Fenton Degradation of Ofloxacin Using a Montmorillonite–Fe3O4 Composite
Abstract
1. Introduction
2. Results and Discussion
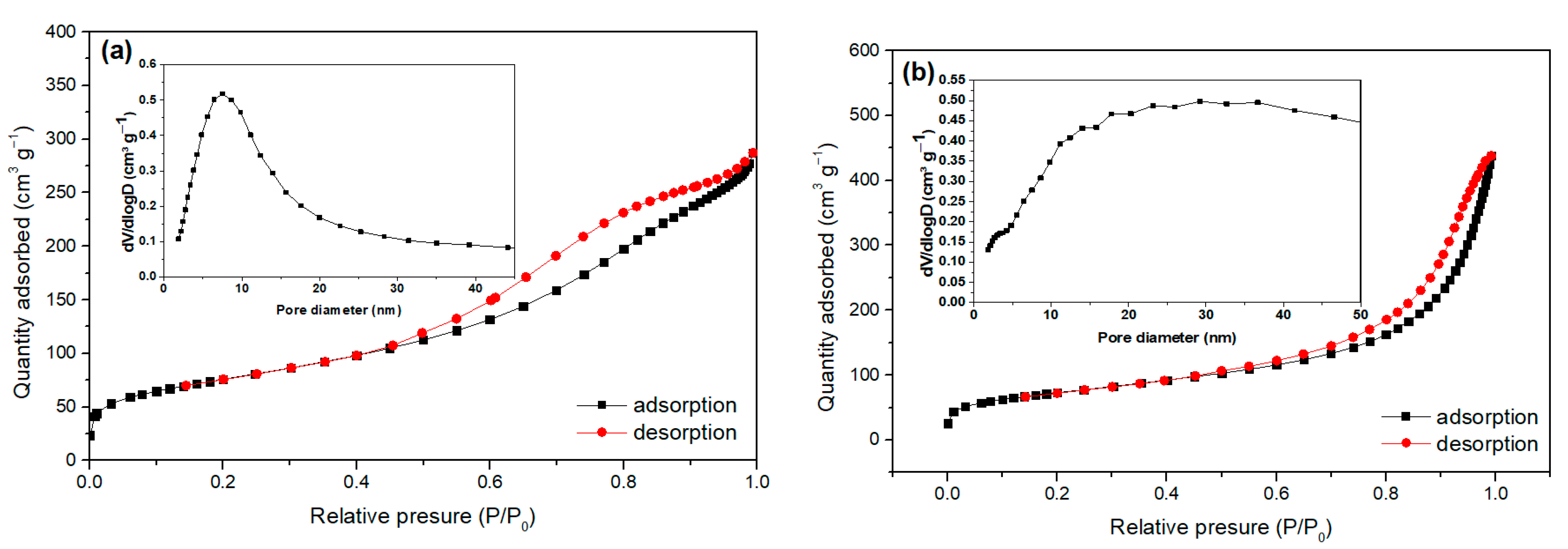
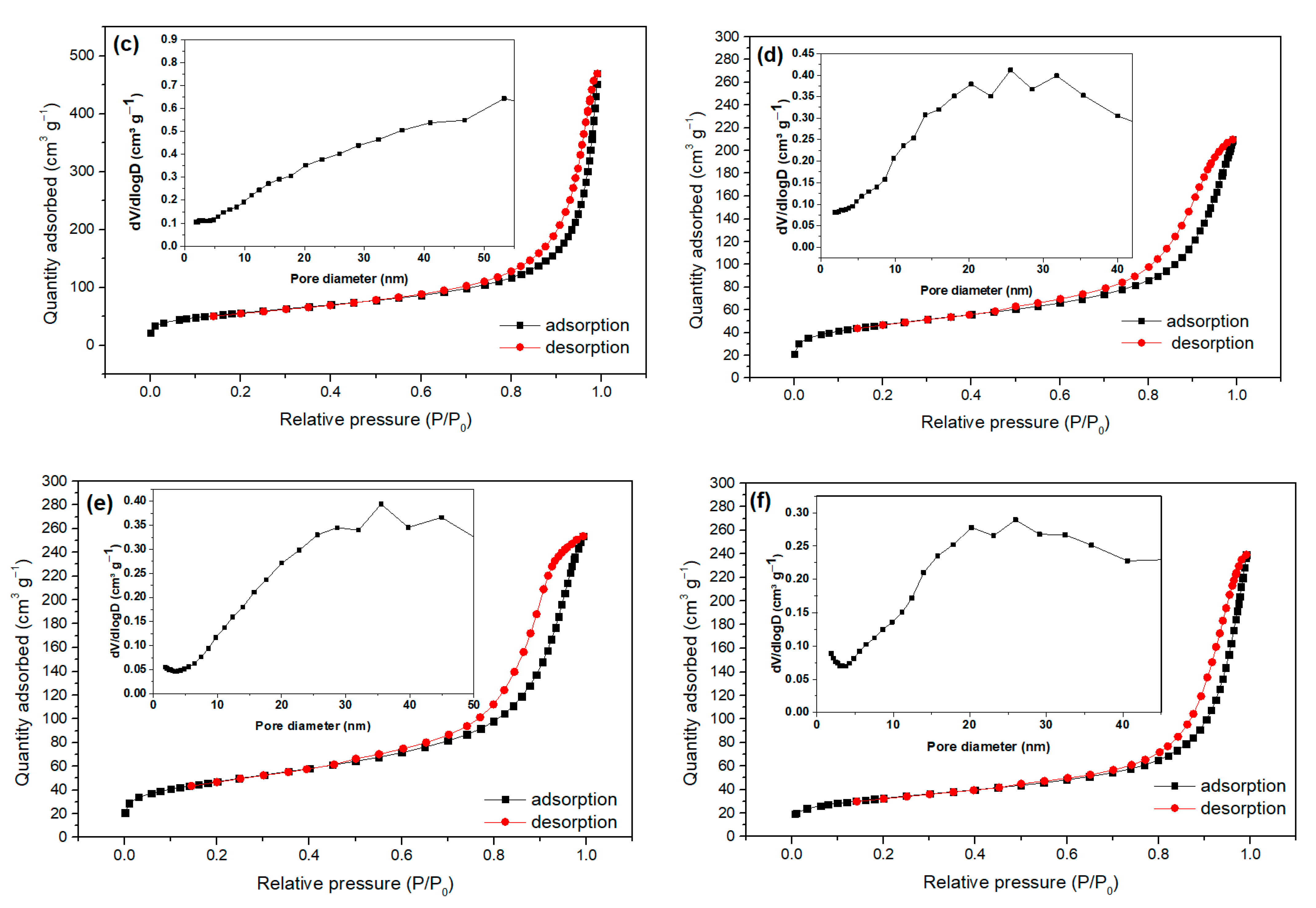
2.1. Characterization of Bare Montmorillonite and Fe3O4–Montmorillonite Composites
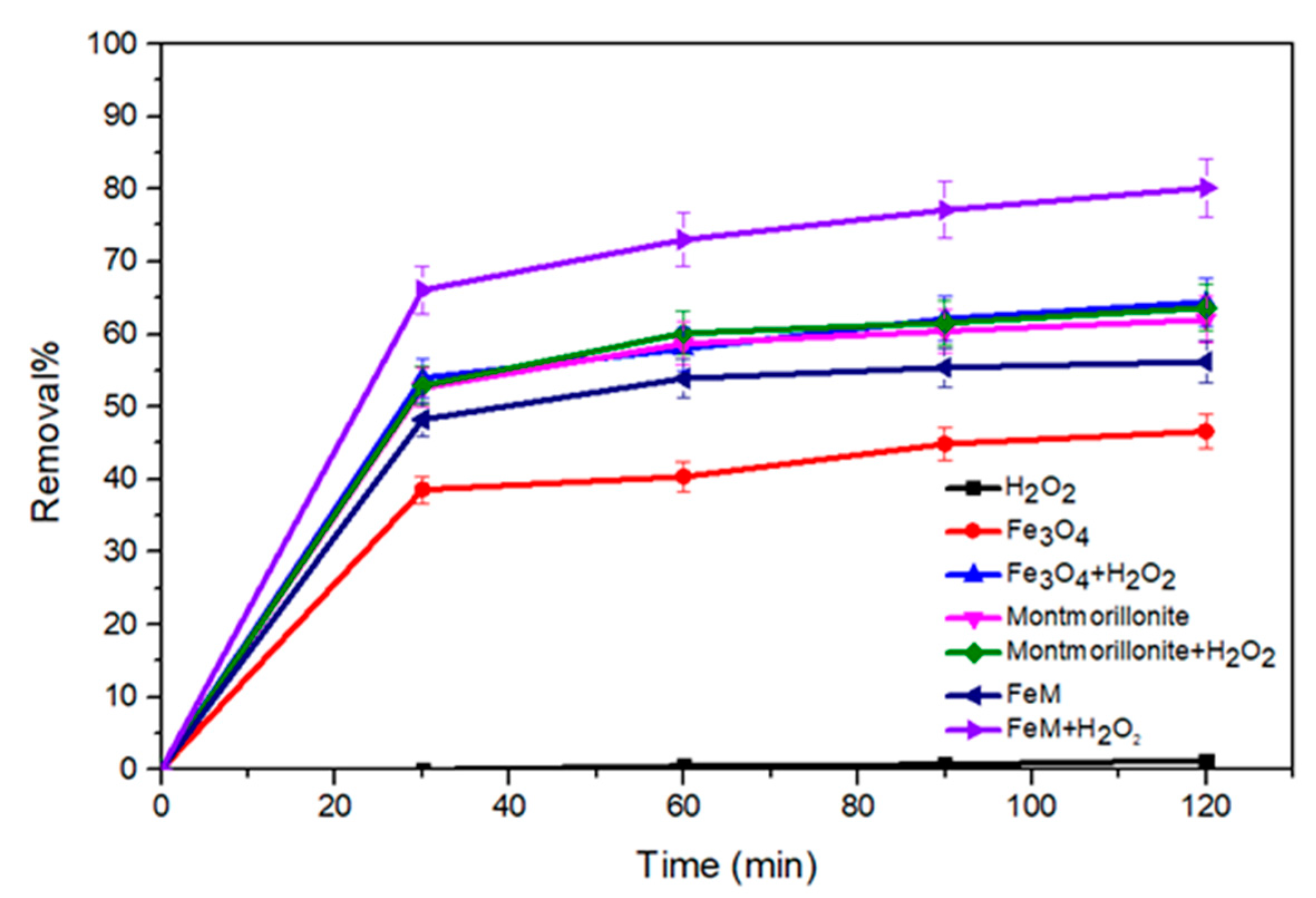
2.2. Fenton Reaction
2.2.1. OFL Removal in Different Processes
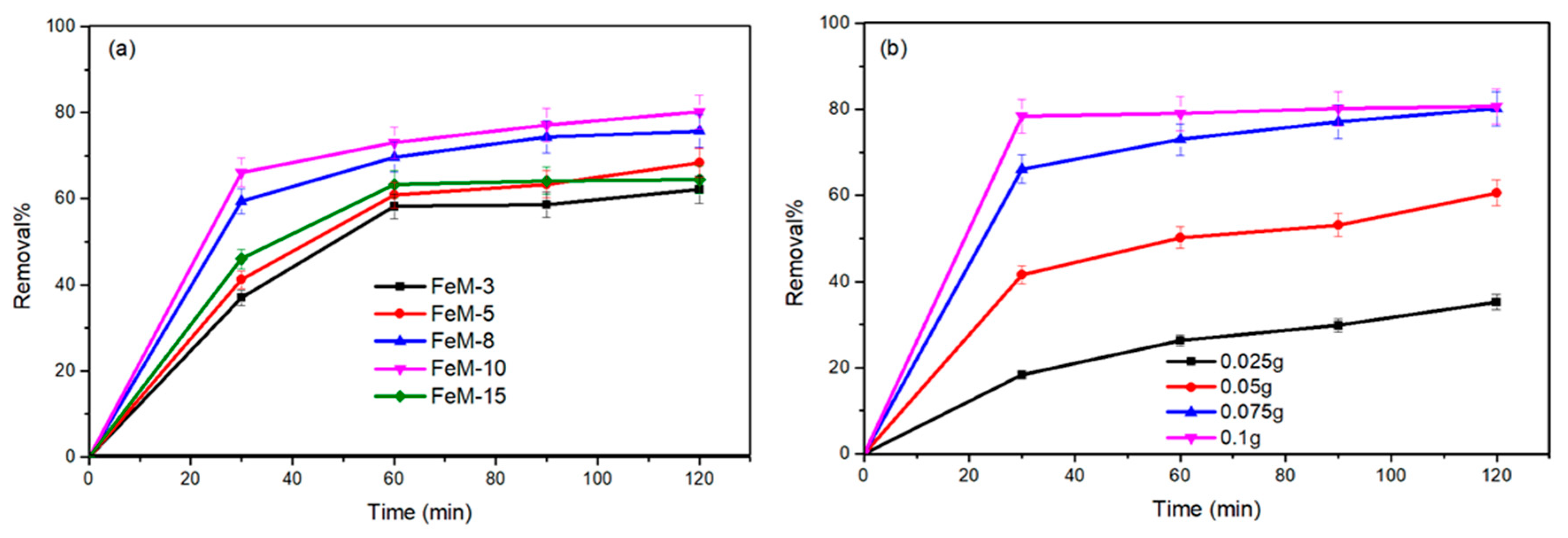
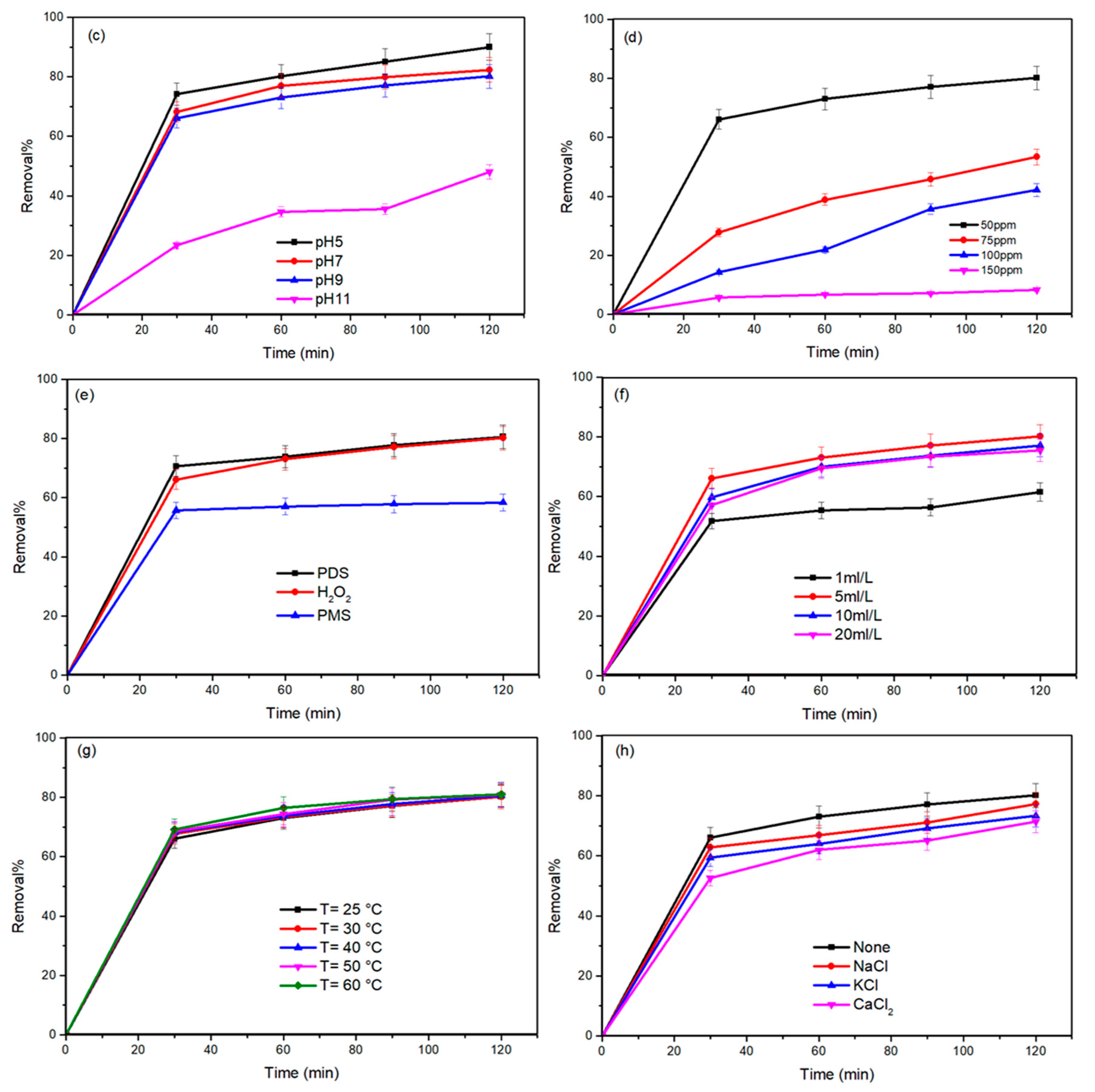
2.2.2. Influence of Process Variables
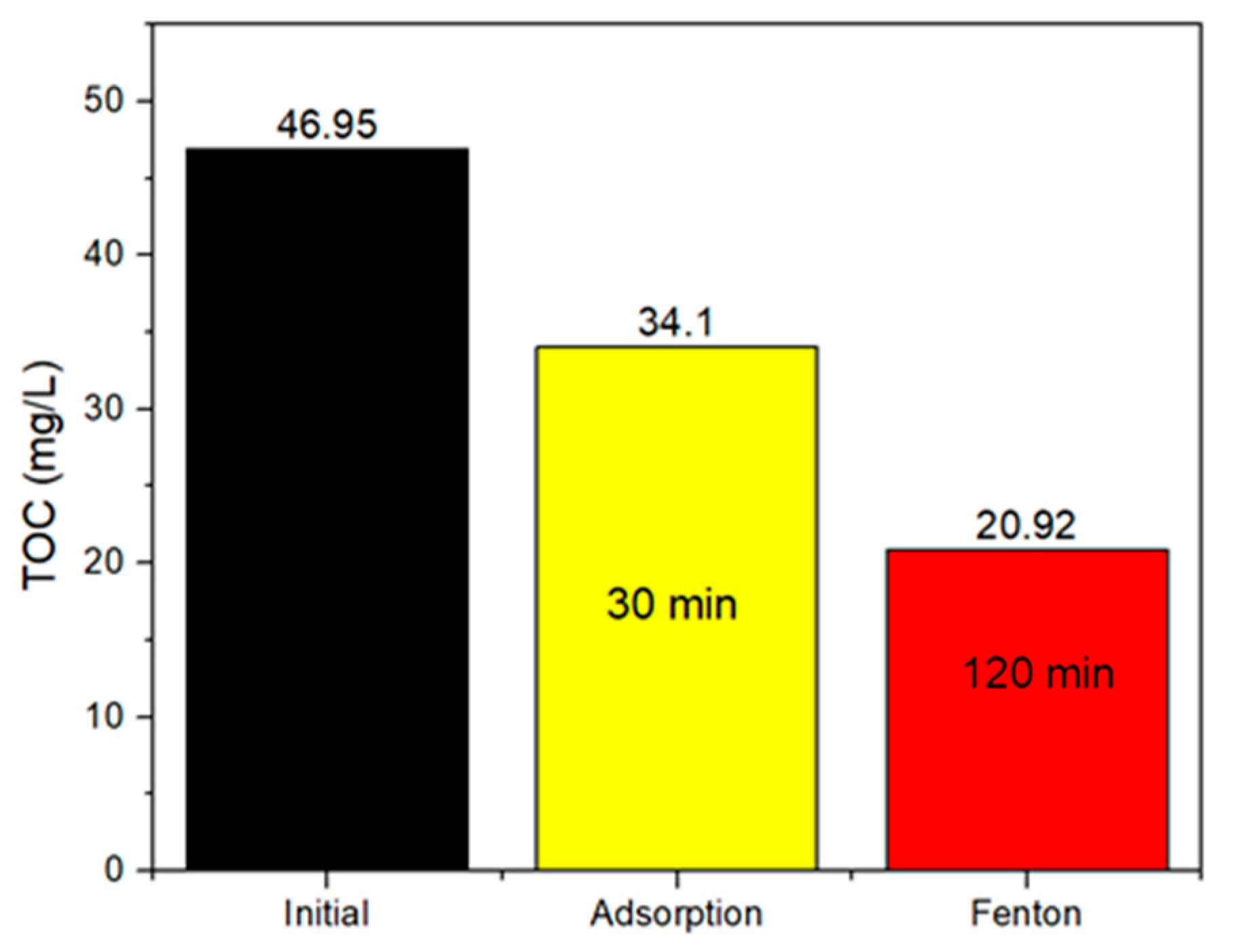
2.2.3. Total Organic Carbon (TOC) Removal of the OFL Solution
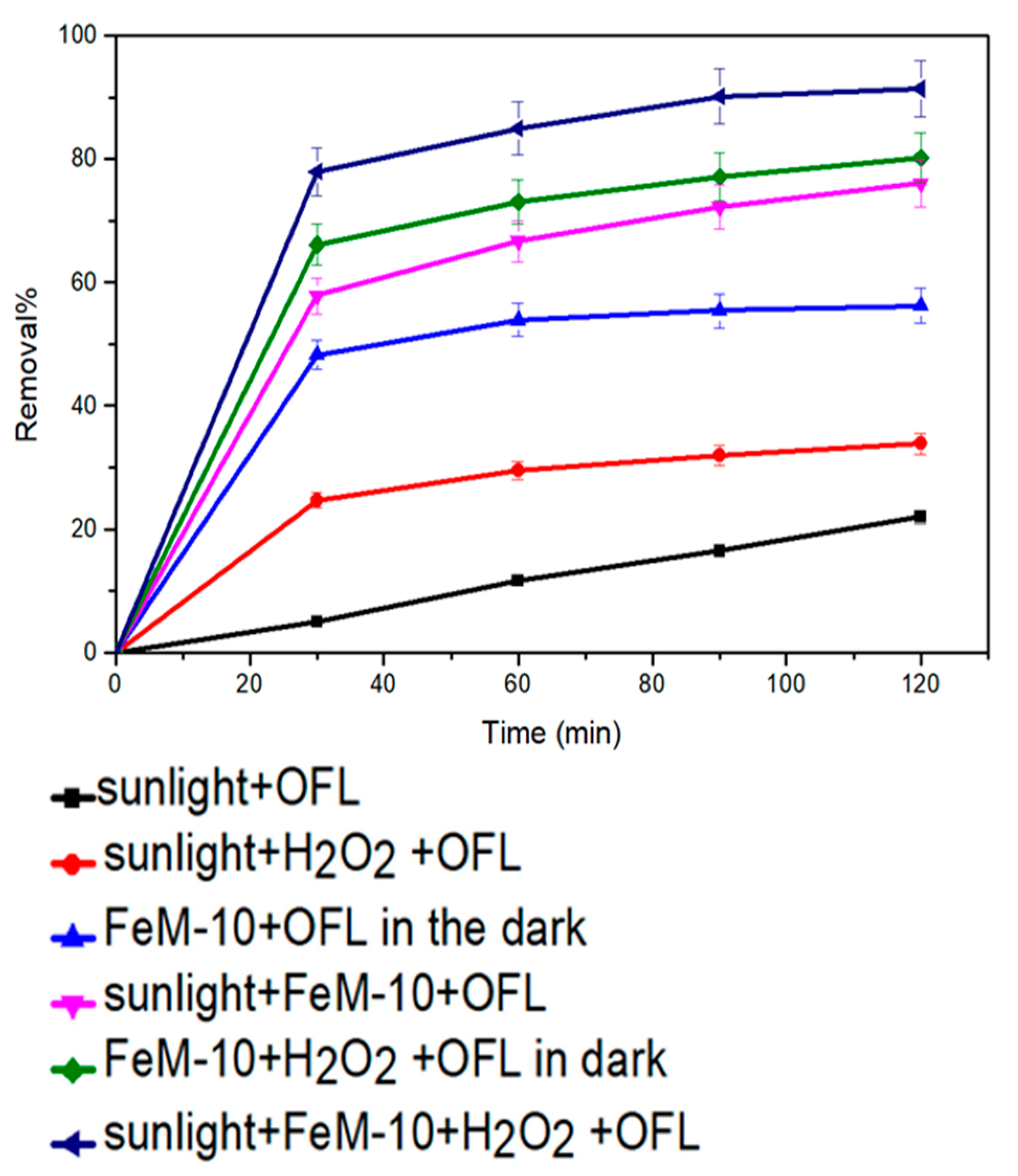
2.2.4. Photo-Fenton Catalytic Activity
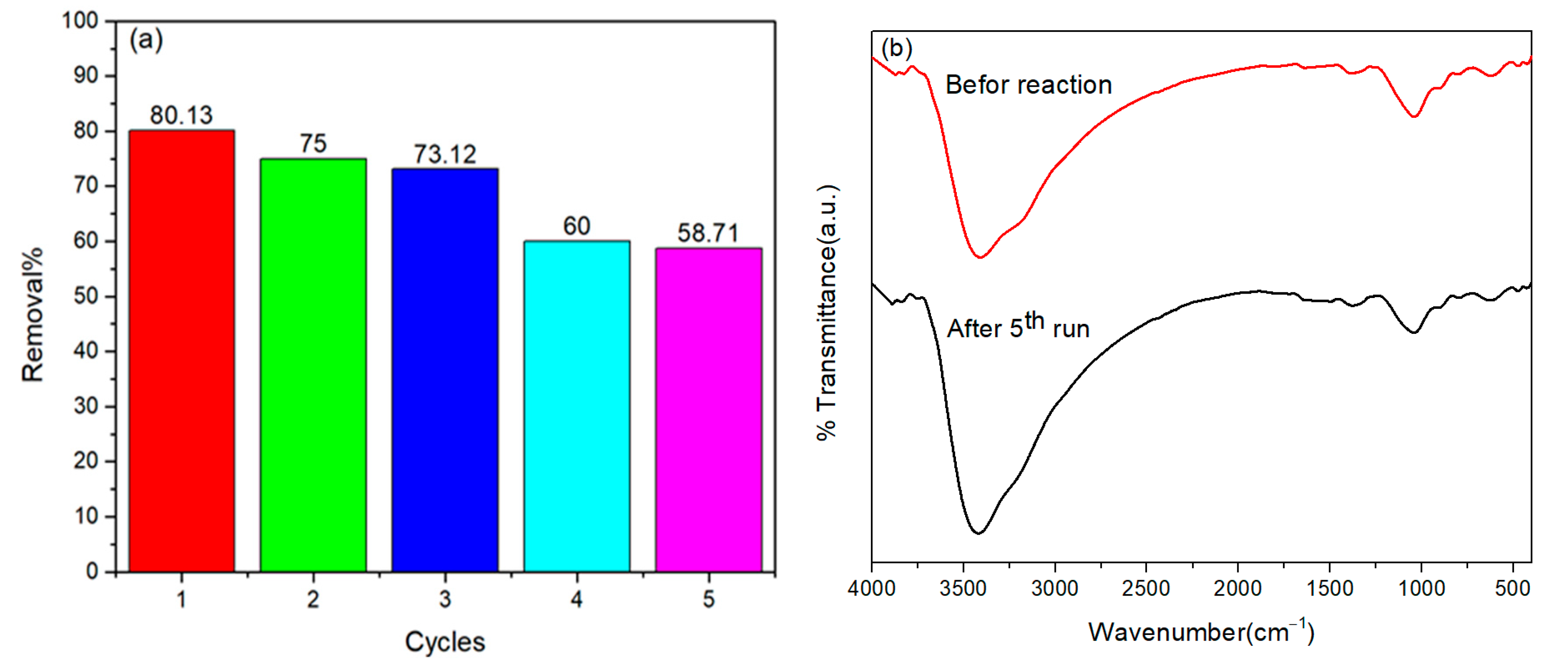
2.2.5. Reusability and Stability Studies
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fe3O4–Montmorillonite (FeM) Composites
3.3. Characterization Techniques
3.4. Fenton Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, P.; Blaney, L.; Cagnetta, G.; Huang, J.; Wang, B.; Wang, Y.; Deng, S.; Yu, G. Degradation of ofloxacin by perylene diimide supramolecular nanofiber sunlight-driven photocatalysis. Environ. Sci. Technol. 2019, 53, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wei, M.; Pan, Z.; Li, L.; Liang, J.; Yu, K.; Zhang, Y. Ultraviolet/peroxydisulfate degradation of ofloxacin in seawater: Kinetics, mechanism and toxicity of products. Sci. Total Environ. 2020, 705, 135960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Ma, Z.; Zhang, W. Influences of cetyltrimethyl ammonium bromide template on photocatalytic degradation of ofloxacin on porous La. Desalination Water Treat. 2019, 159, 310–317. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; Liu, J.; Zhang, C.; Hu, Q.; Hou, X. Ofloxacin degradation by Fe3O4-CeO2/AC Fenton-like system: Optimization, kinetics, and degradation pathways. Mol. Catal. 2019, 465, 61–67. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, J.; Zhou, F.; Chen, X.; Wei, L.; Gao, Q.; Wang, K.; Zhao, Q. Construction of a visible-light-driven magnetic dual Z-scheme BiVO4/g-C3N4/NiFe2O4 photocatalyst for effective removal of ofloxacin: Mechanisms and degradation pathway. Chem. Eng. J. 2021, 405, 126704. [Google Scholar] [CrossRef]
- Mushtaq, K.; Saeed, M.; Gul, W.; Munir, M.; Firdous, A.; Yousaf, T.; Khan, K.; Sarwar, H.M.R.; Riaz, M.A.; Zahid, S. Synthesis and characterization of TiO2 via sol-gel method for efficient photocatalytic degradation of antibiotic ofloxacin. Inorg. Nano-Met. Chem. 2020, 50, 1–7. [Google Scholar] [CrossRef]
- Kaur, R.; Kushwaha, J.P.; Singh, N. Electro-catalytic oxidation of ofloxacin antibiotic in continuous reactor: Evaluation, transformation products and pathway. J. Electrochem. Soc. 2019, 166, H250. [Google Scholar] [CrossRef]
- Dhahawi Ahmad, A.R.; Imam, S.S.; Oh, W.D.; Adnan, R. Fe3O4-Zeolite Hybrid Material as Hetero-Fenton Catalyst for Enhanced Degradation of Aqueous Ofloxacin Solution. Catalysts 2020, 10, 1241. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, H.-Y. Feasibility of using UV and persulfate in degrading ofloxacin in a large reactor. Desalination Water Treat. 2019, 167, 170–175. [Google Scholar] [CrossRef]
- Wuana, R.A.; Sha’Ato, R.; Iorhen, S. Aqueous phase removal of ofloxacin using adsorbents from Moringa oleifera pod husks. Adv. Environ. Res 2015, 4, 49–68. [Google Scholar] [CrossRef]
- Changotra, R.; Guin, J.P.; Khader, S.A.; Varshney, L.; Dhir, A. Electron beam induced degradation of ofloxacin in aqueous solution: Kinetics, removal mechanism and cytotoxicity assessment. Chem. Eng. J. 2019, 356, 973–984. [Google Scholar] [CrossRef]
- Tian, Y.; He, X.; Zhou, H.; Tian, X.; Nie, Y.; Zhou, Z.; Yang, C.; Li, Y. Efficient fenton-like degradation of ofloxacin over bimetallic Fe–Cu@ Sepiolite composite. Chemosphere 2020, 2020, 127209. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J. Fenton-like degradation of 2, 4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B Environ. 2012, 123, 117–126. [Google Scholar] [CrossRef]
- Li, L.; Lai, C.; Huang, F.; Cheng, M.; Zeng, G.; Huang, D.; Li, B.; Liu, S.; Zhang, M.; Qin, L. Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: Synergism of bio-char and Fe–Mn binary oxides. Water Res. 2019, 160, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Liu, Y.; Huang, D.; Lai, C.; Zeng, G.; Huang, J.; Liu, Z.; Zhang, C.; Zhou, C.; Qin, L. Prussian blue analogue derived magnetic Cu-Fe oxide as a recyclable photo-Fenton catalyst for the efficient removal of sulfamethazine at near neutral pH values. Chem. Eng. J. 2019, 362, 865–876. [Google Scholar] [CrossRef]
- He, H.; Gao, C. Supraparamagnetic, conductive, and processable multifunctional graphene nanosheets coated with high-density Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 3201–3210. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, B.; Deng, Y.; Chen, H.; Luo, S.; Sun, C.; Yang, P.; Yang, S. Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nano Fe3O4/MWCNTs in aqueous solution. Appl. Catal. B Environ. 2011, 107, 274–283. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Zhou, L.; Wu, P.; Zhao, Y.; Lai, Y.; Wang, F. Heterogeneous Fenton degradation of bisphenol A using Fe3O4@β-CD/rGO composite: Synergistic effect, principle and way of degradation. Environ. Pollut. 2019, 244, 93–101. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X. Adsorption and desorption of nickel (II) ions from aqueous solution by a lignocellulose/montmorillonite nanocomposite. PLoS ONE 2015, 10, e0117077. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wu, H.; Gu, F.L. Efficient adsorption and photocatalytic degradation of Rhodamine B under visible light irradiation over BiOBr/montmorillonite composites. J. Hazard. Mater. 2014, 275, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Fan, M.; Yang, D.; He, H.; Liu, D.; Yuan, A.; Zhu, J.; Chen, T. Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr (VI)] from aqueous solutions. J. Hazard. Mater. 2009, 166, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Jiang, L.; Zeng, G.; Li, Y.; Zeng, Z.; Wang, X.; Ning, Q. Removal of 17β-Estradiol from water by adsorption onto montmorillonite-carbon hybrids derived from pyrolysis carbonization of carboxymethyl cellulose. J. Environ. Manag. 2019, 236, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Langell, M. XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, L.; Jiang, Z.; Pei, Y.; Xie, S.; Qiao, M.; Fan, K. Heteroepitaxial growth of gold on flowerlike magnetite: An efficacious and magnetically recyclable catalyst for chemoselective hydrogenation of crotonaldehyde to crotyl alcohol. J. Catal. 2011, 281, 106–118. [Google Scholar] [CrossRef]
- Huang, R.; Fang, Z.; Yan, X.; Cheng, W. Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4 magnetic nanoparticles under neutral condition. Chem. Eng. J. 2012, 197, 242–249. [Google Scholar] [CrossRef]
- Tireli, A.A.; do Rosário Guimarães, I.; de Souza Terra, J.C.; da Silva, R.R.; Guerreiro, M.C. Fenton-like processes and adsorption using iron oxide-pillared clay with magnetic properties for organic compound mitigation. Environ. Sci. Pollut. Res. 2015, 22, 870–881. [Google Scholar] [CrossRef]
- Caglar, B.; Guner, E.K.; Keles, K.; Özdokur, K.V.; Cubuk, O.; Coldur, F.; Caglar, S.; Topcu, C.; Tabak, A. Fe3O4 nanoparticles decorated smectite nanocomposite: Characterization, photocatalytic and electrocatalytic activities. Solid State Sci. 2018, 83, 122–136. [Google Scholar] [CrossRef]
- Sharma, P.; Borah, D.J.; Das, P.; Das, M.R. Cationic and anionic dye removal from aqueous solution using montmorillonite clay: Evaluation of adsorption parameters and mechanism. Desalination Water Treat. 2016, 57, 8372–8388. [Google Scholar] [CrossRef]
- Barreca, S.; Colmenares, J.J.V.; Pace, A.; Orecchio, S.; Pulgarin, C. Neutral solar photo-Fenton degradation of 4-nitrophenol on iron-enriched hybrid montmorillonite-alginate beads (Fe-MABs). J. Photochem. Photobiol. A Chem. 2014, 282, 33–40. [Google Scholar] [CrossRef]
- Ashiq, A.; Sarkar, B.; Adassooriya, N.; Walpita, J.; Rajapaksha, A.U.; Ok, Y.S.; Vithanage, M. Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 2019, 236, 124384. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, W.; He, S.; Yu, S.; Chen, Y.; Lu, L.; Shu, Z.; Cui, H.; Zhang, Y.; Jin, H. Rapid adsorption of cationic dye-methylene blue on the modified montmorillonite/graphene oxide composites. Appl. Clay Sci. 2019, 168, 304–311. [Google Scholar] [CrossRef]
- Azmi, N.; Ayodele, O.; Vadivelu, V.; Asif, M.; Hameed, B. Fe-modified local clay as effective and reusable heterogeneous photo-Fenton catalyst for the decolorization of Acid Green 25. J. Taiwan Inst. Chem. Eng. 2014, 45, 1459–1467. [Google Scholar] [CrossRef]
- Wan, D.; Wang, G.; Li, W.; Wei, X. Investigation into the morphology and structure of magnetic bentonite nanocomposites with their catalytic activity. Appl. Surf. Sci. 2017, 413, 398–407. [Google Scholar] [CrossRef]
- Orolínová, Z.; Mockovčiaková, A. Structural study of bentonite/iron oxide composites. Mater. Chem. Phys. 2009, 114, 956–961. [Google Scholar] [CrossRef]
- Hassan, H.; Hameed, B. Fe–clay as effective heterogeneous Fenton catalyst for the decolorization of Reactive Blue 4. Chem. Eng. J. 2011, 171, 912–918. [Google Scholar] [CrossRef]
- Pradhan, A.C.; Nanda, B.; Parida, K.; Das, M. Quick photo-Fenton degradation of phenolic compounds by Cu/Al2O3–MCM-41 under visible light irradiation: Small particle size, stabilization of copper, easy reducibility of Cu and visible light active material. Dalton Trans. 2013, 42, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, M.; Panahi, A.H.; Al-Musawi, T.J.; Ehrampoush, M.; Mahvi, A. The catalytic activity of FeNi3@SiO2 magnetic nanoparticles for the degradation of tetracycline in the heterogeneous Fenton-like treatment method. J. Water Process Eng. 2019, 32, 100943. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Fan, S.; Yang, T. Enhanced visible light assisted Fenton-like degradation of dye via metal-doped zinc ferrite nanosphere prepared from metal-rich industrial wastewater. J. Taiwan Inst. Chem. Eng. 2019, 96, 185–192. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, J.; Gao, L.; Zhang, Y.; Gu, N.; Feng, J.; Yang, D.; Zhu, J.; Yan, X. Decomposing phenol by the hidden talent of ferromagnetic nanoparticles. Chemosphere 2008, 73, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, L.; Jiang, M.; Yang, Y.; Yang, P.; Lu, J.; Ferronato, C.; Chovelon, J.-M. Ferrous-activated peroxymonosulfate oxidation of antimicrobial agent sulfaquinoxaline and structurally related compounds in aqueous solution: Kinetics, products, and transformation pathways. Environ. Sci. Pollut. Res. 2017, 24, 19535–19545. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Zeng, G.-M. An in depth mechanism insight of the degradation of multiple refractory pollutants via a novel SrTiO3/BiOI heterojunction photocatalysts. J. Catal. 2017, 356, 283–299. [Google Scholar] [CrossRef]
- Tsuneda, S.; Ishihara, Y.; Hamachi, M.; Hirata, A. Inhibition effect of chlorine ion on hydroxyl radical generation in UV-H2O2 process. Water Sci. Technol. 2002, 46, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Warang, T.; Patel, N.; Santini, A.; Bazzanella, N.; Kale, A.; Miotello, A. Pulsed laser deposition of Co3O4 nanoparticles assembled coating: Role of substrate temperature to tailor disordered to crystalline phase and related photocatalytic activity in degradation of methylene blue. Appl. Catal. A Gen. 2012, 423, 21–27. [Google Scholar] [CrossRef]
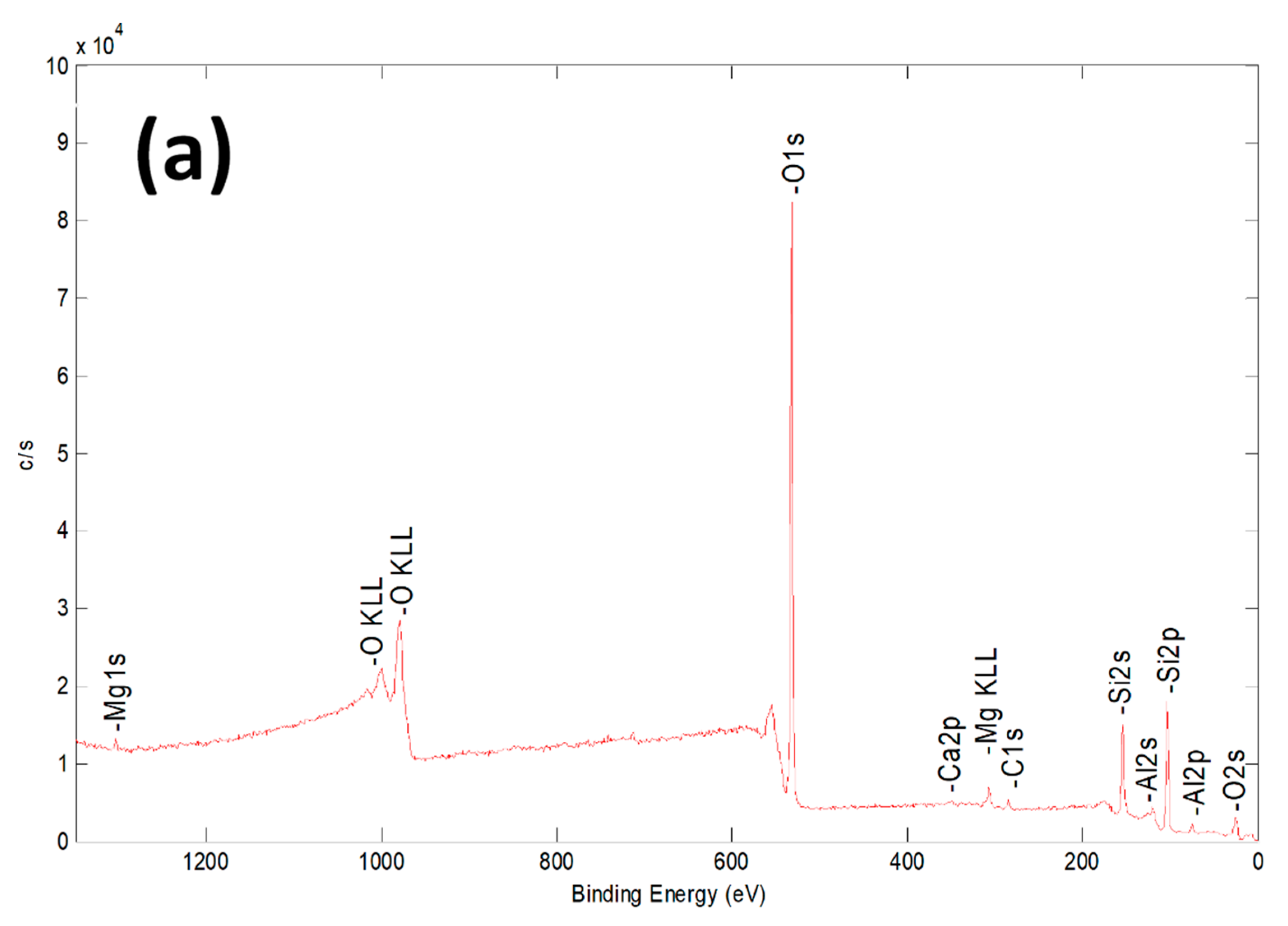
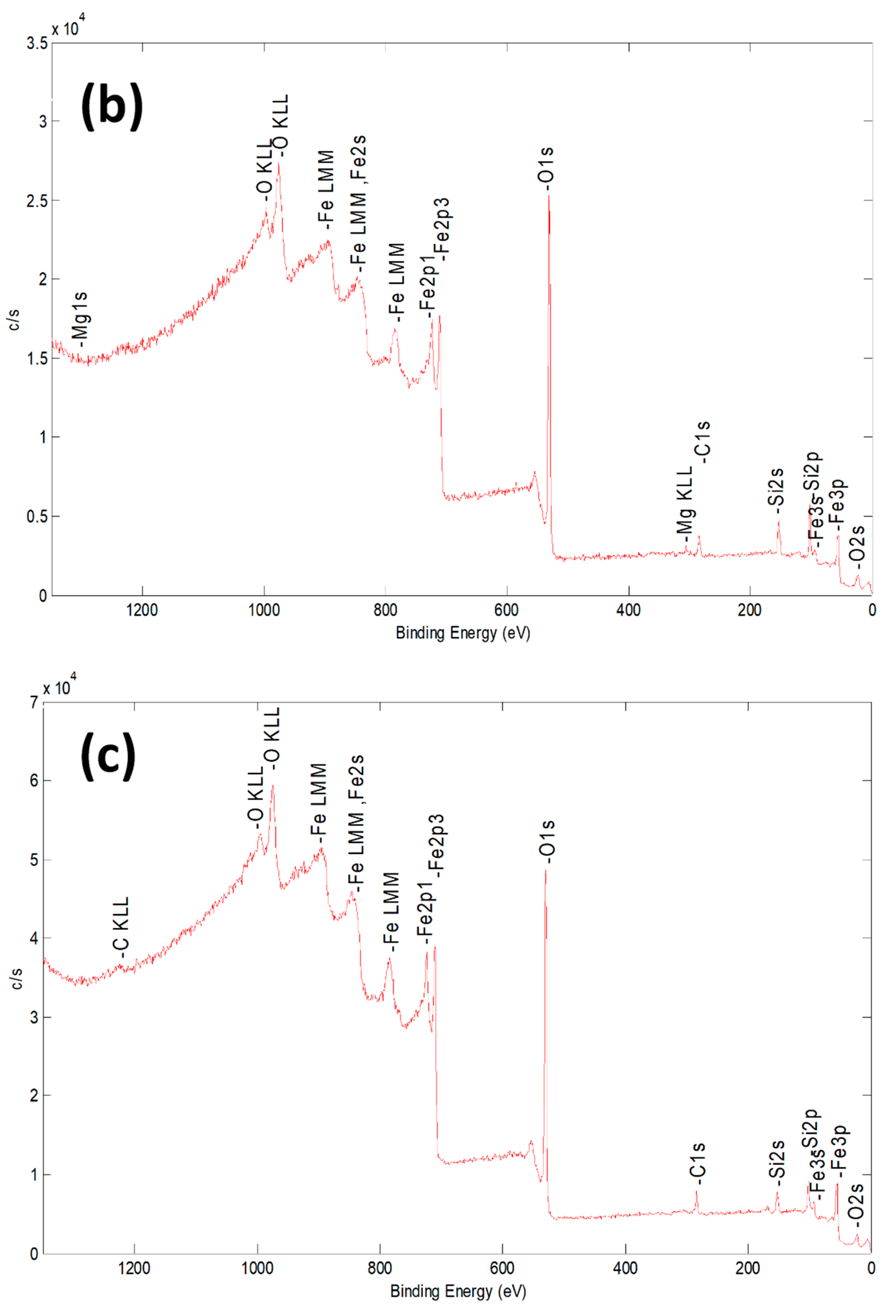
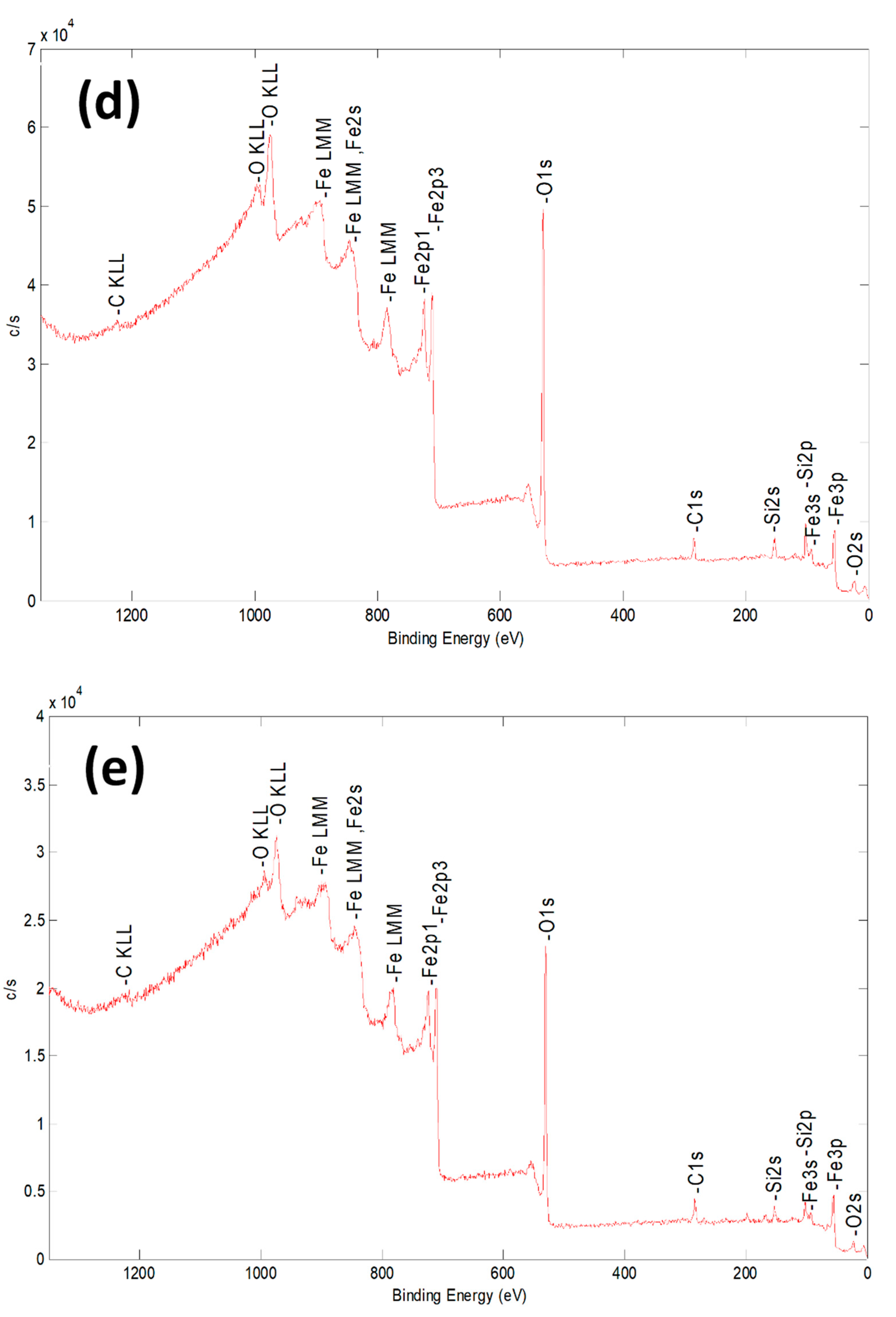
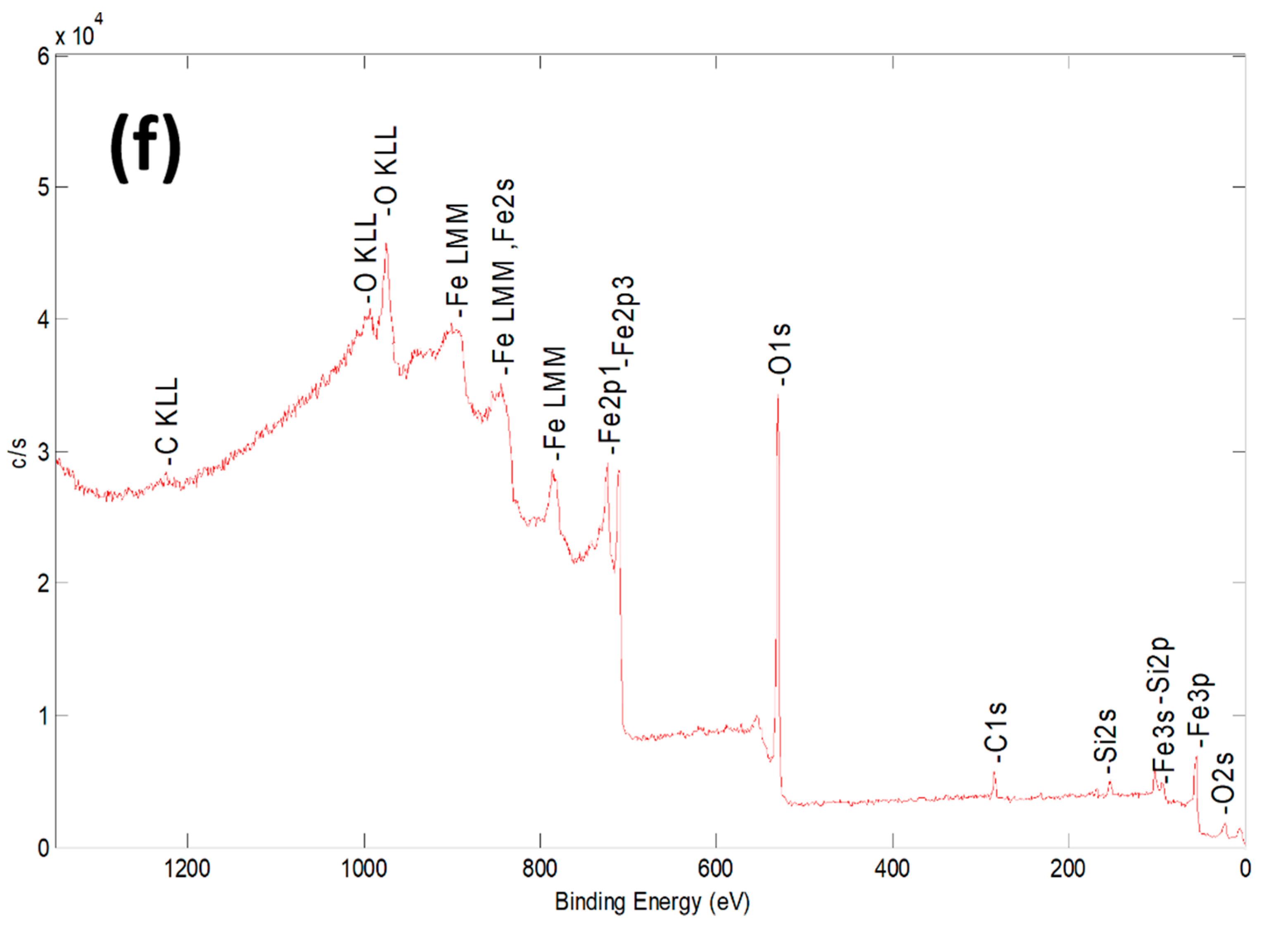
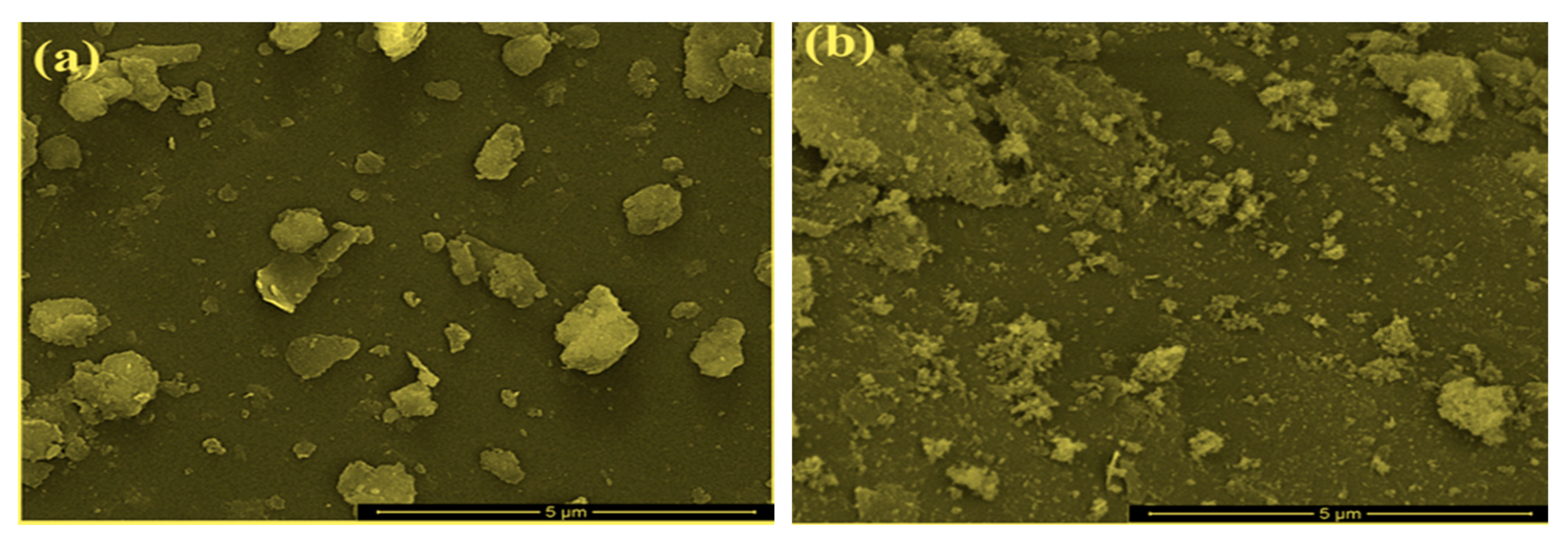
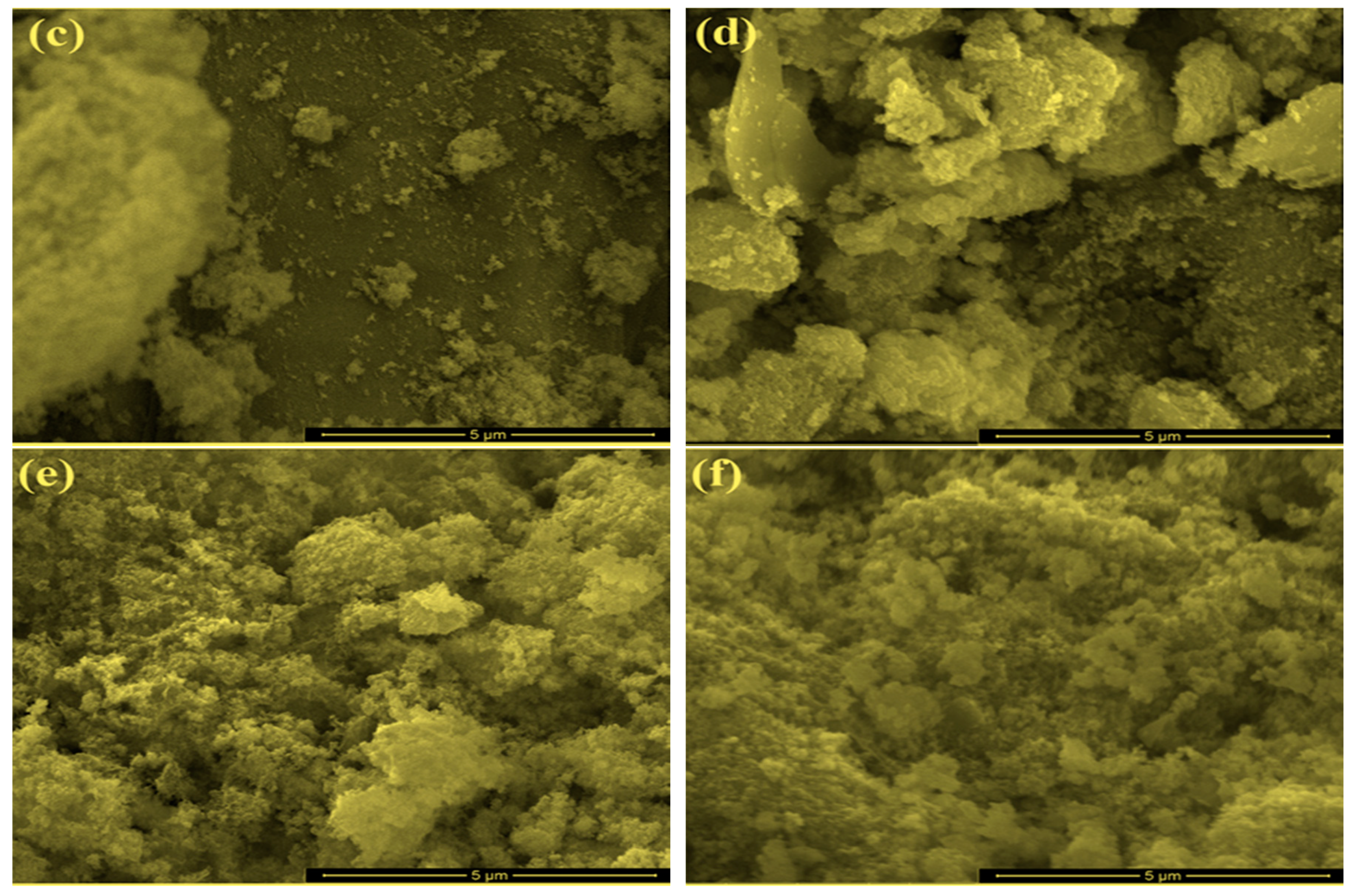
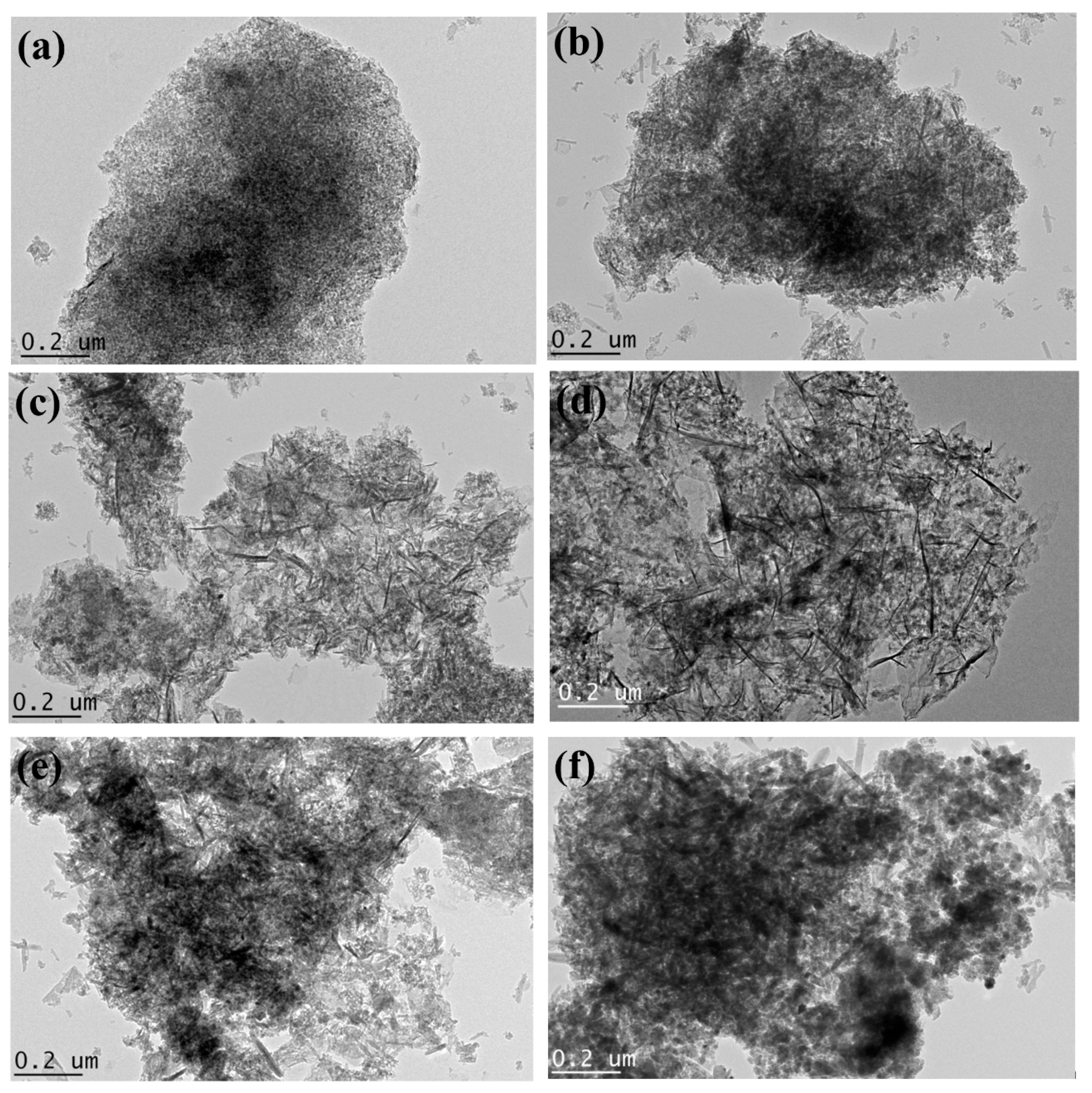
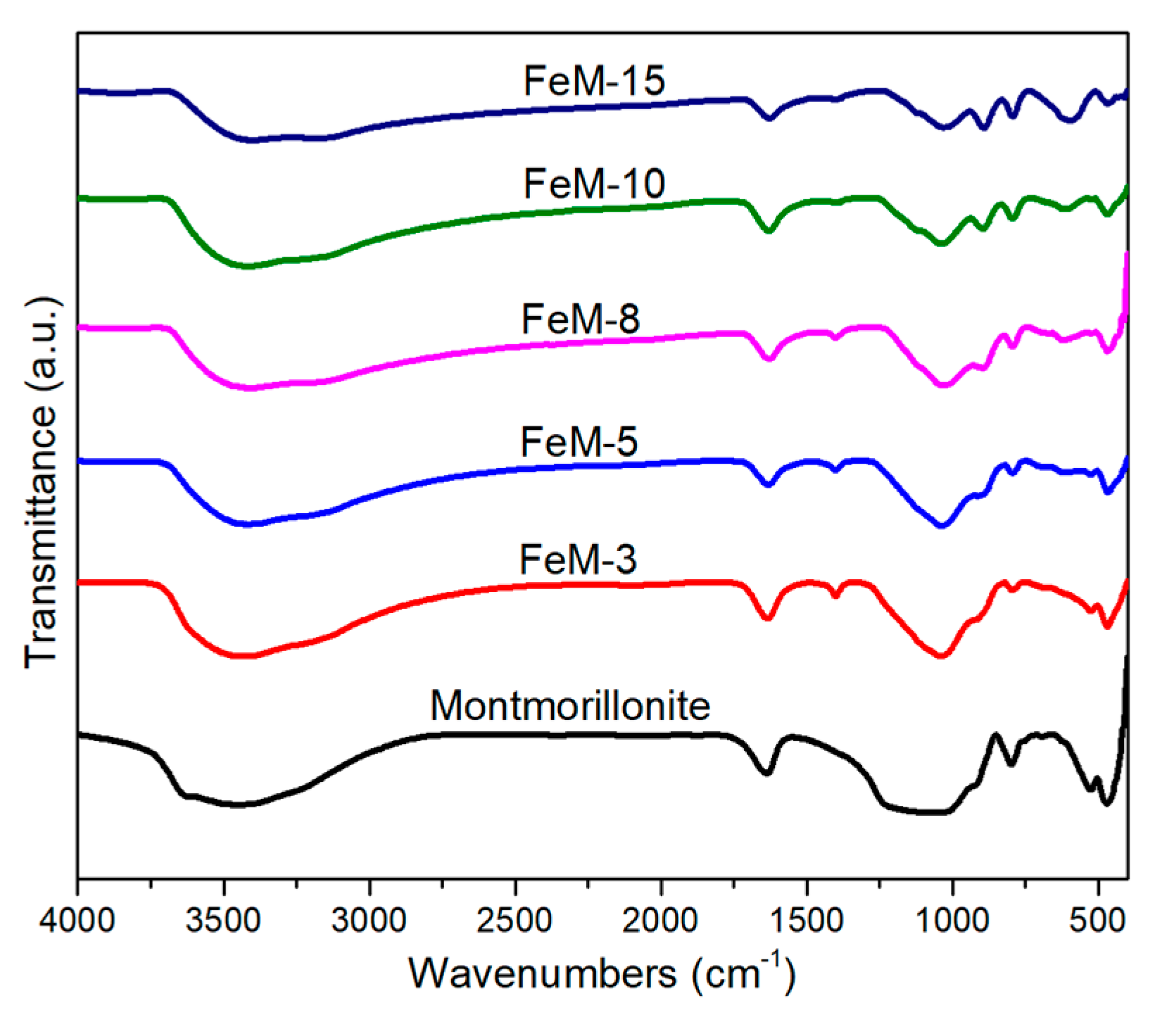
| Sample | Al % | Si % | K % | Mg % | O % | Fe % |
|---|---|---|---|---|---|---|
| Montmorillonite | 9.21 | 33.06 | 4.10 | 1.11 | 52.52 | - |
| FeM-3 | 1.61 | 17.76 | - | - | 37.36 | 43.19 |
| FeM-5 | 1.31 | 10.17 | - | - | 36.92 | 51.60 |
| FeM-8 | 0.83 | 6.58 | - | - | 33.93 | 58.66 |
| FeM-10 | 0.47 | 5.24 | - | - | 33.73 | 60.54 |
| FeM-15 | 0.18 | 2.71 | - | - | 32.96 | 64.15 |
| Samples | Brunauer–Emmett–Teller BET Surface Area (m2/g) | Pore Volume (cm3/g) | Particle Size (nm) |
|---|---|---|---|
| Montmorillonite | 258.108 | 0.423 | 8.092 |
| FeM-3 | 247.944 | 0.634 | 32.532 |
| FeM-5 | 192.118 | 0.602 | 48.290 |
| FeM-8 | 163.552 | 0.384 | 55.028 |
| FeM-10 | 161.800 | 0.363 | 66.101 |
| FeM-15 | 113.186 | 0.312 | 69.735 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.R.D.; Imam, S.S.; Oh, W.D.; Adnan, R. Fenton Degradation of Ofloxacin Using a Montmorillonite–Fe3O4 Composite. Catalysts 2021, 11, 177. https://doi.org/10.3390/catal11020177
Ahmad ARD, Imam SS, Oh WD, Adnan R. Fenton Degradation of Ofloxacin Using a Montmorillonite–Fe3O4 Composite. Catalysts. 2021; 11(2):177. https://doi.org/10.3390/catal11020177
Chicago/Turabian StyleAhmad, Alamri Rahmah Dhahawi, Saifullahi Shehu Imam, Wen Da Oh, and Rohana Adnan. 2021. "Fenton Degradation of Ofloxacin Using a Montmorillonite–Fe3O4 Composite" Catalysts 11, no. 2: 177. https://doi.org/10.3390/catal11020177
APA StyleAhmad, A. R. D., Imam, S. S., Oh, W. D., & Adnan, R. (2021). Fenton Degradation of Ofloxacin Using a Montmorillonite–Fe3O4 Composite. Catalysts, 11(2), 177. https://doi.org/10.3390/catal11020177








