Synthesis, Characterization and Enhanced Visible Light Photocatalytic Performance of ZnWO4-NPs@rGO Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ZnWO4 Nanoparticles
2.2. Preparation of ZnWO4-NPs@rGO Nanocomposites
2.3. Characterization
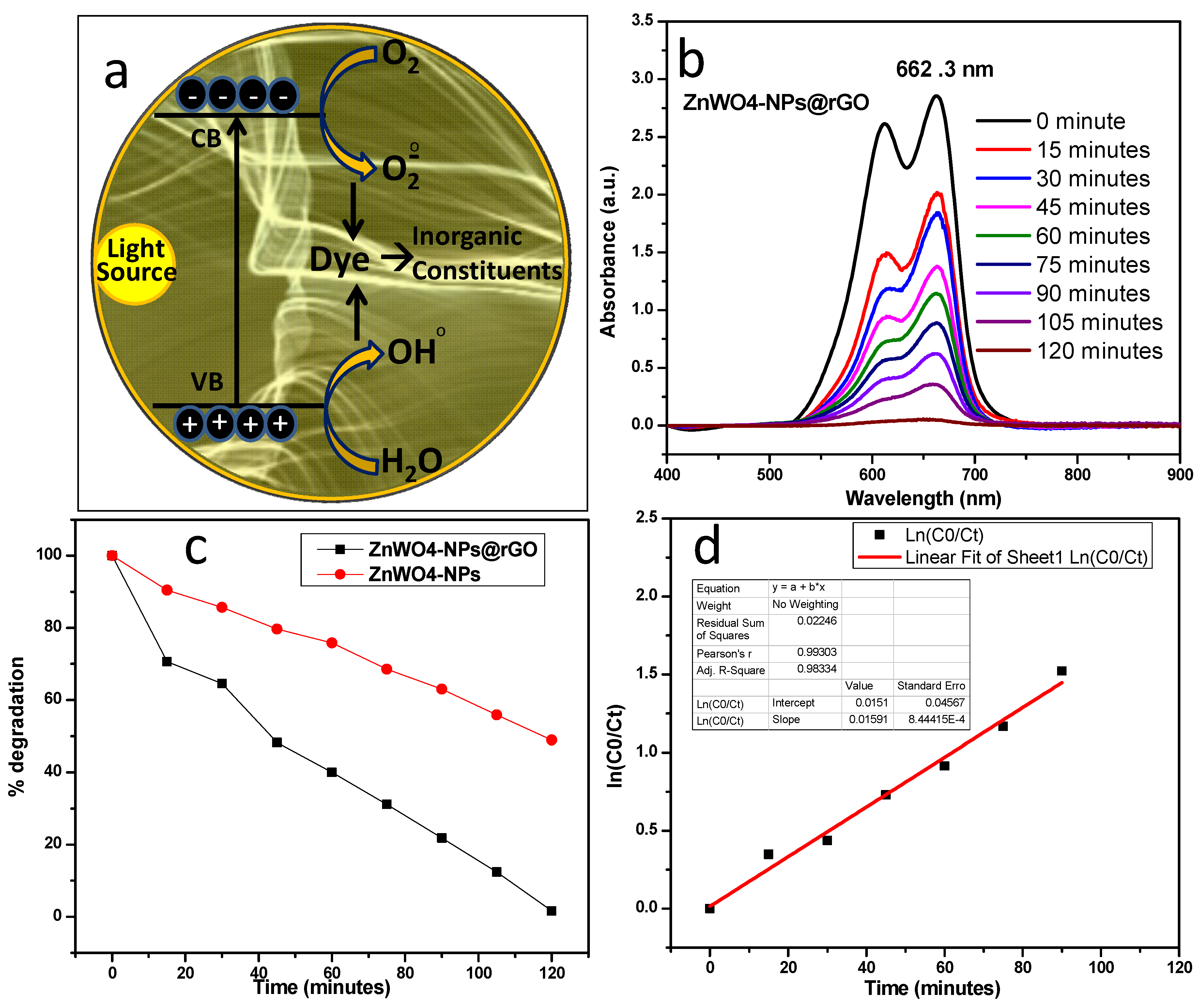
2.4. Photocatalytic Studies
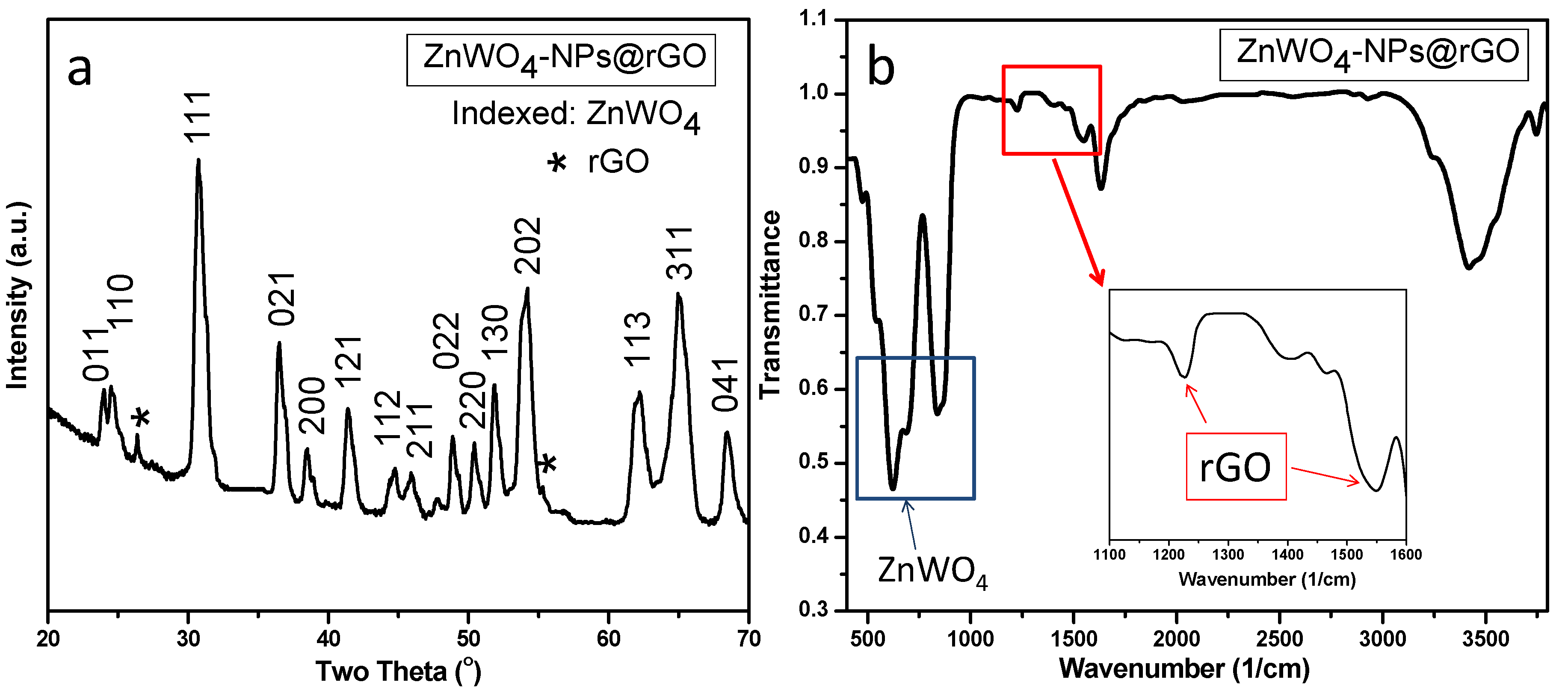
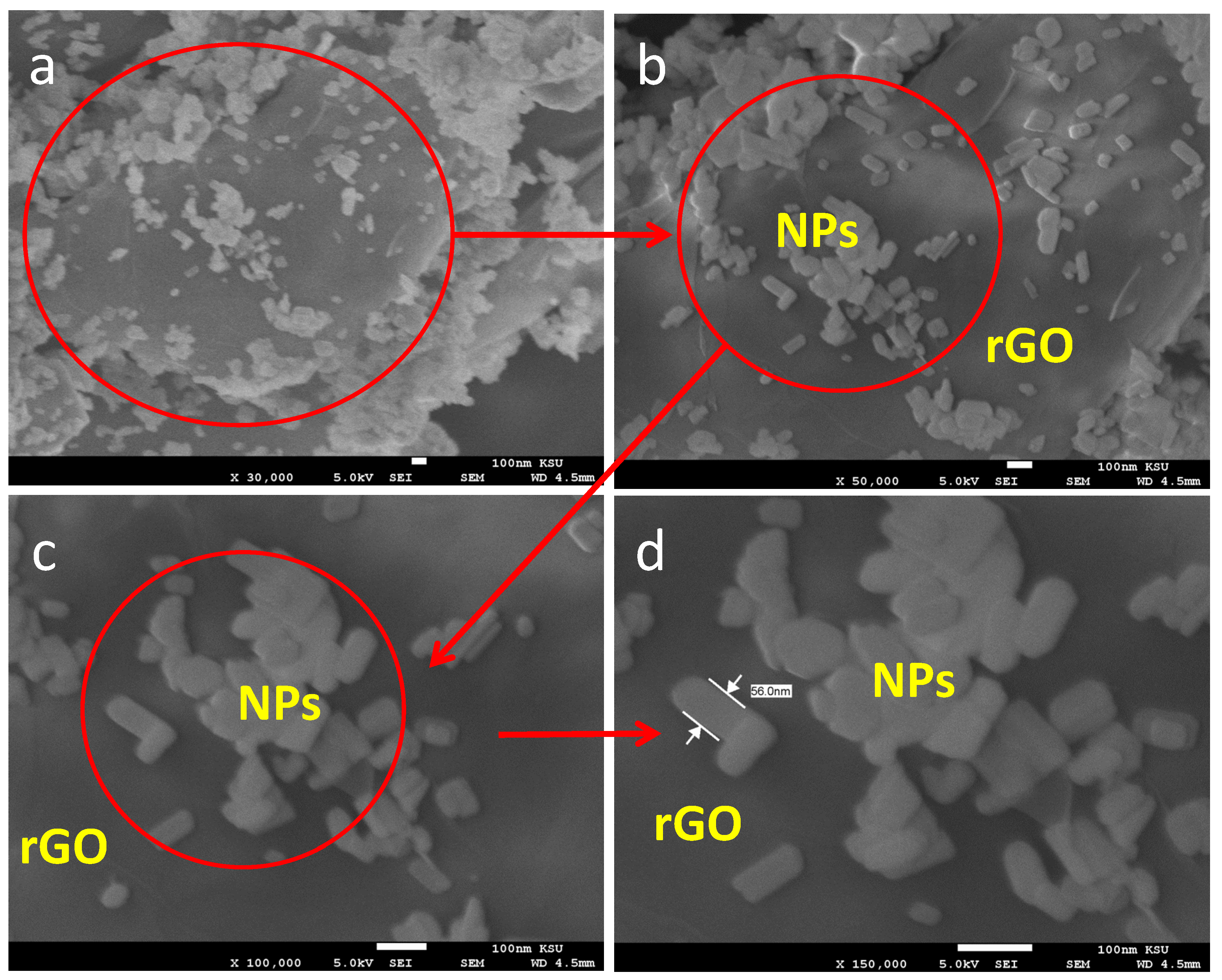
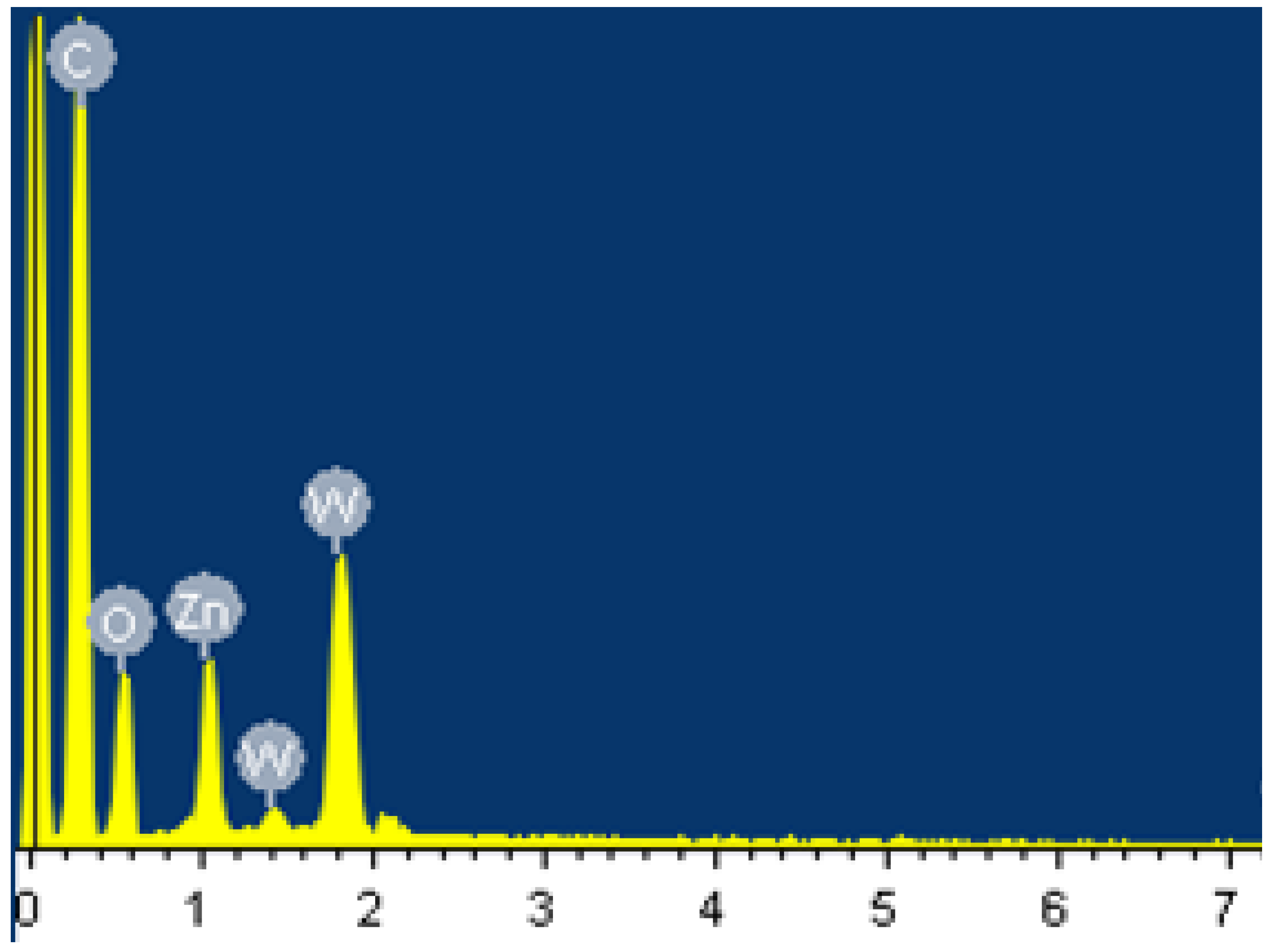
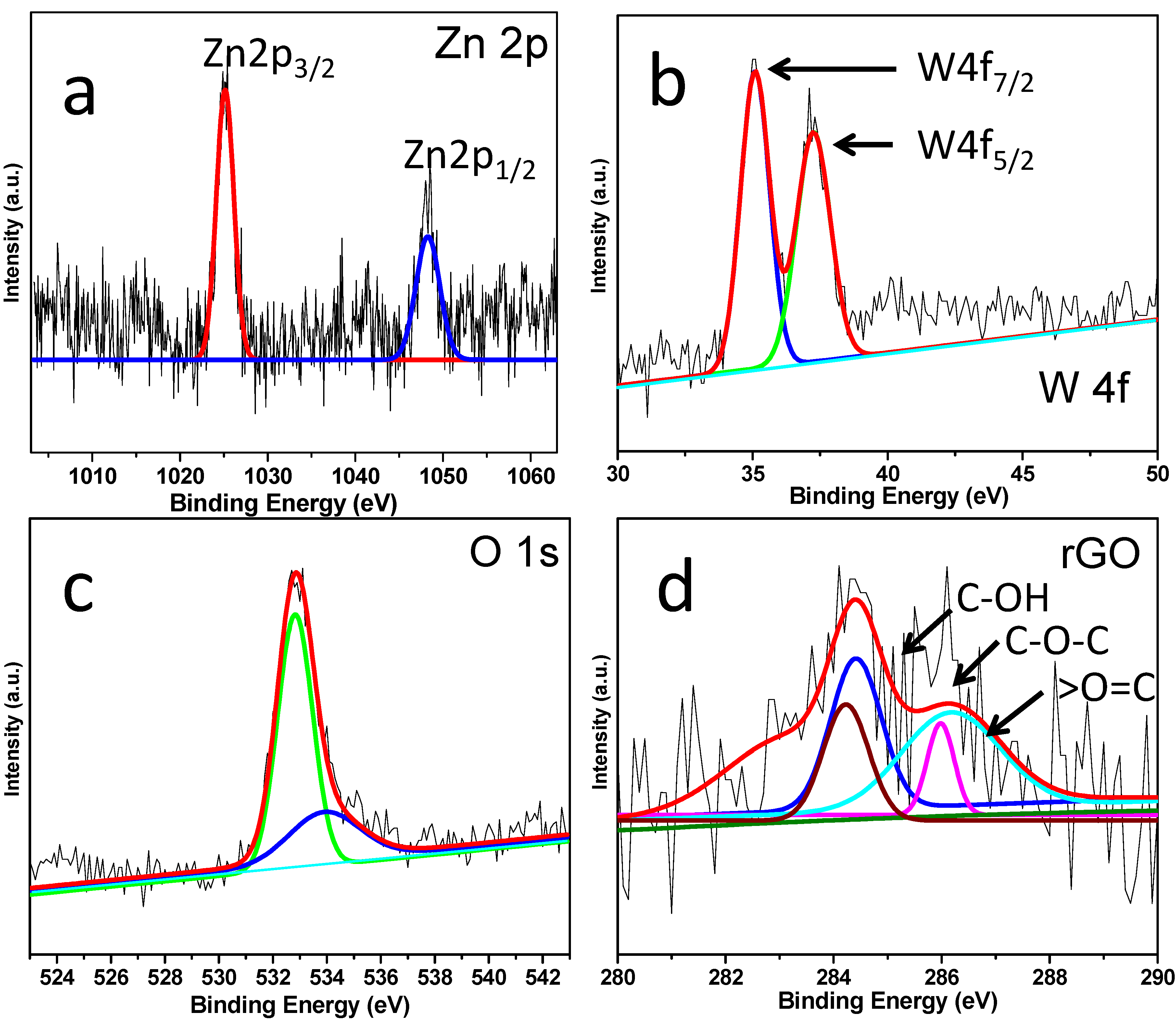
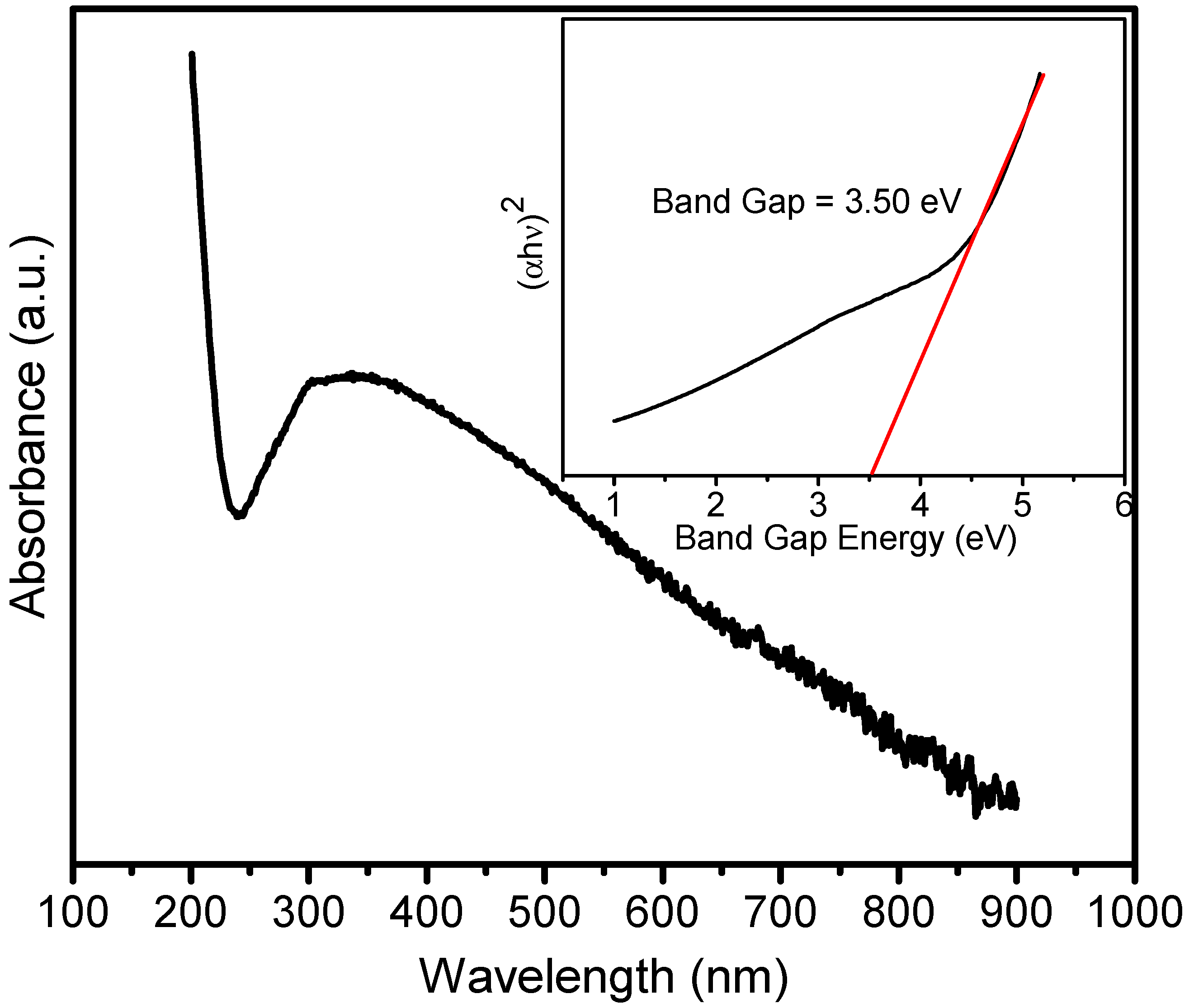
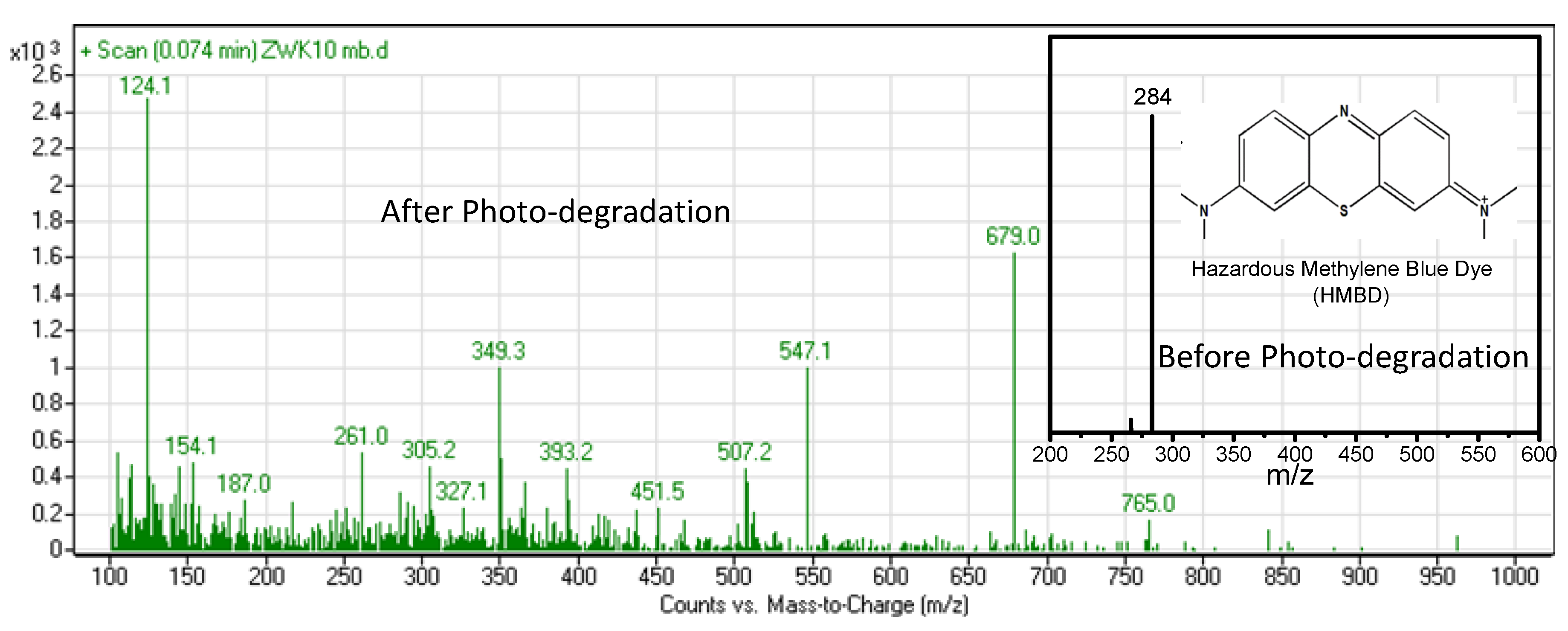
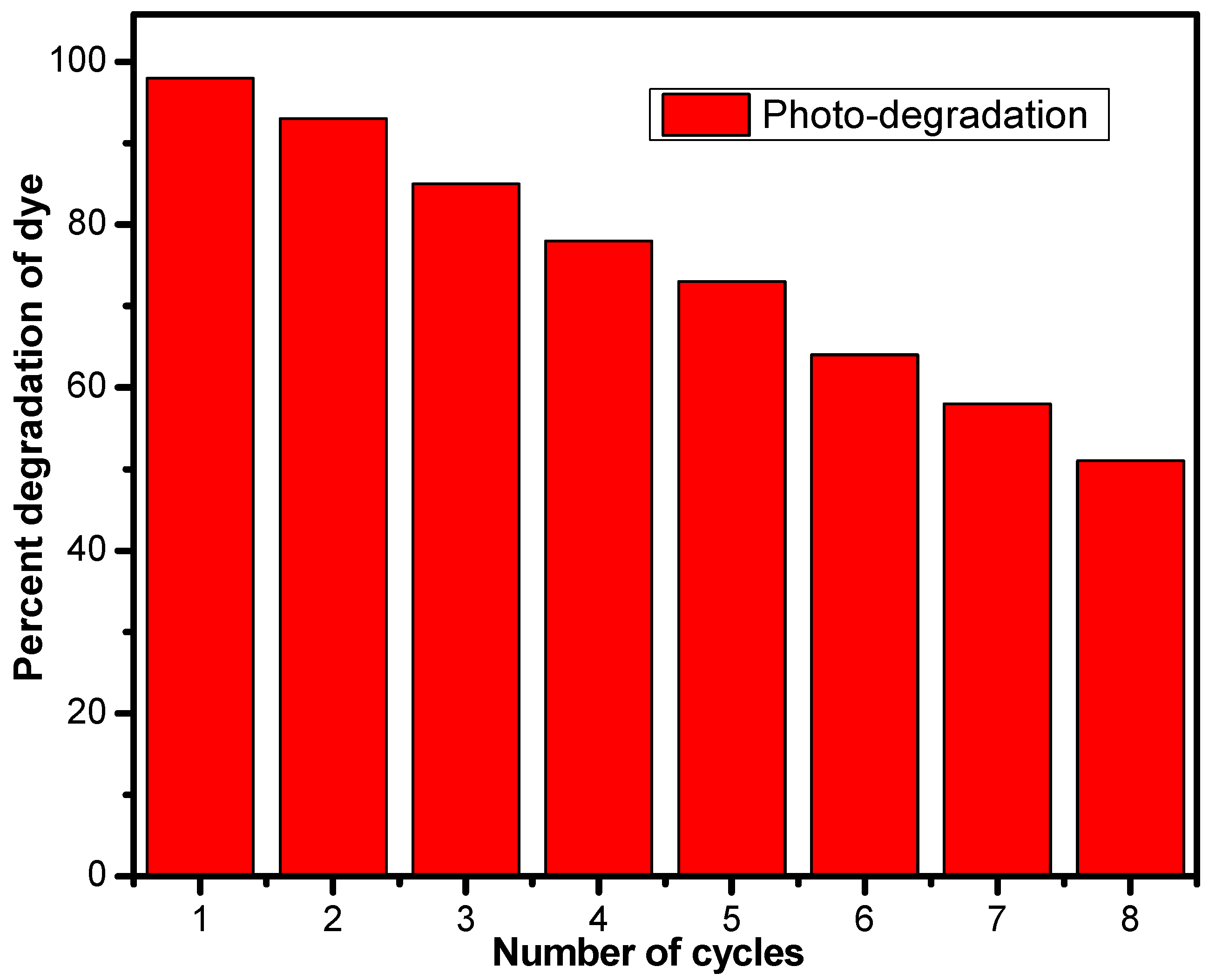
3. Results and Discussion
- Photo-catalyst + hυ → Photo-catalyst (e− + h+) → Photo-catalyst + H+ + OH• + O2•−
- Photo-catalyst (h+) + OH− → Photo-catalyst + OH•
- Photo-catalyst + O2 + e− → Photo-catalyst + O2•
- O2•− + H+ → HO2•
- 2HO2•− → H2O2 + O2
- H2O2 + e− → OH− + OH•
- OH• + HMBD → HMBD• + H2O
- HMBD + h+ → HMBD+• (degraded products, i.e., NH4+, H2O, CO2 etc.)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alshehri, S.M.; Ahmed, J.; Ahamad, T.; Alhokbany, N.; Arunachalam, P.; Al-Mayouf, A.M.; Ahmad, T. Synthesis, characterization, multifunctional electrochemical (OGR/ORR/SCs) and photodegradable activities of ZnWO4 nanobricks. J. Sol-Gel Sci. Technol. 2018, 87, 137–146. [Google Scholar] [CrossRef]
- AlShehri, S.M.; Ahmed, J.; Alzahrani, A.M.; Ahamad, T. Synthesis, characterization, and enhanced photocatalytic properties of NiWO4 nanobricks. New J. Chem. 2017, 41, 8178–8186. [Google Scholar] [CrossRef]
- Amouzegar, Z.; Naghizadeh, R.; Rezaie, H.R.; Ghahari, M.; Aminzare, M. Cubic ZnWO4 nano-photocatalysts synthesized by the microwave-assisted precipitation technique. Ceram. Int. 2015, 41, 1743–1747. [Google Scholar] [CrossRef]
- Barzgari, Z.; Askari, S.Z.; Ghazizadeh, A. Fabrication of nanostructured CuWO4 for photocatalytic degradation of organic pollutants in aqueous solution. J. Mater. Sci. Mater. Electron. 2017, 28, 3293–3298. [Google Scholar] [CrossRef]
- Ahmed, J.; Ahamad, T.; Ubaidullah, M.; Al-Enizi, A.M.; Alhabarah, A.N.; Alhokbany, N.; Alshehri, S.M. rGO supported NiWO4 nanocomposites for hydrogen evolution reactions. Mater. Lett. 2019, 240, 51–54. [Google Scholar] [CrossRef]
- Ahmed, J.; Alhokbany, N.; Ahamad, T.; AlShehri, S.M. Investigation of enhanced electro-catalytic HER/OER performances of copper tungsten oxide@reduced graphene oxide nanocomposites in alkaline and acidic media. New J. Chem. 2022. [Google Scholar] [CrossRef]
- Dhilip Kumar, R.; Andou, Y.; Sathish, M.; Karuppuchamy, S. Synthesis of nanostructured Cu-WO3 and CuWO4 for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2016, 27, 2926–2932. [Google Scholar] [CrossRef]
- Kumar, R.D.; Andou, Y.; Karuppuchamy, S. Synthesis and characterization of nanostructured Ni-WO3 and NiWO4 for supercapacitor applications. J. Alloys Compd. 2016, 654, 349–356. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, L.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Ag/ZnO Heterostructure Nanocrystals: Synthesis, Characterization, and Photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef]
- Yu, C.; Yu, J.C. Sonochemical fabrication, characterization and photocatalytic properties of Ag/ZnWO4 nanorod catalyst. Mater. Sci. Eng. B 2009, 164, 16–22. [Google Scholar] [CrossRef]
- Anandan, S.; Sivasankar, T.; Lana-Villarreal, T. Synthesis of TiO2/WO3 nanoparticles via sonochemical approach for the photocatalytic degradation of methylene blue under visible light illumination. Ultrason. Sonochem. 2014, 21, 1964–1968. [Google Scholar] [CrossRef]
- Fujii, A.; Meng, Z.; Yogi, C.; Hashishin, T.; Sanada, T.; Kojima, K. Preparation of Pt-loaded WO3 with different types of morphology and photocatalytic degradation of methylene blue. Surf. Coat. Technol. 2015, 271, 251–258. [Google Scholar] [CrossRef]
- Keereeta, Y.; Thongtem, S.; Thongtem, T. Enhanced photocatalytic degradation of methylene blue by WO3/ZnWO4 composites synthesized by a combination of microwave-solvothermal method and incipient wetness procedure. Powder Technol. 2015, 284, 85–94. [Google Scholar] [CrossRef]
- Sivaganesh, D.; Saravanakumar, S.; Sivakumar, V.; Rajajeyaganthan, R.; Arunpandian, M.; Nandha Gopal, J.; Thirumalaisamy, T.K. Surfactants-assisted synthesis of ZnWO4 nanostructures: A view on photocatalysis, photoluminescence and electron density distribution analysis. Mater. Charact. 2020, 159, 110035. [Google Scholar] [CrossRef]
- Fu, H.; Lin, J.; Zhang, L.; Zhu, Y. Photocatalytic activities of a novel ZnWO4 catalyst prepared by a hydrothermal process. Appl. Catal. A Gen. 2006, 306, 58–67. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, C.; Zhu, Y. ZnWO4 photocatalyst with high activity for degradation of organic contaminants. J. Alloys Compd. 2007, 432, 269–276. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Zhang, L.; Zhu, Y. Synthesis, characterization and photocatalytic properties of nanosized Bi2WO6, PbWO4 and ZnWO4 catalysts. Mater. Res. Bull. 2007, 42, 696–706. [Google Scholar] [CrossRef]
- Garadkar, K.M.; Ghule, L.A.; Sapnar, K.B.; Dhole, S.D. A facile synthesis of ZnWO4 nanoparticles by microwave assisted technique and its application in photocatalysis. Mater. Res. Bull. 2013, 48, 1105–1109. [Google Scholar] [CrossRef]
- Hosseinpour-Mashkani, S.M.; Maddahfar, M.; Sobhani-Nasab, A. Precipitation Synthesis, Characterization, Morphological Control, and Photocatalyst Application of ZnWO4 Nanoparticles. J. Electron. Mater. 2016, 45, 3612–3620. [Google Scholar] [CrossRef]
- Leonard, K.C.; Nam, K.M.; Lee, H.C.; Kang, S.H.; Park, H.S.; Bard, A.J. ZnWO4/WO3 Composite for Improving Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2013, 117, 15901–15910. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Q.; Li, J.-G.; Kim, B.-N. Elongation of ZnWO4 nanocrystals for enhanced photocatalysis and the effects of Ag decoration. Appl. Surf. Sci. 2020, 515, 146011. [Google Scholar] [CrossRef]
- Dumrongrojthanath, P.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Facile sonochemical synthesis and photocatalysis of Ag nanoparticle/ZnWO4-nanorod nanocomposites. Rare Met. 2019, 38, 601–608. [Google Scholar] [CrossRef]
- He, H.; Luo, Z.; Yu, C. Multifunctional ZnWO4 nanoparticles for photocatalytic removal of pollutants and disinfection of bacteria. J. Photochem. Photobiol. A Chem. 2020, 401, 112735. [Google Scholar] [CrossRef]
- He, H.; Luo, Z.; Tang, Z.-Y.; Yu, C. Controllable construction of ZnWO4 nanostructure with enhanced performance for photosensitized Cr(VI) reduction. Appl. Surf. Sci. 2019, 490, 460–468. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, B.; Chen, D.; Guo, Z.; Peng, Z. Simultaneous Reduction of Vanadium (V) and Chromium (VI) in Wastewater by Nanosized ZnWO4 Photocatalysis. J. Nanosci. Nanotechnol. 2016, 16, 2847–2852. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Wang, X.; Zhu, Q.; Li, J.-G.; Kim, B.-N. Crystallization and architecture engineering of ZnWO4 for enhanced photoluminescence. CrystEngComm 2020, 22, 6398–6406. [Google Scholar] [CrossRef]
- Brijesh, K.; Vinayraj, S.; Dhanush, P.C.; Bindu, K.; Nagaraja, H.S. ZnWO4/SnO2@r-GO nanocomposite as an anode material for high capacity lithium ion battery. Electrochim. Acta 2020, 354, 136676. [Google Scholar] [CrossRef]
- Brijesh, K.; Nagaraja, H.S. ZnWO4/r-GO nanocomposite as high capacity anode for lithium-ion battery. Ionics 2020, 26, 2813–2823. [Google Scholar] [CrossRef]
- Harichandran, G.; Divya, P.; Yesuraj, J.; Muthuraaman, B. Sonochemical synthesis of chain-like ZnWO4 nanoarchitectures for high performance supercapacitor electrode application. Mater. Charact. 2020, 167, 110490. [Google Scholar] [CrossRef]
- Vinayaraj, S.; Brijesh, K.; Dhanush, P.C.; Nagaraja, H.S. ZnWO4/SnO2 composite for supercapacitor applications. Physica B Condens. Matter. 2020, 596, 412369. [Google Scholar] [CrossRef]
- Yan, X.; Ohno, T.; Nishijima, K.; Abe, R.; Ohtani, B. Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 2006, 429, 606–610. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z—What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Ahmed, J.; Ahamad, T.; Alhokbany, N.; Almaswari, B.M.; Ahmad, T.; Hussain, A.; Al-Farraj, E.S.S.; Alshehri, S.M. Molten Salts Derived Copper Tungstate Nanoparticles as Bifunctional Electro-Catalysts for Electrolysis of Water and Supercapacitor Applications. ChemElectroChem 2018, 5, 3938–3945. [Google Scholar] [CrossRef]
- Ahmed, J.; Poltavets, V.V.; Prakash, J.; Alshehri, S.M.; Ahamad, T. Sol–gel synthesis, structural characterization and bifunctional catalytic activity of nanocrystalline delafossite CuGaO2 particles. J. Alloys Compd. 2016, 688, 1157–1161. [Google Scholar] [CrossRef]
- Ahmed, J.; Ubiadullah, M.; Alhokbany, N.; Alshehri, S.M. Synthesis of ultrafine NiMoO4 nano-rods for excellent electro-catalytic performance in hydrogen evolution reactions. Mater. Lett. 2019, 257, 126696. [Google Scholar] [CrossRef]
- AlShehri, S.M.; Ahmed, J.; Ahamad, T.; Arunachalam, P.; Ahmad, T.; Khan, A. Bifunctional electro-catalytic performances of CoWO4 nanocubes for water redox reactions (OER/ORR). RSC Adv. 2017, 7, 45615–45623. [Google Scholar] [CrossRef]
- Ahmed, J.; Alam, M.; Majeed Khan, M.A.; Alshehri, S.M. Bifunctional electro-catalytic performances of NiMoO4-NRs@RGO nanocomposites for oxygen evolution and oxygen reduction reactions. J. King Saud Univ. Sci. 2021, 33, 101317. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Ahmed, J.; Alhabarah, A.N.; Ahamad, T.; Ahmad, T. Nitrogen-Doped Cobalt Ferrite/Carbon Nanocomposites for Supercapacitor Applications. ChemElectroChem 2017, 4, 2952–2958. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.H.; Lin, S.; Liang, C.; Wang, H.; Ye, C.X.; Wang, Y.J.; Zhang, R.; Fan, D.Y.; Yang, H.J.; et al. A facile route to reduced graphene oxide–zinc oxide nanorod composites with enhanced photocatalytic activity. Powder Technol. 2014, 257, 113–119. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, Y.; Deng, Y.; Xiang, Y.; Zhang, Y.; Zhao, Z.; Zeng, D. A Novel and Reusable RGO/ZnO with Nanosheets/Microparticle Composite Photocatalysts for Efficient Pollutants Degradation. ChemistrySelect 2018, 3, 8740–8747. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Suvorova, A.; Wang, S. One-pot hydrothermal synthesis of ZnO-reduced graphene oxide composites using Zn powders for enhanced photocatalysis. Chem. Eng. J. 2013, 229, 533–539. [Google Scholar] [CrossRef]
- Ahmed, J.; Ubiadullah, M.; Khan, M.A.M.; Alhokbany, N.; Alshehri, S.M. Significant recycled efficiency of multifunctional nickel molybdenum oxide nanorods in photo-catalysis, electrochemical glucose sensing and asymmetric supercapacitors. Mater. Charact. 2021, 171, 110741. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Zhang, K.; Li, X.; Leng, Q.; Hu, C. Photocatalytic Activity of ZnWO4: Band Structure, Morphology and Surface Modification. ACS Appl. Mater. Interfaces 2014, 6, 14423–14432. [Google Scholar] [CrossRef] [PubMed]
- Montini, T.; Gombac, V.; Hameed, A.; Felisari, L.; Adami, G.; Fornasiero, P. Synthesis, characterization and photocatalytic performance of transition metal tungstates. Chem. Phys. Lett. 2010, 498, 113–119. [Google Scholar] [CrossRef]
- Ahmed, B.; Kumar, S.; Ojha, A.K.; Donfack, P.; Materny, A. Facile and controlled synthesis of aligned WO3 nanorods and nanosheets as an efficient photocatalyst material. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 250–261. [Google Scholar] [CrossRef]
- Molla, A.; Sahu, M.; Hussain, S. Under dark and visible light: Fast degradation of methylene blue in the presence of Ag-In-Ni-S nanocomposites. J. Mater. Chem. A 2015, 3, 15616–15625. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhokbany, N.; Alshehri, S.M.; Ahmed, J. Synthesis, Characterization and Enhanced Visible Light Photocatalytic Performance of ZnWO4-NPs@rGO Nanocomposites. Catalysts 2021, 11, 1536. https://doi.org/10.3390/catal11121536
Alhokbany N, Alshehri SM, Ahmed J. Synthesis, Characterization and Enhanced Visible Light Photocatalytic Performance of ZnWO4-NPs@rGO Nanocomposites. Catalysts. 2021; 11(12):1536. https://doi.org/10.3390/catal11121536
Chicago/Turabian StyleAlhokbany, Norah, Saad M. Alshehri, and Jahangeer Ahmed. 2021. "Synthesis, Characterization and Enhanced Visible Light Photocatalytic Performance of ZnWO4-NPs@rGO Nanocomposites" Catalysts 11, no. 12: 1536. https://doi.org/10.3390/catal11121536
APA StyleAlhokbany, N., Alshehri, S. M., & Ahmed, J. (2021). Synthesis, Characterization and Enhanced Visible Light Photocatalytic Performance of ZnWO4-NPs@rGO Nanocomposites. Catalysts, 11(12), 1536. https://doi.org/10.3390/catal11121536






