Abstract
We conducted photocatalytic experiments focusing on the peptidoglycan layer to elucidate the details of the mechanism of photocatalytic sterilization. The previous study of our laboratory suggested that the presence of the peptidoglycan layer increases the bactericidal effect. To further verify it, the following experiments were performed: experiments on cells with different peptidoglycan layer thickness used Lactobacillus plantarum cells with different growth phases, experiments on cells with the thin peptidoglycan layer used Escherichia coli cells and spheroplast cells from which the peptidoglycan layer was removed from E. coli cells. The bactericidal effects increased as the growth progresses of L. plantarum. It was confirmed by TEM that the thickness of the peptidoglycan layer increased with cell growth. The survival rates of E. coli intact cells were significantly lower than those of spheroplast cells. These results strongly suggest that the peptidoglycan layer enhances the photocatalytic bactericidal effect. As a result of allowing the photocatalytic reaction to act on peptidoglycan, the amount of hydroxyl radical was smaller, and the amount of hydrogen peroxide was higher than in the absence of peptidoglycan. It is suggested that peptidoglycan may convert produced hydroxyl radical to hydrogen peroxide.
1. Introduction
Recently, environmental pollution in water and air by microorganisms has come to be regarded as a problem, and among the problems, particular attention has been allocated to the influence of microorganisms on the human body such as nosocomial infections and infectious diseases caused by airborne bacteria [1,2,3,4,5]. The photocatalyst, which is inexpensive and has a low environmental impact, is attracting attention as a solution to these problems.
The photocatalyst is a substance that promotes a chemical change (oxidation-reduction reaction) of another substance by using the energy of light, and the most common one is TiO2. When anatase-type TiO2 is irradiated with UV light, reactive oxygen species (ROS) such as hydroxyl radicals are generated on the surface, and they decompose organic matters existing around the photocatalyst into water and carbon dioxide [6,7,8,9,10]. It is the photocatalytic sterilization that utilizes this reaction. So far, various studies have been conducted on the sterilization mechanism of photocatalysts [11,12,13,14,15,16,17] and its application [3,5,18,19,20,21]. However, there is a problem that the details of the sterilization mechanism by the photocatalytic reaction have not been clarified.
Although some studies have reported that higher bactericidal efficiency on Gram-negative bacteria than Gram-positive bacteria, these studies do not deeply study the effect of the peptidoglycan layer [22,23,24,25]. In addition, these studies do not consider the effects of spores resistant to physicochemical stress and the effects of extracellular secretory substances on photocatalytic sterilization. A study by Sunada et al. (2003) focused on the cell wall, the outer structure of the bacterial cells [26]. This study reported that ROS generated by the photocatalytic reaction acts on the outer membrane and the cell membrane of bacteria but does not act on the peptidoglycan layer and only passes through. In response to the study, a study in our laboratory was conducted with the aim of clarifying if ROS really does not act on the peptidoglycan layer and whether the bactericidal effect differs depending on the presence or absence of the peptidoglycan layer [27]. Using Lactobacillus plantarum, Gram-positive bacterium, the protoplast cells without the peptidoglycan layer were prepared, and the effects of the peptidoglycan layer on the photocatalytic bactericidal effect were analyzed by comparing the survival rate of the protoplast cells and the intact cells. As the results, the survival rate of the intact cells was significantly lower than the protoplast cells, it is suggested that the peptidoglycan layer enhances the bactericidal effect of the photocatalytic reaction.
Therefore, the purpose of this study is to clarify the mechanism of photocatalytic sterilization by multifaceted analyses of the effect of the peptidoglycan layer on the photocatalytic bactericidal effect. This study is structured as follows, (i) the effect of the photocatalytic sterilization on Gram-positive bacterium differing in the thickness of the peptidoglycan layer with the growth phase, (ii) the effect of the photocatalytic sterilization on spheroplast cell which is Gram-negative bacterium cell made by removing the peptidoglycan layer from the cell wall, (iii) the quantitative determination of hydroxyl radical and hydrogen peroxide generated when the peptidoglycan is photocatalytically treated.
2. Results
2.1. The Survival Rate of L. Plantarum as a Gram-Positive Bacterium on the Photocatalytic Reaction
2.1.1. The Survival Rates of L. plantarum JCM1142T in Each Growth Phase
To verify whether the bactericidal effect of the photocatalytic reaction differs depending on the thickness of the peptidoglycan layer, we performed the photocatalytic reaction with TiO2-coated glass on the L. plantarum cells in each growth phase for 0, 20, 40, 60, and 120 min. The survival rates of the early log phase, the medium log phase, the late log phase, the early stationary phase cells decreased to about 54%, about 30%, about 28%, and about 16% after 40 min of the photocatalytic reaction, and about 20%, about 13%, about 7%, and about 8% after 1 h of the photocatalytic reaction, respectively (Figure 1). As a result of the significance test, it was shown that there were significant differences between the survival rates in the early log phase and the early stationary phase after 40 min (p < 0.001) and after 1 h (p < 0.01) of the photocatalytic reaction.
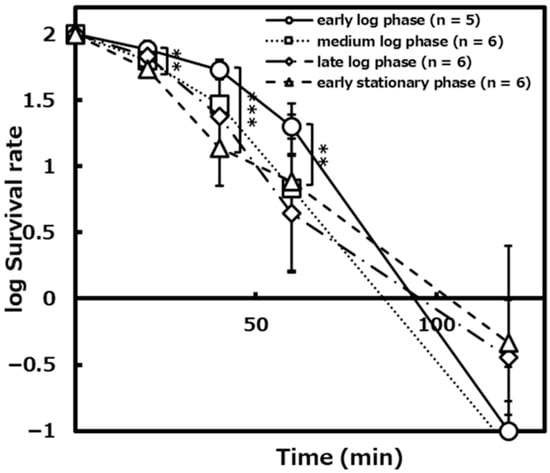
Figure 1.
The survival rates of L. plantarum JCM1142T at each growth phase on the photocatalytic reaction with TiO2-coated glass for 0, 20, 40, 60, and 120 min.([early log phase]: n = 5, [medium log phase]: n = 6, [late log phase]: n = 6, [early stationary phase]: n = 5). Error bars indicate the mean value ± standard deviation of experimental results repeated n times. ([20 min]: p < 0.01, [40 min]: p < 0.001, [60 min]: p < 0.01). The double asterisk (**) and triple asterisk (***) indicate significant differences in the survival rates of the early log phase and early stationary phase at p < 0.01 and p < 0.001, respectively.
2.1.2. The Comparison of Survival Rates of L. plantarum JCM1142T with and without the Photocatalytic Reaction
The survival rates of L. plantarum cells under negative control conditions ([TiO2 (−), UVA (−), early log phase], [TiO2 (−), UVA (−), early stationary phase], [TiO2 (−), UVA (+), early log phase], [TiO2 (−), UVA (+), early stationary phase], [TiO2(+), UVA (−), early log phase], and [TiO2 (+), UVA (−), early stationary phase]) indicated about 86%, 96%, 80%, 98%, 89%, and 81% after 2 h of the photocatalytic reaction, respectively (Figure 2).
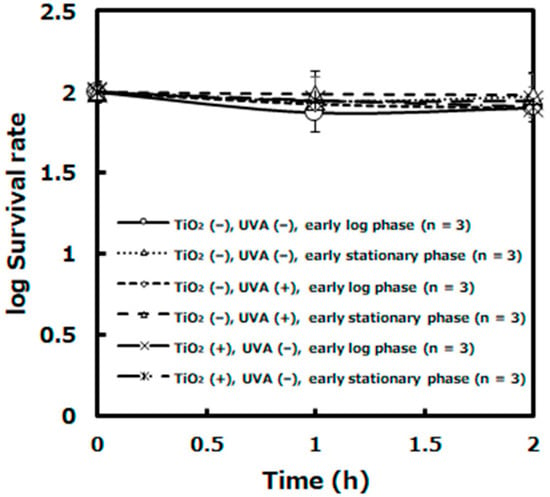
Figure 2.
The survival rates of L. plantarum JCM1142T on the photocatalytic reaction under negative control conditions. ([TiO2 (−), UVA (−), log early phase] [TiO2 (−), UVA (−), stationary early phase]. [TiO2 (−), UVA (+), log early phase] [TiO2 (−), UVA (+), stationary early phase]. [TiO2 (+), UVA (−), log early phase] [TiO2 (+), UVA (−), stationary early phase]: n = 3). Error bars indicate the mean value ± standard deviation of experimental results repeated n times.
2.2. TEM Observation of L. plantarum Cells in Each Growth Phase
In order to verify whether the results that L. plantarum JCM1142T cells on the photocatalytic reaction tended to be killed with cell growth were due to the thickening of the peptidoglycan layer with cell growth, the early log phase and the early stationary phase of L. plantarum cells were observed with a transmission electron microscope (Figure 3).
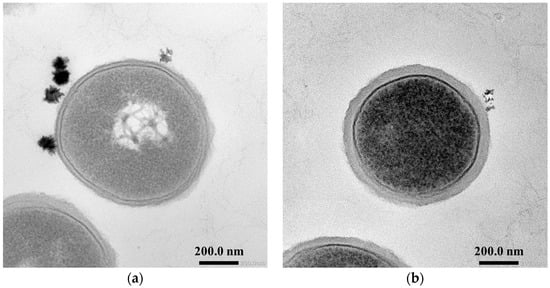
Figure 3.
Transmission electron microscopic images of L. plantarum JCM1142T. (a) early log phase cells, (b) early stationary phase cells.
The thickness of the peptidoglycan layer of the cells in each growth phase was measured by ImageJ. Then, the average thickness of the peptidoglycan layers was calculated and compared (Figure 4). The peptidoglycan layer of L. plantarum cells in the early log phase was about 17 nm, and that in the early stationary phase was about 45 nm. As a result of the significance test, it was shown that there was a significant difference in the thickness of the peptidoglycan layer between the early log phase and the early stationary phase (p < 0.001).
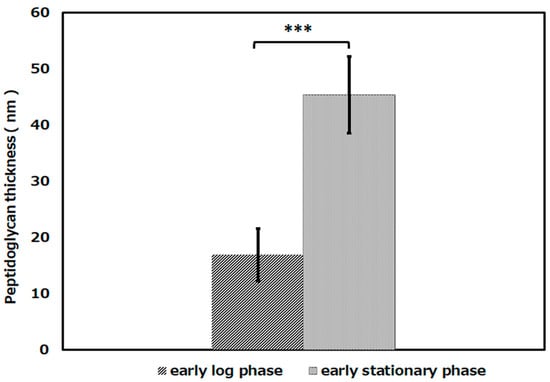
Figure 4.
The thickness of the peptidoglycan layer of L. plantarum JCM1142T in the early log phase and the early stationary phase. ([early log phase]: n = 29, [early stationary phase]: n = 64). Error bars indicate the mean value ± standard deviation of experimental results repeated n times (p < 0.001). The triple asterisk (***) indicates a significant difference in the peptidoglycan thickness of early log phase and early stationary phase at p < 0.001.
2.3. The Survival Rate of E. coli IAM12119T as A Gram-Negative Bacterium on the Photocatalytic Reaction
2.3.1. The Survival Rate of Spheroplast Cells of E. coli
Photocatalytic sterilization was performed on spheroplast cells of E. coli IAM12119T from which the peptidoglycan layer was removed by the enzyme (Lysozyme) and ethylenediaminetetraacetic acid (EDTA), and the survival rate was evaluated (Figure 5 and Figure 6). The survival rates of spheroplast cells decreased to about 95%, about 63%, and about 57% corresponding to after 1 h, 2 h, and 3 h of the photocatalytic reaction. In negative control experiments ([TiO2 (−), UVA (+)], [TiO2 (+), UVA (−)]), the survival rates after 3 h were about 95% and 99%, respectively.
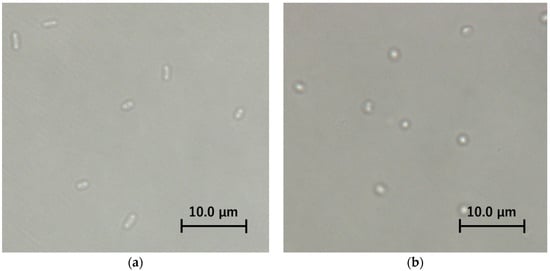
Figure 5.
Light microscopic bright field images of E. coli IAM12119T, (a) intact cells, (b) spheroplast cells.
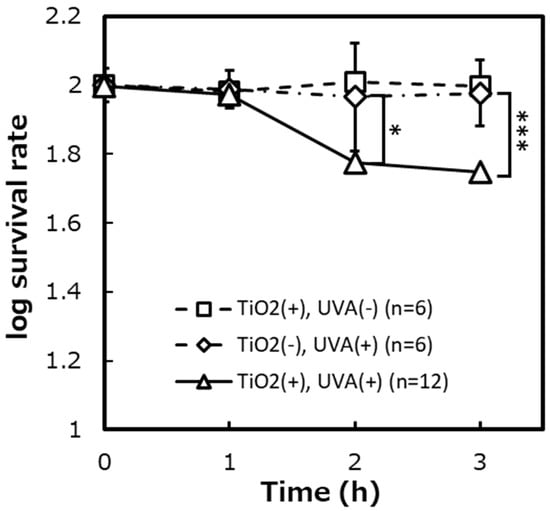
Figure 6.
The survival rates of spheroplast cells of E. coli IAM12119T on the photocatalytic reaction. ([TiO2 (+), UVA (+)]: n = 12, [TiO2 (+), UVA (−)]: n = 6, [TiO2 (−), UVA (+)]: n = 6). Error bars indicate the mean value ± standard deviation of experimental results repeated n times. ([1 h]: p < 0.05, [3 h]: p < 0.001). The asterisk (*) and triple asterisk (***) indicate significant differences in the survival rates of [TiO2 (+), UVA (+)] and [TiO2 (−), UVA (+)] at p < 0.05 and at p < 0.001, respectively.
2.3.2. The Comparison of Survival Rates of E. coli IAM12119T with and without Peptidoglycan Layer
Figure 7 summarizes the survival rates of E. coli when the photocatalytic reaction (TiO2 (+), UVA (+)) was performed between the intact cells and the spheroplast cells. The survival rates after 3 h of the photocatalytic reaction were about 41% for the intact cells and about 57% for the spheroplast cells. As a result of the significance test, it was shown that there were significant differences in the survival rates between the intact cells and the spheroplast (p < 0.001).
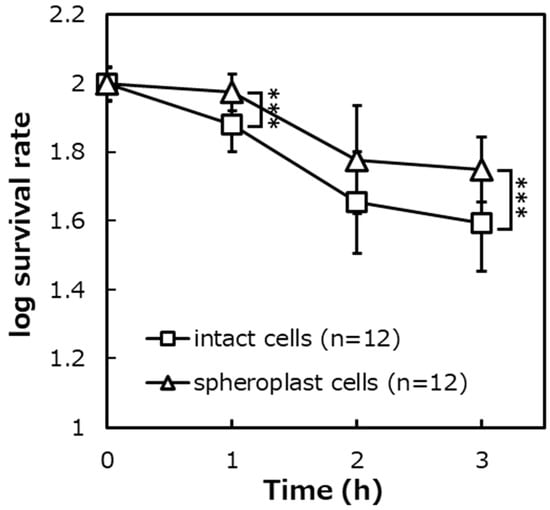
Figure 7.
The survival rates of E. coli intact cells and spheroplast cells on the photocatalytic reaction ([intact cells]: n = 12, [spheroplast cells]: n = 12). Error bars indicate the mean value ± standard deviation of experimental results repeated n times, ([1 h] [3 h]: p < 0.001). The triple asterisk (***) indicates a significant difference in the survival rates of intact cells and spheroplast cells at p < 0.001.
2.3.3. The Effect of Protein Addition on the Photocatalytic Sterilization
To verify the effect of the enzyme added during the preparation of spheroplast cells on the photocatalytic sterilization, albumin without enzyme activity was added to the intact cells and the photocatalytic reaction was carried out (Figure 8).
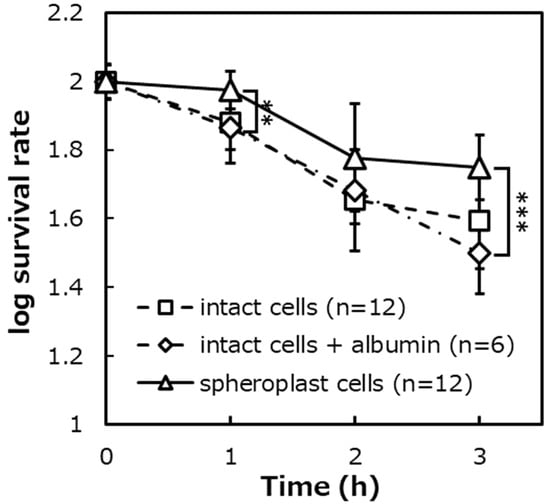
Figure 8.
The survival rates of protein-added E. coli IAM12119T intact cells on the photocatalytic reaction, ([intact cells + albumin]: n = 6, [intact cells]: n = 12, [spheroplast cells]: n = 12). Error bars indicate the mean value ± standard deviation of experimental results repeated n times, ([1 h]: p < 0.01, [3 h]: p < 0.001). The double asterisk (**) and triple asterisk (***) indicate significant differences in the survival rates of spheroplast cells and intact cells + albumin at p < 0.01 and at p < 0.001, respectively.
First, to evaluate the effect of the presence or absence of protein addition on the photocatalytic bactericidal effect, the survival rate of albumin-added intact cells was compared with the survival rate of albumin-free intact cells. The survival rates of the intact cells with albumin were about 75%, about 49%, and about 32%, corresponding to after 1 h, 2 h, and 3 h of the photocatalytic reaction. Additionally, the survival rates of intact cells with albumin were about 77% after 1 h of the photocatalytic reaction, about 47% after 2 h, and about 41% after 3 h. As a result of the significance test, it was shown that there was no significant difference in the survival rates between the presence and absence of protein addition.
Next, to evaluate the effect of the presence or absence of the peptidoglycan layer on the photocatalytic sterilization under protein addition conditions, the survival rates of albumin-added intact cells and the spheroplast cells were compared. The survival rates of the intact cells with albumin were added was about 75%, about 49%, and about 32%, corresponding to after 1 h, 2 h, and 3 h of the photocatalytic reaction. The survival rates of the spheroplast cells were about 95%, about 63%, and about 57% after 1 h, 2 h, and 3 h of the photocatalytic reaction. As a result of the significance test, it was shown that there was a significant difference in survival rates between albumin-added intact cells and the spheroplast cells after 3 h of the photocatalytic reaction (p < 0.001).
2.3.4. The Effect of EDTA Addition on the Photocatalytic Sterilization
Similar to the experimental objective in Section 2.3.3., to verify the effect of EDTA used during spheroplast cells preparation on the photocatalytic effect, only EDTA was added to the intact cells and the photocatalytic reaction was carried out (Figure 9).
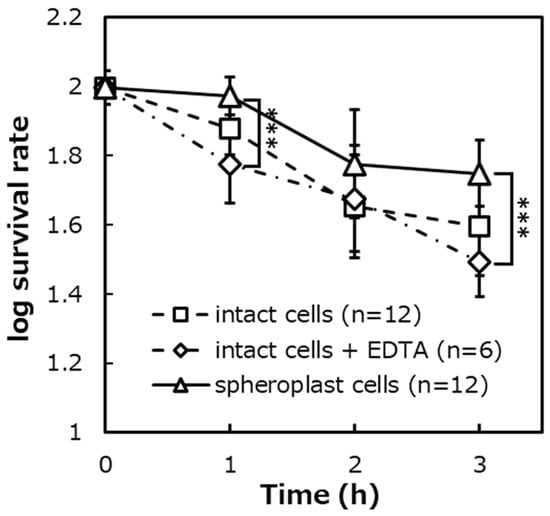
Figure 9.
The survival rates of EDTA-added E. coli IAM12119T intact cells on the photocatalytic reaction, ([intact cells + EDTA]: n = 6, [intact cells]: n = 12, [spheroplast cells]: n = 12). Error bars indicate the mean value ± standard deviation of experimental results repeated n times, ([1 h], [3 h]: p < 0.001). The triple asterisk (***) indicates a significant difference in the survival rates of spheroplast cells and intact cells + EDTA at p < 0.001.
The survival rates of the intact cells with EDTA were about 61%, about 50%, and about 32% after 1 h, 2 h, and 3 h of the photocatalytic reaction. As a result of the significant difference test, it was shown that there was no significant difference in the survival rates between the intact cells with EDTA and without EDTA.
Moreover, as a result of the significant difference test, it was shown that there were significant differences in the survival rate between the intact cells with EDTA and the spheroplast cells (p < 0.001).
2.3.5. Evaluation of the Survival Rate of the Spheroplast Cells Added Peptidoglycan
To clarify the cause of the enhancement of the photocatalytic bactericidal effect of the peptidoglycan layer, it was investigated whether the cause of the enhancement was the presence of the peptidoglycan layer in the cell wall or the substance peptidoglycan itself. Therefore, the survival rates of the spheroplast cells added peptidoglycan and the intact cells were compared (Figure 10).
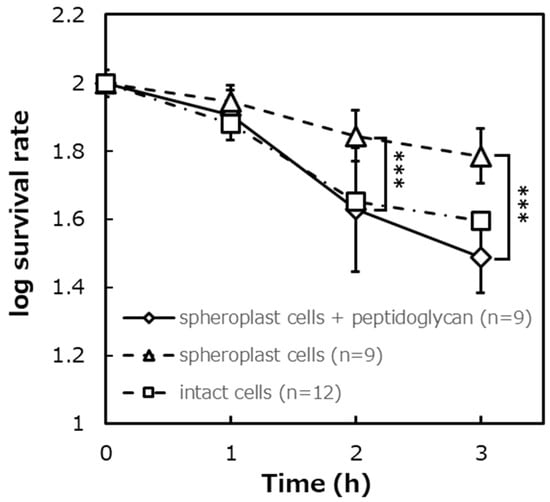
Figure 10.
The survival rate after the photocatalytic reaction in E. coli IAM12119T spheroplast cells added peptidoglycan, ([Spheroplast cells + peptidoglycan]: n = 9, [spheroplast cells]: n = 9, [intact cells]: n = 12). Error bars indicate the mean value ± standard deviation of experimental results repeated n times, ([2 h], [3 h]: p < 0.001). The triple asterisk (***) indicates a significant difference in the survival rates of spheroplast cells and spheroplast cells + peptidoglycan at p < 0.001.
Figure 10 shows the results of comparing the survival rates of spheroplast cells to which peptidoglycan was added, spheroplast cells to which peptidoglycan was not added, and intact cells when the photocatalytic reaction was performed. The survival rates of spheroplast cells to which peptidoglycan was not added were about 81% after 1 h of the photocatalytic reaction, about 45% after 2 h, and about 32% after 3 h. The survival rate of spheroplast cells added peptidoglycan was about 89% after 1 h, about 71% after 2 h, and about 62% after 3 h. The survival rate of intact cells was about 77% after 1 h, about 47% after 2 h, and about 41% after 3 h. As a result of a significant difference test between spheroplast cells to which peptidoglycan was added and spheroplast cells to which peptidoglycan preparation was not added, it was shown that there was a significant difference in survival rate between the two conditions after 2 and 3 h (p < 0.001). Moreover, as a result of a significant difference test between spheroplast cells to which peptidoglycan was added and intact cells, it was shown that there was no significant difference.
2.4. Quantitative Determination of Hydroxyl Radical and Hydrogen Peroxide under Conditions of the Presence and Absence of Peptidoglycan
From the previous study of our laboratory and this study, it was suggested that peptidoglycan promotes the photocatalytic bactericidal effect. However, it is not clear how the peptidoglycan promotes photocatalytic sterilization. Photogenerated reactive oxygen species (ROS) such as hydroxyl radical and hydrogen peroxide are considered to be the major bactericidal agents [22], and we investigated the quantitative determination of hydroxyl radical in the photocatalytic reaction under the condition of different amounts of the peptidoglycan.
The amount of hydroxyl radical generated by the photocatalytic reaction with TiO2 and peptidoglycan was significantly lower than the condition of only TiO2 alone (Figure 11). In the negative control experiments without TiO2, no hydroxyl radical was generated by the peptidoglycan under UVA irradiation.
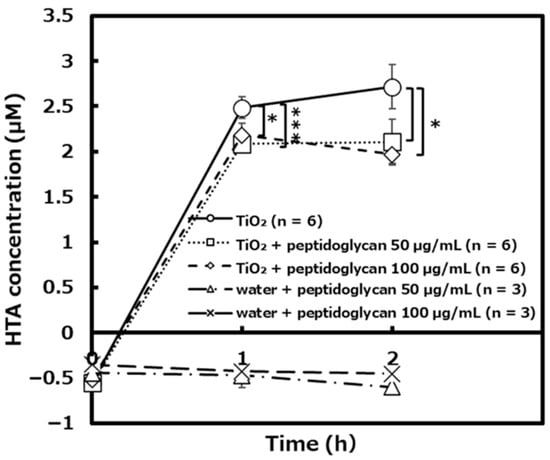
Figure 11.
The amount of hydroxyl radical generated by TiO2 the photocatalytic reaction in the presence and absence of peptidoglycan ([TiO2]: n = 6, [TiO2 + peptidoglycan 50 µg/mL]: n = 6, [TiO2 + peptidoglycan 100 µg/mL]: n = 6, [water + peptidoglycan 50 µg/mL]: n = 3, [water + peptidoglycan 100 µg/mL]: n = 3). Error bars indicate the mean value ± standard deviation of experimental results repeated n times, ([1 h, TiO2: TiO2 + peptidoglycan 50 µg/mL]: p < 0.001, [1 h, TiO2: TiO2 + peptidoglycan 100 µg/mL] [2 h, TiO2: TiO2 + peptidoglycan 50 µg/mL or peptidoglycan 100 µg/mL]: p < 0.05). The asterisk (*) and triple asterisk (***) indicate significant differences in HTA concentration of TiO2 and TiO2 + peptidoglycan 50 or 100 µg/mL at p < 0.05 and at p < 0.001, respectively.
Next, we focused on hydrogen peroxide as active oxygen species that acts on sterilization, and quantified hydrogen peroxide generated when peptidoglycan was photocatalytically treated for 3 h. It was also verified whether the amount of hydrogen peroxide generated changed depending on the amount of peptidoglycan.
The photocatalytic reaction with 50 and 1000 µg/mL of peptidoglycan suspensions for 3 h resulted in significantly higher amounts of hydrogen peroxide generated than in the absence of peptidoglycan (Figure 12). The amount of hydrogen peroxide generated increased in proportion to the amount of peptidoglycan added.
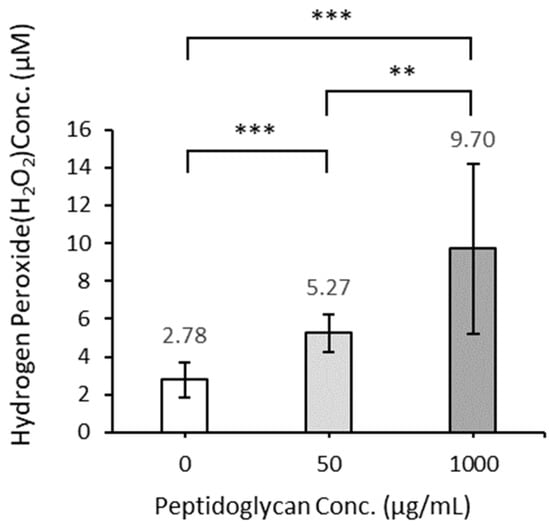
Figure 12.
The amount of hydrogen peroxide generated by TiO2 photocatalytic reaction in the presence and absence of peptidoglycan ([0 µg/mL]: n = 21, [50 µg/mL]: n = 21, [1000 µg/mL]: n = 16). Error bars indicate the mean value ± standard deviation of experimental results repeated n times, ([0 µg/mL: 50 µg/mL], [0 µg/mL: 1000 µg/mL]: p < 0.001, [50 µg/mL: 1000 µg/mL]: p < 0.01). The double asterisk (**) and triple asterisk (***) indicate significant differences in H2O2 conc. of each peptidoglycan conc. at p < 0.01 and at p < 0.001, respectively.
3. Discussion
A previous study in our laboratory suggested that the peptidoglycan layer enhances the photocatalytic bactericidal effect by the results of photocatalytic experiments using Gram-positive bacterium [27]. However, the quantitative relationship between the thickness of the peptidoglycan layer and the enhancement of photocatalytic bactericidal effect and the photocatalytic bactericidal effect of the peptidoglycan layer using Gram-negative bacterium were not clarified. In this study, we attempted to clarify these factors.
3.1. The Quantitative Relationship between the Thickness of the Peptidoglycan Layer on a Gram-Positive or Gram-Negative Bacterium and the Enhancement of Photocatalytic Bactericidal Effect
To verify whether the bactericidal effect of the photocatalytic reaction differs depending on the thickness of the peptidoglycan layer, we hypothesized that the peptidoglycan layer became thicker as the cell grew and performed the photocatalytic reaction on the L. plantarum cells in each growth phase. The hypothesis was verified by the observation of transmission electron microscopic observation. The thickness of the peptidoglycan layer of L. plantarum cells in the early stationary phase was about three times that in the early log phase (Figure 3 and Figure 4). This result shows that the peptidoglycan layer increase thickens as the cells grow.
From the start of UVA irradiation to 1 h, the survival rate of the early stationary phase of L. plantarum cells was lower than that in the early log phase cells (Figure 1). As the cause of significantly reducing the survival rate of early stationary phase cells, it was also thought that the influence of the cell aging or lactic acid produced by L. plantarum. The results of the verification showed that L. plantarum cells under negative control conditions without photocatalytic reaction rarely died compared to the condition in the photocatalytic reaction even if time passed (Figure 2). From these results, it was suggested that the lactic acid produced by L. plantarum during the photocatalytic reaction did not affect the photocatalytic sterilization. In addition, since there is no significant difference between the results of the early log phase and the results of the more advanced early stationary phase, it is considered that cell aging does not affect photocatalytic sterilization. It is suggested that the peptidoglycan layer promotes the photocatalytic bactericidal effect in the quantitative relationship.
To verify whether the bactericidal effect of the photocatalytic reaction differs depending on the presence or absence of the peptidoglycan layer, the photocatalytic reaction was carried out on intact cells and spheroplast cells of E. coli, which is a Gram-negative bacterium. Since the spheroplast cells without the peptidoglycan layer are vulnerable to physical stress when the cells are spread on the agar medium plate, we used LIVE/DEAD® BacLightTM Bacterial Viability which determined survival and dead cell based on the membrane damage instead of the colony counting method. After preparing the spheroplast cells, the cell morphology was observed in the bright field of a light microscope to confirm whether the peptidoglycan layer was removed (Figure 5). The untreated intact cells were short rod-shaped, whereas the enzyme-treated spheroplast cells were spherical, suggesting that the peptidoglycan layer was removed. Before comparing the survival rate of intact cells and spheroplast cells, negative control experiments were performed to confirm that TiO2-coated glass and UVA irradiation did not affect intact cells and spheroplast cells of E. coli (Figure 6). As a result, it was shown that TiO2-coated glass and UVA irradiation itself did not show a significant bactericidal effect on intact cells and spheroplast cells.
The survival rates of intact cells and spheroplast cells after the photocatalytic reaction were compared. The result was that the survival rates of intact cells with a peptidoglycan layer were significantly lower than that of spheroplast cells that had lost the peptidoglycan layer (Figure 7). The possibility that the photocatalytic bactericidal effect was suppressed by lysozyme and EDTA added during the preparation of spheroplast cells was denied from the results of verifying experiments (Figure 8 and Figure 9).
3.2. Evaluation of Survival Rate of Spheroplast Cells Added Peptidoglycan Preparation
From the above, it was strongly suggested that the peptidoglycan layer enhanced the photocatalytic bactericidal effect. Then, to evaluate whether the state in which the peptidoglycan layer is present in the cell wall is important or simply the component of the peptidoglycan is important, the peptidoglycan was added to spheroplast cells and the photocatalytic reaction was carried out (Figure 10). Negative control experiments were performed to show that the addition of peptidoglycan did not negatively affect spheroplast cells. As a result, the addition of the peptidoglycan did not show a significant negative effect on spheroplast cells.
Then, the survival rates of the spheroplast cells added peptidoglycan, and the spheroplast cells without peptidoglycan were compared. Three hours after the photocatalytic reaction, the survival rate of the spheroplast cells added peptidoglycan was significantly lower than that of the spheroplast cells without peptidoglycan. Furthermore, there was no significant difference in the survival rate of spheroplast cells added peptidoglycan and intact cells. This suggests that for peptidoglycan to affect the bactericidal effect, the peptidoglycan layer does not need to be present in the cell as the cell wall, and the component of peptidoglycan is important.
3.3. Quantification of Hydroxyl Radical and Hydrogen Peroxide
From the above results, it is suggested that the peptidoglycan layer promotes the photocatalytic bactericidal effect in proportion. However, since it is not clear how peptidoglycan enhances photocatalytic sterilization, it was quantitatively confirmed whether the addition of peptidoglycan to the photocatalytic reaction increased the amount of hydroxyl radical and hydrogen peroxide. As a result, when peptidoglycan was added to TiO2, the amount of hydroxyl radical produced was lower than that of TiO2 alone (Figure 11). It was also shown that the amount of hydrogen peroxide generated during the photocatalytic reaction increased in proportion to the concentration of peptidoglycan (Figure 12). These results suggest that peptidoglycan consumed the hydroxyl radical produced by the photocatalytic reaction to produce hydrogen peroxide. The enhancing effect of photocatalytic sterilization by peptidoglycan is thought to be achieved by the production of ROS such as hydrogen peroxide instead of hydroxyl radical. We think that the decrease in hydroxyl radicals is due to peptidoglycan, but the relationship between that reaction and the reaction that leads to the increase in hydrogen peroxide cannot be speculated at this time. We are currently considering ways to find out more.
To summarize this study, like the study of the Gram-positive bacterium in our previous study, the Gram-negative bacterium was shown to be a high bactericidal effect in the presence of the peptidoglycan layer. It was also revealed that the thicker the peptidoglycan layer, the higher the photocatalytic bactericidal effect. It was shown that the cause of the photocatalytic sterilization enhancing effect of peptidoglycan may be the production of new ROS such as hydrogen peroxide by peptidoglycan.
4. Materials and Methods
4.1. Bacterial Strains
L. plantarum JCM1149T and E. coli IAM12119T were used for the photocatalytic bactericidal experiments. These strains were obtained from Japan Collection of Microorganisms, RIKEN BioResource Research Center, Japan and Institute of Applied Microbiology, The University of Tokyo, Japan.
4.2. Preparation of Bacterial Suspension
4.2.1. L. plantarum Cells
L. plantarum JCM1149T cells were statically precultured in de Man, Rogosa, and Sharpe (MRS) broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) at 30 °C for 24 h. In the main culture, the bacterial suspension was inoculated into the new MRS broth, and static-cultured in an incubator at 30 °C until each growth phase. The growth phase begins in the lag phase, goes through the log phase, the stationary phase, and then to the death phase. Each phase is divided into three, early, middle, and late. Each growth phase was divided with reference to L. plantarum growth curve that we measured and created. The cells were washed three times with 1 × PBS, re-suspended in the new 1 × PBS to OD (Optical Density) 660 nm of 1.00. This solution was used as a bacterial suspension of L. plantarum cells.
4.2.2. E. coli Cells and Spheroplast Cells
E. coli IAM12119T cells were precultured with shaking in nutrient broth (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) in an incubator (Tokyo Rika Kikai, Tokyo, Japan) at 37 °C for 18 h. In the main culture, the bacterial suspension was inoculated into the new nutrient broth, and shake-cultured in an incubator at 37 °C for 2.5 h until the early log phase. Furthermore, 1 × PBS was used as a bacterial suspension of E. coli intact cell.
The cells cultured in the same manner as above were washed 3 times with 0.03 M Tris-HCl buffer containing 20% sucrose. The cells were re-suspended in 0.03 M Tris-HCl buffer containing 20% sucrose to OD 660 nm of 0.50. Lysozyme solution was added to the bacterial suspension to a final concentration of 1 mg/mL, and 0.1 M EDTA solution was also added. After mixing by inversion, the mixture stood at 30 °C for 1 h. Then, the mixture was centrifuged and resuspended in 10 mM PBS buffer containing 0.2% MgCl2. This solution was used as a bacterial suspension of spheroplast cells. The concentration of each reagent was determined considering the optimal conditions for the preparation of spheroplast cells.
4.3. Photocatalytic Experimental Device
In this study, the TiO2-coated glass was used to investigate the photocatalytic bactericidal effect, which does not need into consideration in nanoparticle toxicity. The TiO2- coated glass was prepared as follows. A flat glass plate (50 × 50 × 2 mm3, Tokyo Glass Instruments, Tokyo, Japan) was washed with 0.5% NaOH solution and water. An amount of 1 mL of TiO2 coating solution (TKC-304, Tayca Co., Ltd., Osaka, Japan) was dropped onto the washed glass plate, spin-coated (3000 rpm, 5 s) using a spin coater (MS-A200, Mikasa Co., Ltd., Tokyo, Japan), and dried at room temperature.
The photocatalytic reaction was carried out by constructing an evaluation device according to Japanese Industrial Standards (JIS R-1702) described in the previous paper. All parts of the evaluation device were washed with 70% ethanol and exposed to UVC overnight. Filter paper moistened with sterilized water, a plastic stage, and TiO2-coated glass plate were placed in order from the bottom of a petri dish.
4.4. The Photocatalytic Reaction
In the experiments with L. plantarum, 100 µL of bacterial suspension was dropped onto the TiO2-coated glass plate. An OHP film (45 × 45 mm2, KOKUYO Co., Ltd., Osaka, Japan) was placed on it, and the entire petri dish was covered with a wrap. Using the black light FL15BL-B (Panasonic, Osaka, Japan) as light source, the irradiation intensity of UVA was adjusted to 0.25 mW/cm2 as described in the previous paper, and the experimental device was irradiated with UVA for a maximum of 2 h. This irradiation intensity corresponds to the intensity of the sunlight shining into the window during the daytime. After the photocatalytic reaction, the TiO2-coated glass plate with bacterial cells and 10 mL of PBS buffer were placed in a sterile plastic bag and washed by rubbing to recover the bacterial suspension. The serially diluted bacterial suspension was spread on the Nutrient agar (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan), incubated at 37 °C overnight, and the number of surviving cells was determined.
In the experiments with E. coli, 0.3 µL of LIVE/DEAD® BacLight™ Bacterial Viability Kit solution (Molecular Probes, Eugene, OR, USA) was added to 100 µL of bacterial suspension, and 10 µL of this bacterial suspension was dropped to TiO2-coated glass plate. A cover glass (22 × 22 mm2) was placed thereon, and the entire petri dish was covered with a wrap. The irradiation intensity of UVA was adjusted to 0.25 mW/cm2 as described in the previous paper. The photocatalytic reaction was performed for a maximum of 3 h as the fluorescent pigments of the LIVE/DEAD® BacLightTM Bacterial Viability Kit faded with prolonged UVA irradiation. After the photocatalytic reaction, the fluorescent cell was observed using an all-in-one fluorescence microscope BZ-8100 (KEYENCE) to calculate the cell membrane non-damage rate, that is, the survival rate.
4.5. Evaluation of the Survival Rate of the Spheroplast Cells Added Peptidoglycan
In the experiments of peptidoglycan addition, the peptidoglycan prepared from Bacillus subtilis (Sigma-Aldrich, St. Louis, MO, USA) was added to the bacterial suspension of spheroplast cells to a final concentration of 50 µg/mL.
4.6. Transmission Electron Microscopic Observation of L. plantarum Cells in Each Growth Phase
To clarify that the peptidoglycan layer of L. plantarum cell thickens with cells growth, we observed the early log phase cells and the early stationary phase cells with a transmission electron microscope. L. plantarum cells cultured up to the above two growth phases were collected and washed three times with PBS buffer. The cells were fixed in 2% glutaraldehyde (2% glutaraldehyde, 0.1 M NaH2PO4, 0.1 M Na2HPO4) at 4 °C for 1.5 h, washed five times with 0.1 M phosphate buffer (0.1 M sucrose, 0.1 M NaH2PO4, 0.1 M Na2HPO4) at 4 °C for 30 min, and postfixed with 1.0% OsO4 (1.0% OsO4, 0.1 M sucrose, 0.1 M NaH2PO4, 0.1 M Na2HPO4) at 4 °C for 1.5 h. Fixed cells were embedded in 2% agarose (Agarose S, FUJIFILM Wako Pure Chemical Co., Ltd., Osaka, Japan) and cut into 1 mm3 cube. The cubes were dehydrated through 50, 70, 80, 90, and 95% ethanol solutions at 4 °C for 10 min at each dilution, followed by dehydration with 99.5% ethanol twice for 30 min. After dehydration, we discarded half of 99.5% ethanol and added QY-1 (n-butyl glycidyl ether, FUJIFILM Wako Pure Chemical Co., Ltd., Osaka, Japan) to replace ethanol in the cubes at room temperature for 5 min. Subsequently, all of the reagents were discarded, and an equal amount of QY-1 was added to replace ethanol completely in the cubes at room temperature for 10 min. After half of the reagents were discarded, an equal amount of epoxy resin (Epon 812, Ouken Trading Co., Ltd., Tokyo, Japan) was added and penetrated with shaking (80 rpm, room temperature) for 2 h. All amounts of the reagents were discarded, followed by completely penetration twice with new epoxy resin for 1 h (120 rpm). New epoxy resin was poured into the mold and the cube was embedded in the tip. Subsequently, the epoxy resin containing the cube was polymerized at 60 °C for 24 h. Ultra-thin section preparation, staining, and observation were performed at the contract-based analysis company (JEOL Ltd., Tokyo, Japan).
4.7. Quantitative Determination of Hydroxyl Radical
In order to quantify the hydroxyl radical generated during the photocatalytic reaction, we used chemical dosimetry based on terephthalic acid (TA) [28]. The hydroxyl radical change terephthalic acid to 2-hydroxyterephthalic acid (HTA), which can be detected by fluorescence measurement. An amount of 100 µL of TiO2 coating solution (Tersus EN, Shin-Etsu Astech Co., Ltd., Tokyo, Japan) and 100 µL of 2 mM TA solution (FUJIFILM Wako Pure Chemical Co., Ltd., Osaka, Japan) were added to the 96well plate. TA solution was prepared by dissolving TA in the distilled water containing 5 mM NaOH (FUJIFILM Wako Pure Chemical Co., Ltd., Osaka, Japan). The peptidoglycan prepared from Bacillus subtilis (Sigma-Aldrich, St. Louis, MO, USA) was added to the well to a final concentration of 50 and 100 µg/mL. After covering the 96 well plate with a wrap, the plate was irradiated with 0.25 mW/cm2 of UVA for a maximum of 2 h. After irradiation, the fluorescence of HTA was measured with FluoroCount (PACKARD, Detroit, MI, USA).
4.8. Quantitative Determination of Hydrogen Peroxide
Quantitative determination of hydrogen peroxide was performed using Hydrogen Peroxide Colorimetric Detection Kit (Arbor Assays LLC, Ann Arbor, MI, USA). An amount of 100 µL of 50, and 1000 ng/mL peptidoglycan suspension or sterilized water was dropped to TiO2-coated glass in the evaluation device. The irradiation intensity of UVA was adjusted to 0.25 mW/cm2, and UVA irradiation to the evaluation device was performed for 3 h. After irradiation, the solution on the glass was collected, and 50 µL of the collected solution was added to the 96 well plate. After reacting with Hydrogen Peroxide Colorimetric Detection Kit solution for 15 min, the absorbance was measured with SpectraMax ABS Plus (Molecular Devices, San Jose, CA, USA).
Author Contributions
Investigation—study with L. plantarum and hydroxyl radical, A.S.; Investigation —study with E. coli and hydrogen peroxide, K.S.; writing—original draft preparation A.S. and K.S.; writing—review and editing, T.S.; supervision, T.S.; project administration, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We are grateful Distinguished Akira Fujishima and Chiaki Terashima for providing us a place to research and useful suggestion. We also thank Tayca Co., Ltd. for providing us TiO2 coating solution (TKC-304).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ross, M.A.; Curtis, L.; Scheff, P.A.; Hryhorczuk, D.O.; Ramakrishnan, V.; Wadden, R.A.; Persky, V.W. Association of asthma symptoms and severity with indoor bioaerosols. Allergy 2000, 55, 705–771. [Google Scholar] [CrossRef] [PubMed]
- Green, C.F.; Scarpino, P.V. The use of ultraviolet germicidal irradiation (UVGI) in disinfection of airborne bacteria. Environ. Eng. Policy 2002, 3, 101–107. [Google Scholar] [CrossRef]
- Unosson, E.; Tsekoura, E.K.; Engqvist, H.; Welch, K. Synergetic inactivation of Staphylococcus epidermidis and Streptococcus mutans in a TiO2 /H2O2 /UV system. Biomatter 2013, 3, e26727. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.P.; MacDougall, C.; Lim, M. Ontario Agency for Health Protection and Promotion 218(Public Health Ontario). Antimicrobial surfaces to prevent healthcare-associated infections: A systematic review. J. Hosp. Infect. 2016, 92, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Caratto, V.; Ball, L.; Sanguineti, E.; Insorsi, A.; Firpo, I.; Alberti, S.; Ferretti, M.; Pelosi, P. Antibacterial activity of standard and N-doped titanium dioxide-coated endotracheal tubes: An in vitro study. Rev. Bras. Ter. Intensiva 2017, 29, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. Photochem. Photobiol. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Sopyan, I.; Watanabe, M.; Murasawa, S.; Hashimoto, K.; Fujishima, A. An efficient TiO2 thin film photocatalyst: Photocatalytic properties in gas-phase acetaldehyde degradation. Photochem. Photobiol. 1996, 98, 79–86. [Google Scholar] [CrossRef]
- Noguchi, T.; Fujishima, A.; Sawunyama, P.; Hashimoto, K. Photocatalytic Degradation of Gaseous Formaldehyde Using TiO2 Film. Environ. Sci. Technol. 1998, 32, 3831–3833. [Google Scholar] [CrossRef]
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal Activity of Photocatalytic TiO2 Reaction: Toward an Understanding of Its Killing Mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004, 38, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Gogniat, G.; Dukan, S. TiO2 Photocatalysis Causes DNA Damage via Fenton Reaction-Generated Hydroxyl Radicals during the Recovery Period. Appl. Environ. Microbiol. 2007, 65, 7740–7743. [Google Scholar] [CrossRef] [PubMed]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.C.; Horvatovich, P.; Gies, J.P.; Keller, V.; Keller, N.; Andre, P. TiO2 Photocatalysis Damages Lipids and Proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xia, D.; An, T.; Ng, T.W.; Yip, H.Y.; Li, G.; Zhao, H.; Wong, P.K. Dual Roles of Capsular Extracellular Polymeric Substances in Photocatalytic Inactivation of Escherichia coli: Comparison of E. coli BW25113 and Isogenic Mutants. Appl. Environ. Microbiol. 2015, 81, 5174–5183. [Google Scholar] [CrossRef]
- Xiao, X.; Zhu, W.W.; Liu, Q.Y.; Yuan, H.; Li, W.W.; Wu, L.J.; Li, Q.; Yu, H.Q. Impairment of Biofilm Formation by TiO2 Photocatalysis through Quorum Quenching. Environ. Sci. Technol. 2016, 50, 11895–11902. [Google Scholar] [CrossRef]
- Huang, G.; Ng, T.W.; An, T.; Li, G.; Xia, D.; Yip, H.Y.; Zhao, H.; Wong, P.K. Probing the intracellular organic matters released from the photocatalytic inactivation of bacteria using fractionation procedure and excitation-emission-matrix fluorescence. Water Res. 2017, 110, 270–280. [Google Scholar] [CrossRef]
- Ouyang, K.; Dai, K.; Walker, S.L.; Huang, Q.; Yin, X.; Cai, P. Efficient Photocatalytic Disinfection of Escherichia coli O157:H7 using C70-TiO2 Hybrid under Visible Light Irradiation. Sci. Rep. 2016, 6, 25702. [Google Scholar] [CrossRef]
- Yoo, S.; Ghafoor, K.; Kim, S.; Sun, Y.W.; Kim, J.U.; Yang, K.; Lee, D.U.; Shahbaz, H.M.; Park, J. Inactivation of pathogenic bacteria inoculated onto a BactoTM agar model surface using TiO2-UVC photocatalysis, UVC and chlorine treatments. Appl. Microbiol. 2015, 119, 688–696. [Google Scholar] [CrossRef][Green Version]
- Tartanson, M.A.; Soussan, L.; Rivallin, M.; Pecastaings, S.; Chis, C.V.; Penaranda, D.; Roques, C.; Faur, C. Dynamic Mechanisms of the Bactericidal Action of an Al2O3-TiO2-Ag Granular Material on an Escherichia coli Strain. Appl. Environ. Microbiol. 2015, 81, 7135–7142. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, H.; Shang, Y.; Zeng, S.; Qin, Z.; Yin, S.; Li, J.; Lu, G.; Liang, S.; Liu, Z. Spiky nanohybrids of titanium dioxide/gold nanoparticles for enhanced photocatalytic degradation and anti-bacterial property. J. Colloid Interface Sci. 2019, 535, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Quintero, R.R.; Acevedo-Merino, A.; Nebot, E. Disinfection performance using a UV/persulfate system: Effects derived from different aqueous matrices. Photochem. Photobiol. Sci. 2019, 18, 878–883. [Google Scholar] [CrossRef]
- Pal, A.; Pehkonen, S.O.; Liya, E.Y.; Ray, M.B. Photocatalytic inactivation of Gram-positive and Gram-negative bacteria using fluorescent light. J. Photochem. Photobiol. A Chem. 2007, 186, 335–341. [Google Scholar] [CrossRef]
- Van Grieken, R.; Marugán, J.; Pablos, C.; Furones, L.; López, A. Comparison between the photocatalytic inactivation of Gram-positive E. faecalis and Gram-negative E. coli faecal contamination indicator microorganisms. Appl. Catal. B Environ. 2010, 100, 212–220. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Takao, A.; Suzuki, T. The Effects of Peptidoglycan on the Photocatalytic Bactericidal Activity of Titanium Dioxide. Biocontrol Sci. 2020, 25, 167–171. [Google Scholar] [CrossRef]
- Kanazawa, S.; Furuki, T.; Nakaji, T.; Akamine, S.; Ichiki, R. Application of chemical dosimetry to hydroxyl radical measurement during underwater discharge. J. Phys. Conf. Ser. 2012, 418, 012012. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).