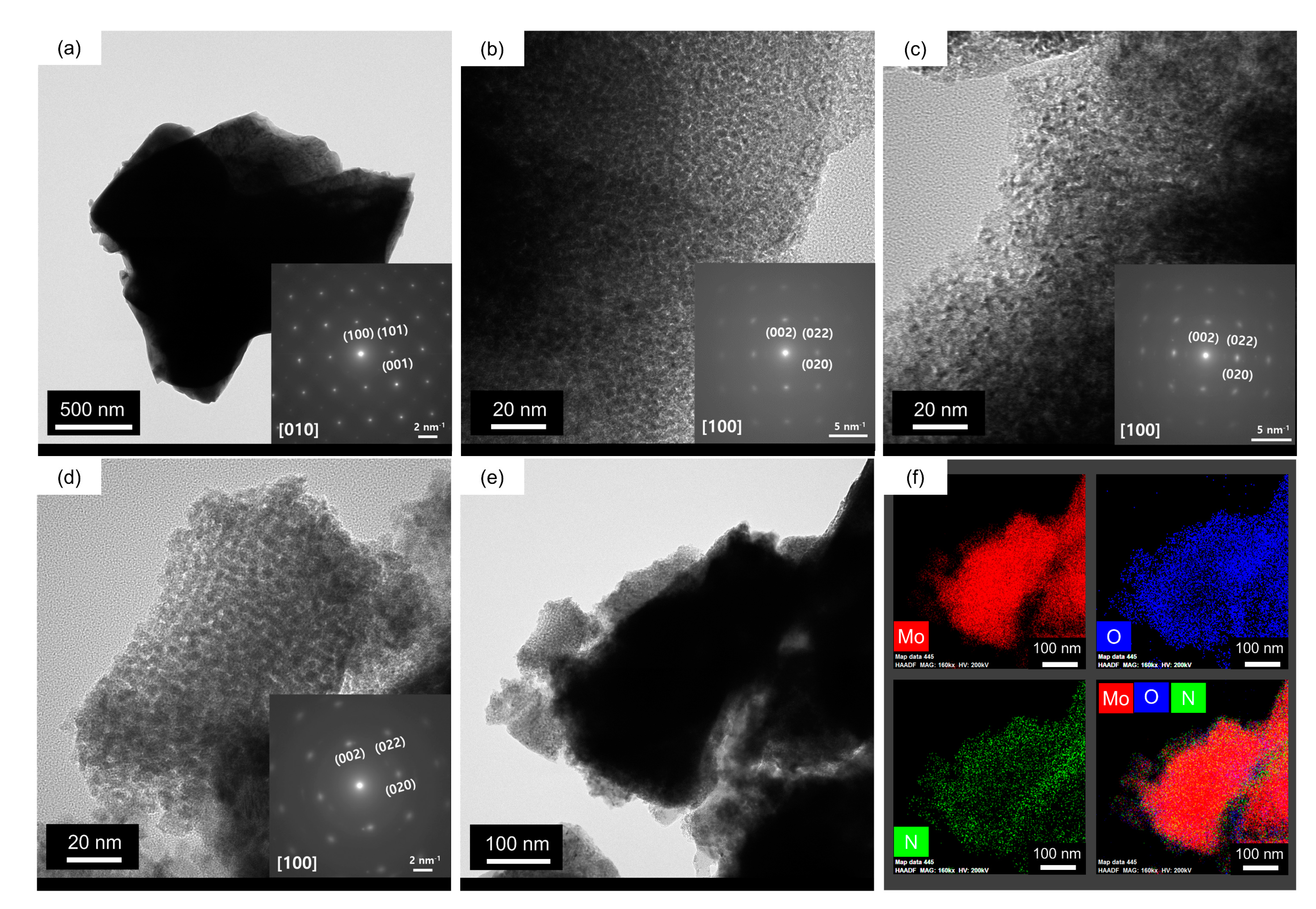
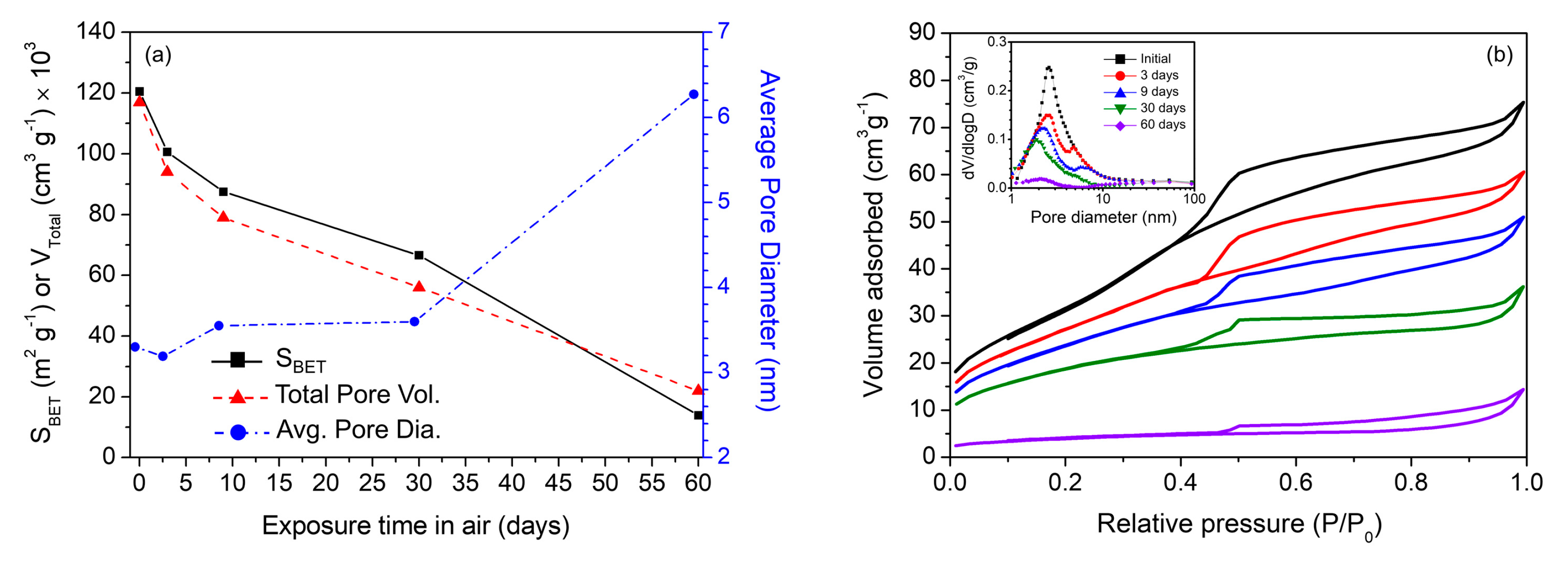
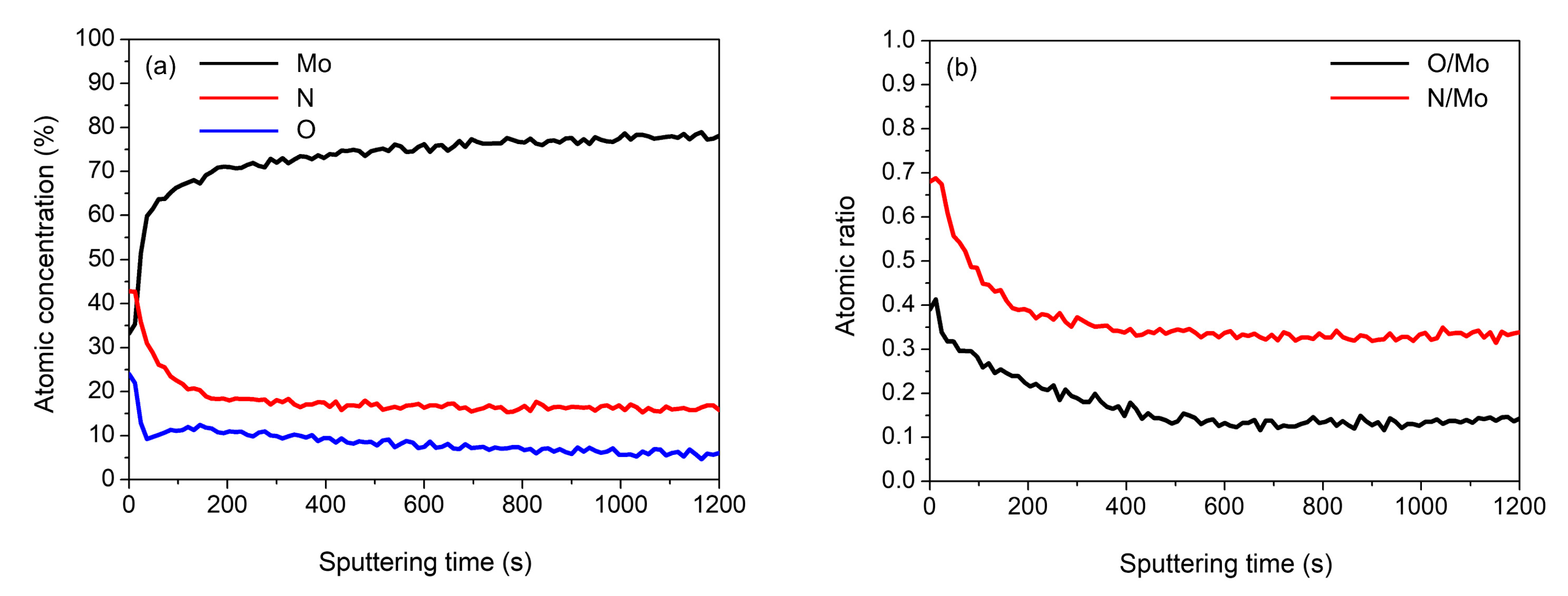
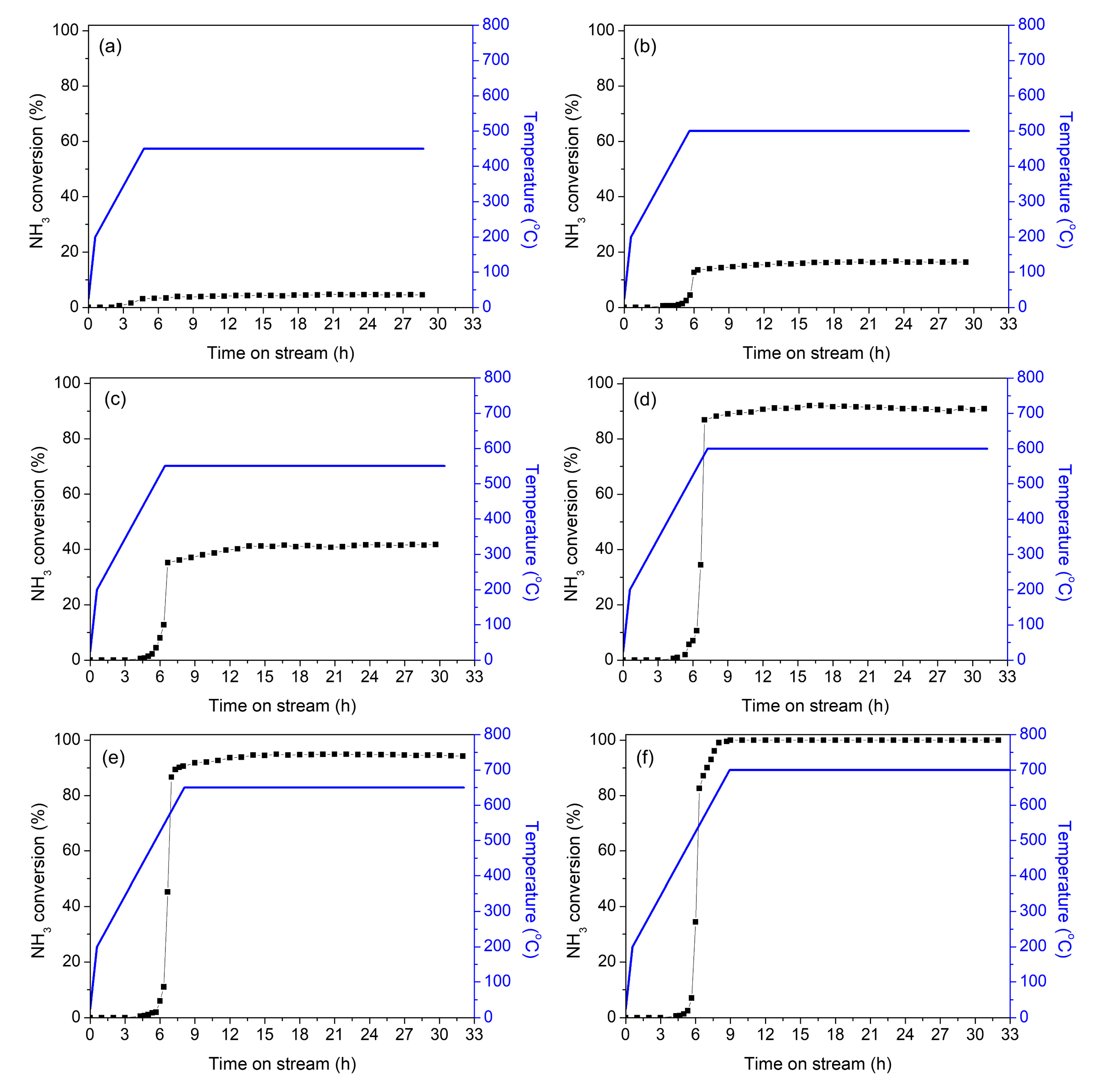
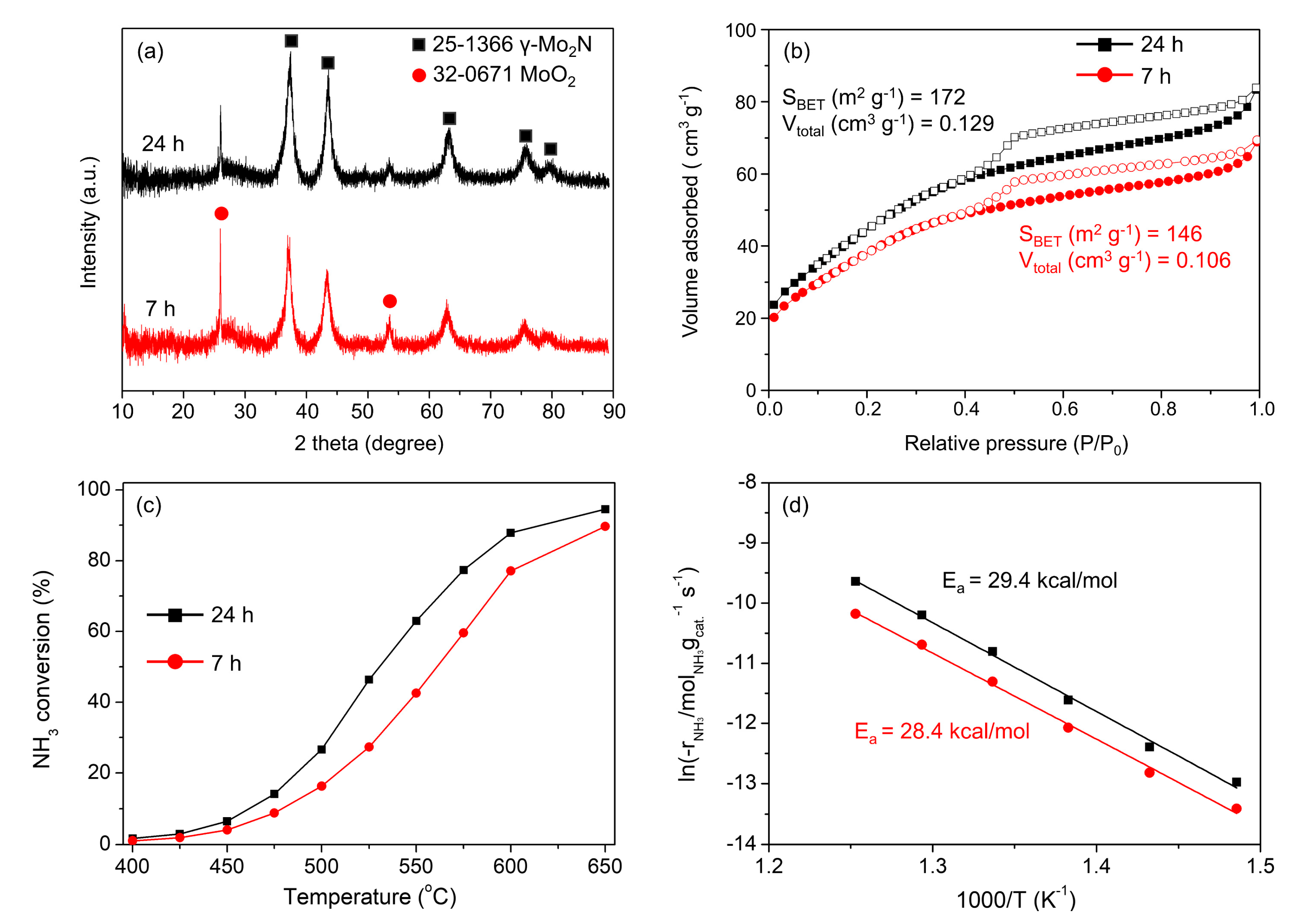
2. Results and Discussion
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, A.-M.; Hargreaves, J.S.J. Alternative catalytic materials: Carbides, nitrides, phosphides and amorphous boron alloys. Chem. Soc. Rev. 2010, 39, 4388–4401. [Google Scholar] [CrossRef] [PubMed]
- Kung, H.H. Transition Metal Oxides: Surface Chemistry and Catalysis; Elsevier: Burlington, MA, USA, 1989; Volume 45. [Google Scholar]
- Kral, C.; Lengauer, W.; Rafaja, D.; Ettmayer, P. Critical review on the elastic properties of transition metal carbides, nitrides and carbonitrides. J. Alloys Compd. 1998, 265, 215–233. [Google Scholar] [CrossRef]
- Oyama, S.T. Transition Metal Carbides, Nitrides, and Phosphides. In Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl, G., Knözinger, H., Weitkamp, J., Eds.; Wiley-VCH, Cop.: Weinheim, Allemagne, 2008; Volume 8, pp. 342–355. [Google Scholar]
- Jaggers, C.H.; Michaels, J.N.; Stacy, A.M. Preparation of high-surface-area transition-metal nitrides: Molybdenum nitrides, Mo2N and MoN. Chem. Mater. 1990, 2, 150–157. [Google Scholar] [CrossRef]
- Volpe, L.; Boudart, M. Compounds of molybdenum and tungsten with high specific surface area: I. Nitrides. J. Solid State Chem. 1985, 59, 332–337. [Google Scholar] [CrossRef]
- Volpe, L.; Boudart, M. Compounds of molybdenum and tungsten with high specific surface area: II. Carbides. J. Solid State Chem. 1985, 59, 338–356. [Google Scholar] [CrossRef]
- Shin, C.-H.; Bugli, G.; Djéga-Mariadassou, G. Preparation and characterization of titanium oxynitrides with high specific surface areas. J. Solid State Chem. 1991, 95, 145–155. [Google Scholar] [CrossRef]
- Tomas-Garcia, A.L.; Li, Q.; Jensen, J.O.; Bjerrum, N.J. High surface area tungsten carbides: Synthesis, characterization and catalytic activity towards the hydrogen evolution reaction in phosphoric acid at elevated temperatures. Int. J. Electrochem. Sci. 2014, 9, 1016–1032. [Google Scholar]
- Iglesia, E.; Ribeirob, F.H.; Boudart, M.; Baumgartner, J.E. Synthesis, characterization, and catalytic properties of clean and oxygen-modified tungsten carbides. Catal. Today 1992, 15, 307–337. [Google Scholar] [CrossRef]
- Podila, S.; Zaman, S.F.; Driss, H.; Alhamed, Y.A.; Al-Zahrani, A.A.; Petrov, L.A. Hydrogen production by ammonia decomposition using high surface area Mo2N and Co3Mo3N catalysts. Catal. Sci. Technol. 2016, 6, 1496–1506. [Google Scholar] [CrossRef]
- Podila, S.; Zaman, S.F.; Driss, H.; Al-Zahrani, A.A.; Daous, M.A.; Petrov, L.A. High performance of bulk Mo2N and Co3Mo3N catalysts for hydrogen production from ammonia: Role of citric acid to Mo molar ratio in preparation of high surface area nitride catalysts. Int. J. Hydrogen Energy 2017, 42, 8006–8020. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Raval, R.; Li, C.; Zhai, R.; Xin, Q. The modification of molybdenum nitrides: The effect of the second metal component. Catal. Lett. 1997, 48, 239–245. [Google Scholar] [CrossRef]
- Srifa, A.; Okura, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. COx-free hydrogen production via ammonia decomposition over molybdenum nitride-based catalysts. Catal. Sci. Technol. 2016, 6, 7495–7504. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Norskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mat. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Using ammonia as a sustainable fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.R.F.; Wu, G. Low-temperature ammonia decomposition catalysts for hydrogen generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Hill, A.K.; Torrente-Murciano, L. Low temperature H2 production from ammonia using ruthenium-based catalysts: Synergetic effect of promoter and support. Appl. Catal. B Environ. 2015, 172–173, 129–135. [Google Scholar] [CrossRef]
- Yin, S.-F.; Zhang, Q.-H.; Xu, B.-Q.; Zhu, W.-X.; Ng, C.-F.; Au, C.-T. Investigation on the catalysis of COx-free hydrogen generation from ammonia. J. Catal. 2004, 224, 384–396. [Google Scholar] [CrossRef]
- Duan, X.; Qian, G.; Zhou, X.; Sui, Z.; Chen, D.; Yuan, W. Tuning the size and shape of Fe nanoparticles on carbon nanofibers for catalytic ammonia decomposition. Appl. Catal. B Environ. 2011, 101, 189–196. [Google Scholar] [CrossRef]
- Bell, T.E.; Torrente-Murciano, L. H2 production via ammonia decomposition using non-noble metal catalysts: A review. Top. Catal. 2016, 59, 1438–1457. [Google Scholar] [CrossRef]
- Gurram, V.R.B.; Enumula, S.S.; Chada, R.R.; Koppadi, K.S.; Burri, D.R.; Kamaraju, S.R.R. Synthesis and industrial catalytic applications of binary and ternary molybdenum nitrides: A review. Catal. Surv. Asia 2018, 22, 166–180. [Google Scholar] [CrossRef]
- Lendzion-Bielun, Z.; Narkiewicz, U.; Arabczyk, W. Cobalt-based catalysts for ammonia decomposition. Materials 2013, 6, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Zeinalipour-Yazdi, C.D.; Hargreaves, J.S.J.; Catlow, C.R.A. Low-T Mechanisms of ammonia synthesis on Co3Mo3N. J. Phys. Chem. C 2018, 122, 6078–6082. [Google Scholar] [CrossRef]
- Mckay, D.; Hargreaves, J.S.J.; Rico, J.L.; Rivera, J.L.; Sun, X.-L. The influence of phase and morphology of molybdenum nitrides on ammonia synthesis activity and reduction characteristics. J. Solid State Chem. 2008, 181, 325–333. [Google Scholar] [CrossRef]
- Nishibyashi, Y. Molybdenum-catalyzed reduction of molecular dinitrogen into ammonia under ambient reaction conditions. C.R. Chimie 2015, 18, 776–784. [Google Scholar] [CrossRef]
- Nagai, M.; Goto, Y.; Miyata, A.; Kiyoshi, M.; Hada, K.; Oshikawa, K.; Omi, S. Temperature-programmed reduction and XRD studies of ammonia-treated molybdenum oxide and its activity for carbazole hydrodenitrogenation. J. Catal. 1992, 182, 292–301. [Google Scholar] [CrossRef]
- Li, S.; Lee, J.S. Molybdenum nitride and carbide prepared from heteropolyacid. II. Hydrodenitrogenation of indole. J. Catal. 1998, 173, 134–144. [Google Scholar]
- Chen, X.; Zhang, T.; Zheng, M.; Wu, Z.; Wu, W.; Li, C. The reaction route and active site of catalytic decomposition of hydrazine over molybdenum nitride catalyst. J. Catal. 2004, 224, 473–478. [Google Scholar] [CrossRef]
- He, H.; Dai, H.X.; Ngan, K.Y.; Au, C.T. Molybdenum nitride for the direct decomposition of NO. Catal. Lett. 2001, 71, 147–153. [Google Scholar] [CrossRef]
- Inumaru, K.; Baba, K.; Yamanaka, S. Synthesis and characterization of superconducting β-Mo2N crystalline phase on a Si substrate: An application of pulsed laser deposition to nitride chemistry. Chem. Mater. 2005, 17, 5935–5940. [Google Scholar] [CrossRef]
- Dewangan, K.; Patil, S.S.; Joag, D.S.; More, M.A.; Gajbhiye, N.S. Topotactical nitridation of α-MoO3 fibers to γ-Mo2N fibers and its field emission properties. J. Phys. Chem. C 2010, 114, 14710–14715. [Google Scholar] [CrossRef]
- Wei, Z.B.; Xin, Q.; Grange, P.; Delmon, B. Surface species and the stability of γ-Mo2N. Solid State Ion. 1997, 101–103, 761–767. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Gómez-Quero, S.; Perret, N.; Kiwi-Minsker, L.; Keane, M.A. β-Molybdenum nitride: Synthesis mechanism and catalytic response in the gas phase hydrogenation of p-chloronitrobenzene. Catal. Sci. Technol. 2011, 1, 794–801. [Google Scholar]
- Cárdenas-Lizana, F.; Lamey, D.; Kiwi-Minsker, L.; Keane, M.A. Molybdenum nitrides: A study of synthesis variables and catalytic performance in acetylene hydrogenation. J. Mater. Sci. 2018, 53, 6707–6718. [Google Scholar] [CrossRef]
- Gong, S.; Chen, H.; Li, W.; Li, B. Synthesis of β-Mo2N0.78 hydrodesulfurization catalyst in mixtures of nitrogen and hydrogen. Appl. Catal. A Gen. 2005, 279, 257–261. [Google Scholar] [CrossRef]
- Choi, J.-G.; Brenner, J.R.; Colling, C.W.; Demczyk, B.G.; Dunning, J.L.; Thompson, L.T. Synthesis and characterization of molybdenum nitride hydrodenitrogenation catalysts. Catal. Today 1992, 15, 201–222. [Google Scholar] [CrossRef]
- Spevack, P.A.; Mclntyre, N.S. Thermal reduction of molybdenum trioxide. J. Phys. Chem. 1992, 96, 9029–9035. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; ISBN 9780123009500. [Google Scholar]
- Chen, J.; Wei, Q. Phase transformation of molybdenum trioxide to molybdenum dioxide: An in-situ transmission electron microscopy investigation. Int. J. Appl. Ceram. Technol. 2017, 14, 1020–1025. [Google Scholar] [CrossRef]
- Choi, J.-G.; Curl, R.L.; Thompson, L.T. Molybdenum nitride catalysts I. Influence of the synthesis factors on structural properties. J. Catal. 1994, 146, 218–227. [Google Scholar] [CrossRef]
- Lee, K.-H.; Lee, Y.-W.; Kwak, D.-H.; Moon, J.-S.; Park, A.-R.; Hwang, E.-T.; Park, K.-W. Single-crystalline mesoporous Mo2N nanobelts with an enhanced electrocatalytic activity for oxygen reduction reaction. Mater. Lett. 2014, 124, 231–234. [Google Scholar]
- Clayton, C.R. Passivity Mechanisms in Stainless Steels: Mo-N Synergism; State University of New York at Stony Brook, Department of Materials Science and Engineering, Stony Brook: New York, NY, USA, 1986. [Google Scholar]
- Yuan, Y.; Zhang, B.; Sun, J.; Jonnard, P.; Le Guen, K.; Tu, Y.; Yan, C.; Lan, R. Structure and optical properties of CrOxNy films with composition modulation. Surf. Eng. 2019, 36, 411–417. [Google Scholar] [CrossRef]
- Su, D.; Zhang, X.; Wu, A. CoO-Mo2N hollow heterostructure for high-efficiency electrocatalytic hydrogen evolution reaction. NPG Asia Mater. 2019, 11, 78. [Google Scholar] [CrossRef]
- Wei, Z.B.Z.; Grange, P.; Delmon, B. XPS and XRD studies of fresh and sulfided Mo2N. Appl. Surf. Sci. 1998, 135, 107–114. [Google Scholar] [CrossRef]
- Zheng, W.; Cotter, T.P.; Kaghazchi, P.; Jacob, T.; Frank, B.; Schlichte, K.; Zhang, W.; Su, D.S.; Schüth, F.; Schlӧgl, R. Experimental and theoretical investigation of molybdenum carbide and nitride as catalysts for ammonia decomposition. J. Am. Chem. Soc. 2013, 135, 3458–3464. [Google Scholar] [CrossRef] [PubMed]

| Sample | N 1s | O 1s | Mo 3d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binding Energy (eV), (at.%) | Binding Energy (eV), (at.%) | Binding Energy (eV), (at.%) | ||||||||||
| Mo 3p3/2 | O-N | NH3 | NH4+ | O2− | OH- | O-N | Mo-N | Mo4+ | Mo5+ | Mo6+ | Mo-OH | |
| (a) MoO3 | 398.7 (100) | - | - | - | 530.7 (88.1) | 531.9 (11.9) | - | - | - | - | 232.8 (77.8) | 233.7 (22.2) |
| (b) MN (550) 1 | 395.6 (41.9) | 397.4 (29.5) | 399.0 (24.1) | 401.2 (4.5) | 530.6 (69.4) | 531.8 (21.7) | 533.1 (8.9) | 229.0 (5.1) | 229.7 (36.5) | 230.8 (20.5) | 232.5 (32.7) | 233.6 (5.2) |
| (c) MN (600) | 395.2 (44.0) | 397.5 (33.6) | 399.2 (17.9) | 401.2 (4.5) | 530.6 (69.2) | 531.8 (21.1) | 532.9 (9.7) | 229.0 (24.2) | 229.7 (20.5) | 230.8 (18.7) | 232.7 (31.5) | 233.8 (5.1) |
| (d) MN (650) | 395.2 (46.9) | 397.6 (33.8) | 399.2 (15.1) | 401.3 (4.2) | 530.7 (68.8) | 531.9 (20.8) | 532.9 (10.4) | 228.9 (32.7) | 229.7 (19.7) | 230.8 (17.2) | 232.7 (25.5) | 233.8 (4.9) |
| (e) MN (700) | 395.2 (49.4) | 397.7 (34.1) | 399.4 (13.1) | 401.3 (3.4) | 530.7 (68.1) | 531.8 (20.5) | 532.9 (11.4) | 228.9 (34.2) | 229.7 (19.3) | 230.7 (17.1) | 232.7 (25.0) | 233.8 (4.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.-H.; Yun, K.; Kang, D.-C.; An, H.; Park, M.B.; Shin, C.-H.; Min, H.-K. Characteristics of High Surface Area Molybdenum Nitride and Its Activity for the Catalytic Decomposition of Ammonia. Catalysts 2021, 11, 192. https://doi.org/10.3390/catal11020192
Baek S-H, Yun K, Kang D-C, An H, Park MB, Shin C-H, Min H-K. Characteristics of High Surface Area Molybdenum Nitride and Its Activity for the Catalytic Decomposition of Ammonia. Catalysts. 2021; 11(2):192. https://doi.org/10.3390/catal11020192
Chicago/Turabian StyleBaek, Seo-Hyeon, Kyunghee Yun, Dong-Chang Kang, Hyejin An, Min Bum Park, Chae-Ho Shin, and Hyung-Ki Min. 2021. "Characteristics of High Surface Area Molybdenum Nitride and Its Activity for the Catalytic Decomposition of Ammonia" Catalysts 11, no. 2: 192. https://doi.org/10.3390/catal11020192
APA StyleBaek, S.-H., Yun, K., Kang, D.-C., An, H., Park, M. B., Shin, C.-H., & Min, H.-K. (2021). Characteristics of High Surface Area Molybdenum Nitride and Its Activity for the Catalytic Decomposition of Ammonia. Catalysts, 11(2), 192. https://doi.org/10.3390/catal11020192







