Hollow TiO2 Microsphere/Graphene Composite Photocatalyst for CO2 Photoreduction
Abstract
:1. Introduction
2. Results and Discussion
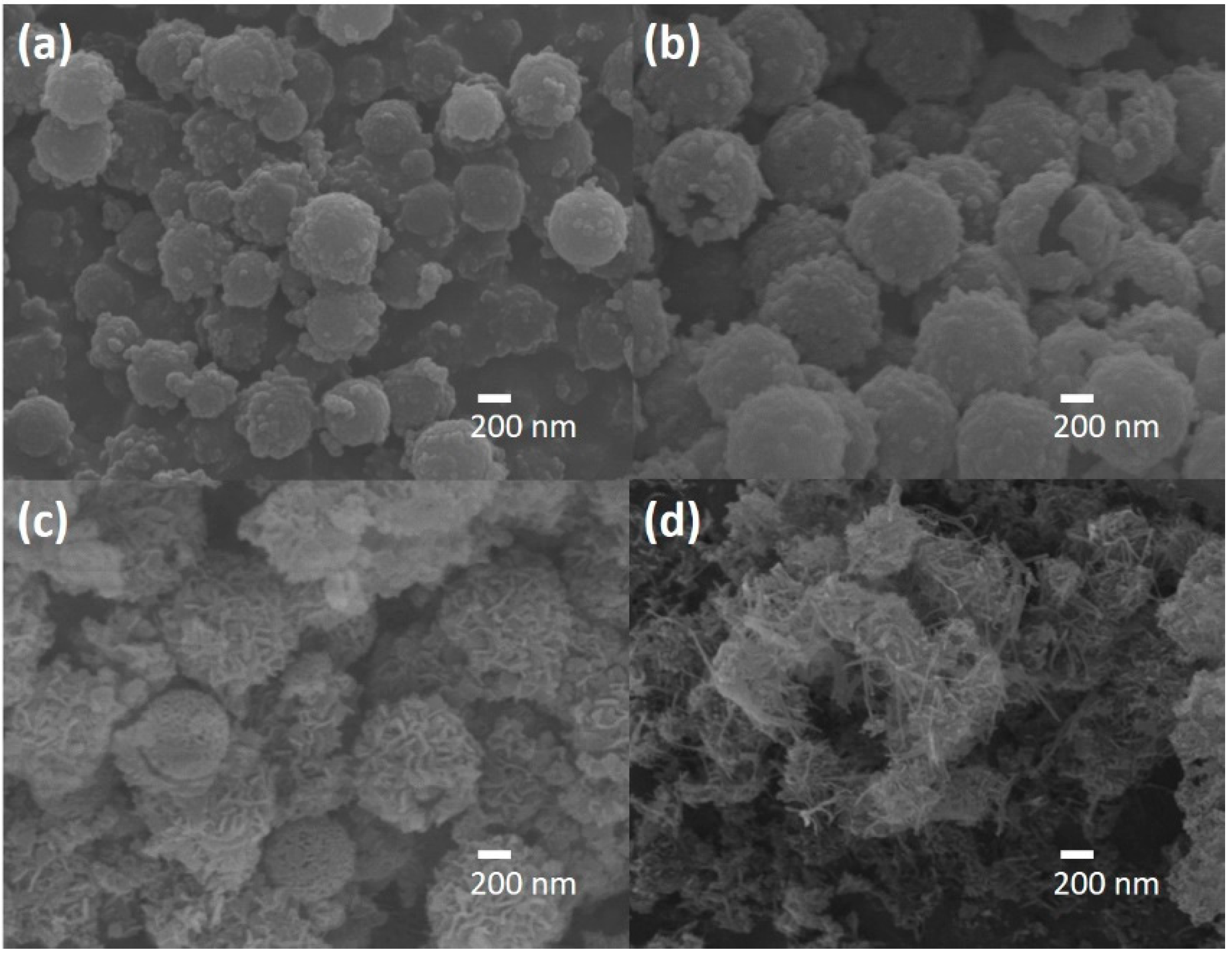
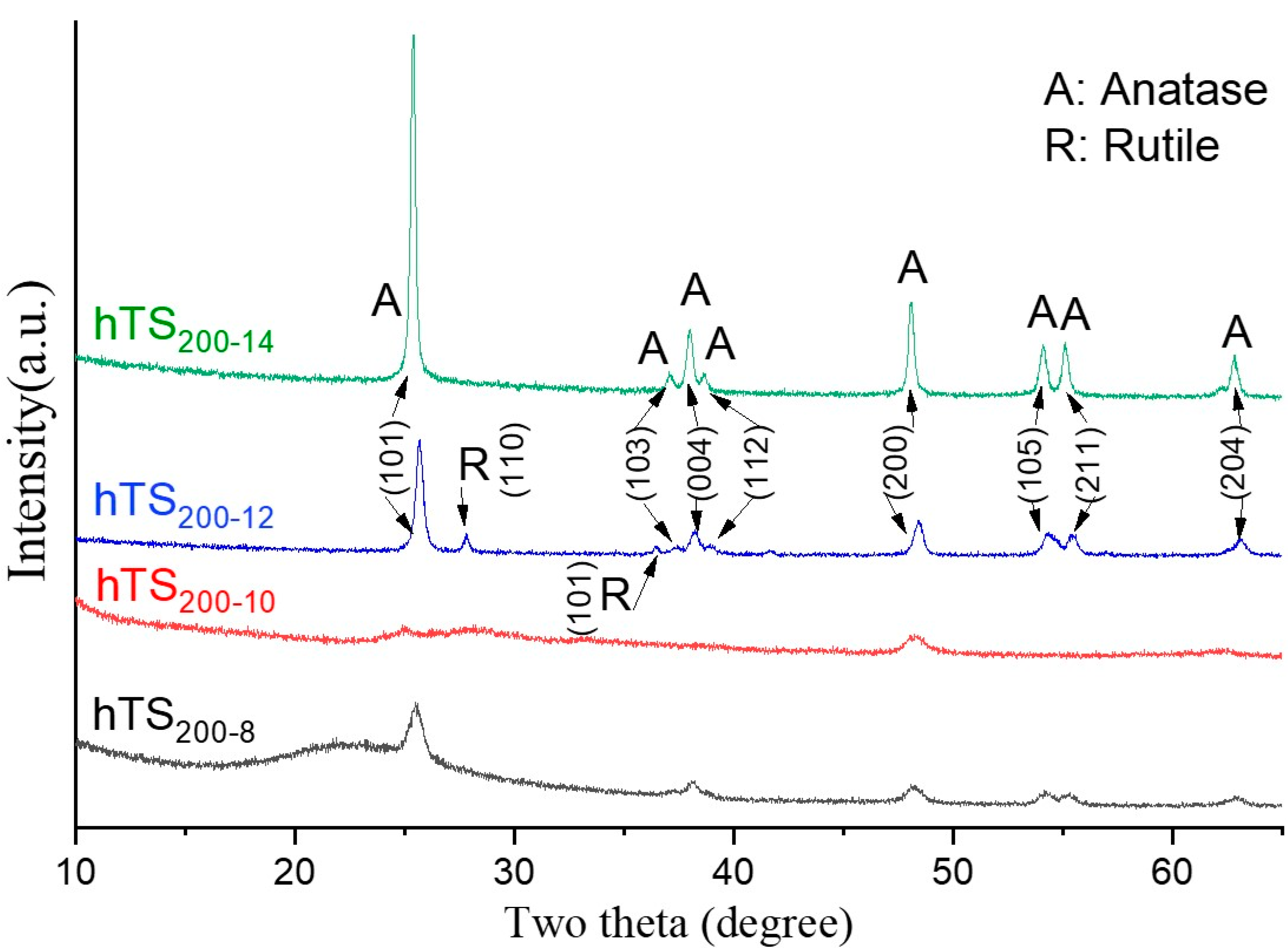
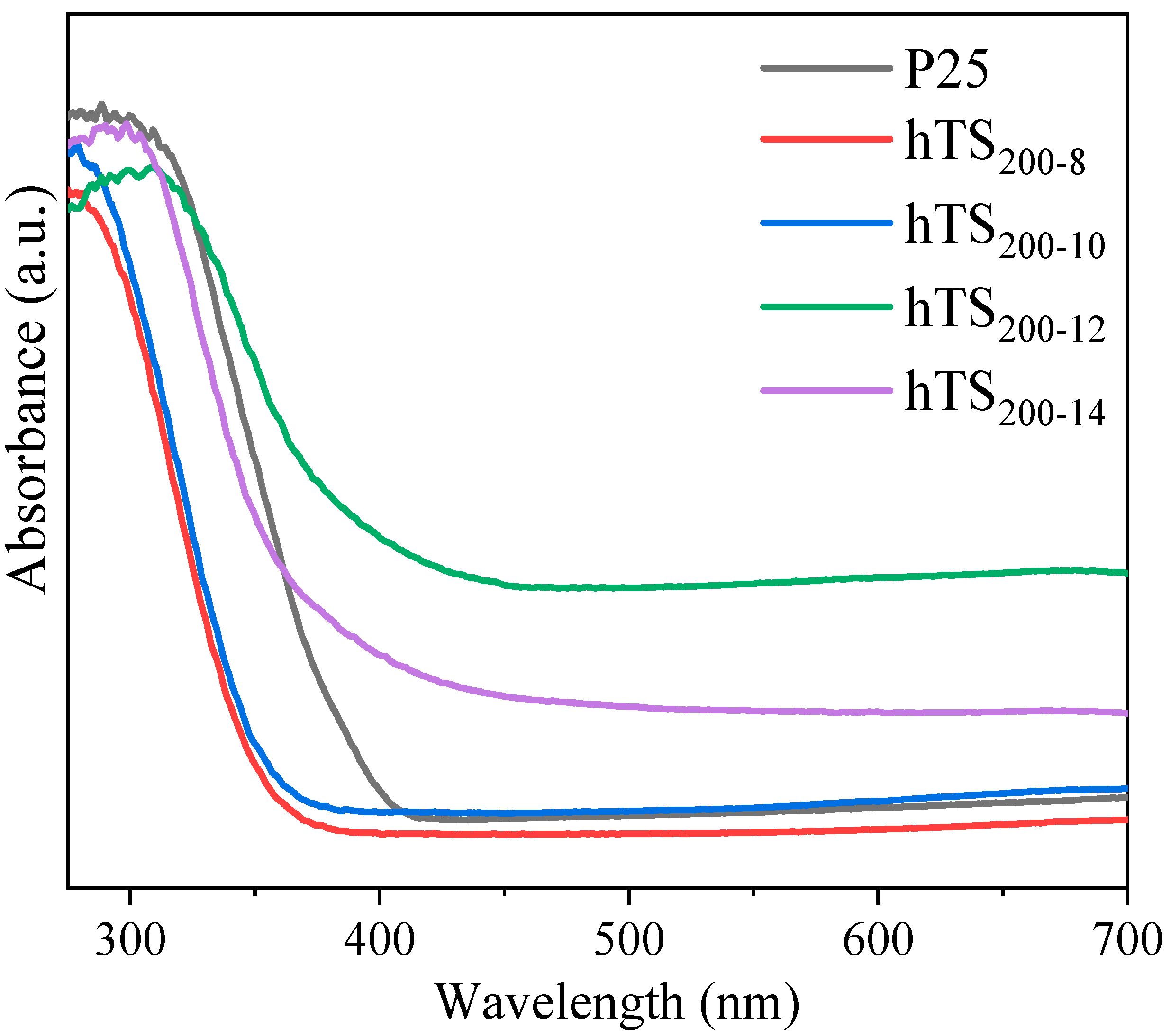
2.1. Structure and Crystalline Properties
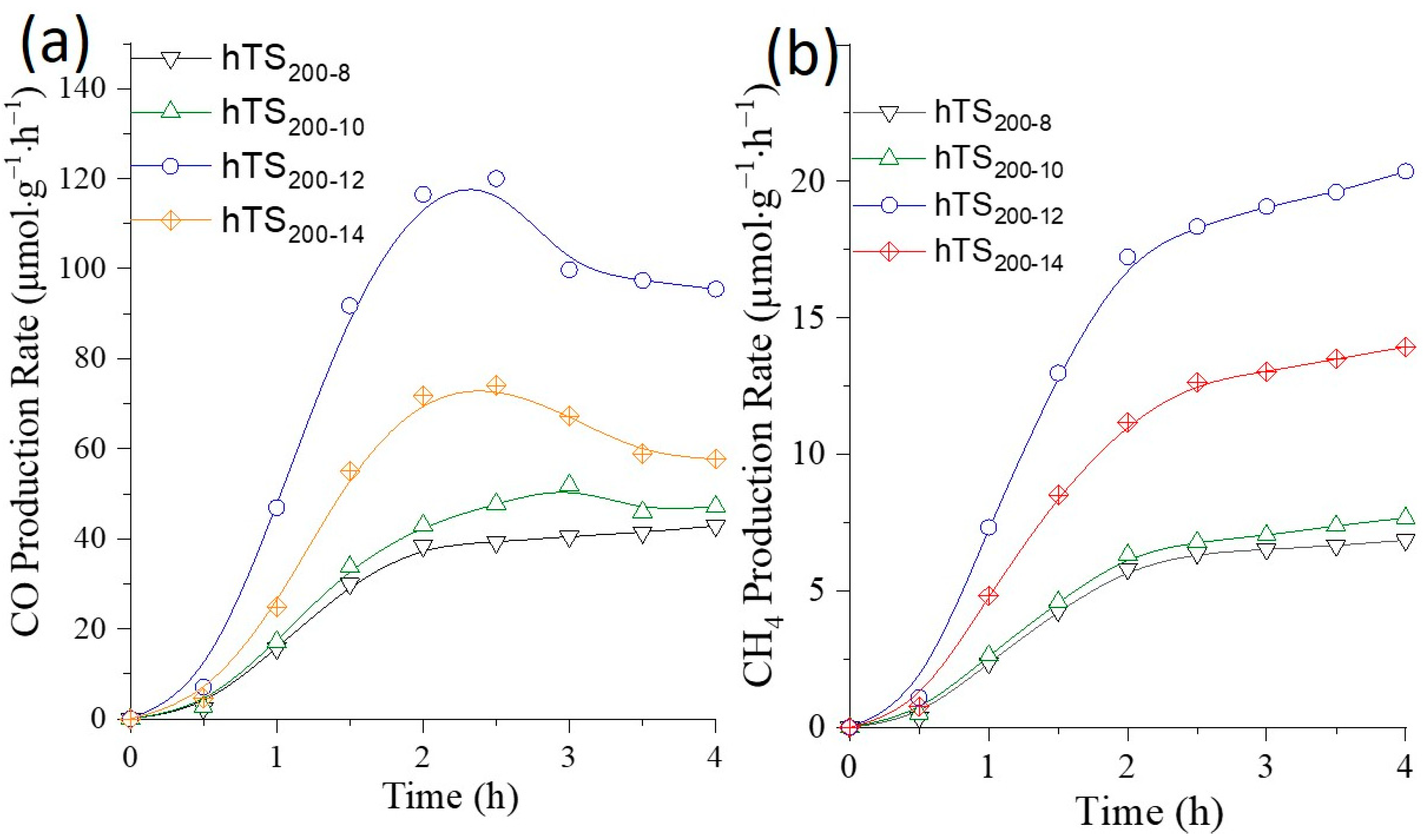
2.2. Photocatalytic CO and CH4 Production
2.2.1. Effect of the Processing pH of the Catalyst on Its Photocatalytic Performance
2.2.2. Effect of the Catalyst Cavity Size on Its Photocatalytic Performance
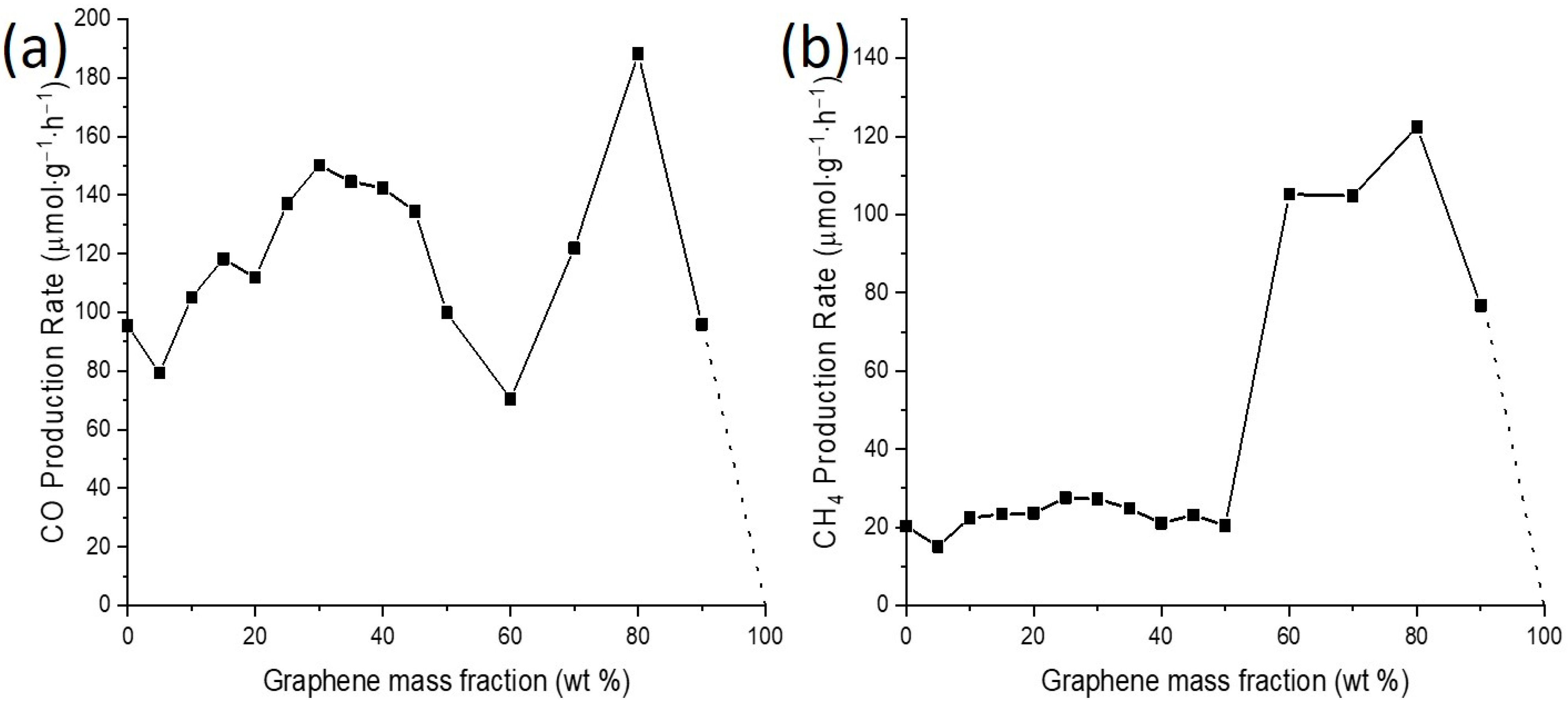
2.3. Effect of the hTS200-12/Graphene Ratio on Its Photocatalytic Performance
3. Materials and Methods
3.1. Preparation of Silica Microspheres (SSx)
3.2. Preparation of TiO2/SiO2 Microspheres (TSx)
3.3. Preparation of Hollow TiO2 Microspheres (hTSx-y)
3.4. Preparation of the Hollow Titanium Dioxide Microsphere/Graphene Composite Photocatalysts on Quartz Wool (hTS200-12Gz)
3.5. Material Characterization
3.6. Photocatalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lo, A.-Y.; Chang, C.-C.; Lai, Y.-W.; Chen, P.-R.; Xu, B.-C. Improving the Supercapacitor Performance by Dispersing SiO2 Microspheres in Electrodes. ACS Omega 2020, 5, 11522–11528. [Google Scholar] [CrossRef]
- Lo, A.-Y.; Saravanan, L.; Tseng, C.-M.; Wang, F.-K.; Huang, J.-T. Effect of Composition Ratios on the Performance of Graphene/Carbon Nanotube/Manganese Oxide Composites toward Supercapacitor Applications. ACS Omega 2020, 5, 578–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, A.-Y.; Huang, C.-Y.; Sung, L.-Y.; Louh, R.-F. Low Humidifying Proton Exchange Membrane Fuel Cells with Enhanced Power and Pt–C–h-SiO2 Anodes Prepared by Electrophoretic Deposition. ACS Sustain. Chem. Eng. 2016, 4, 1303–1310. [Google Scholar] [CrossRef]
- Ran, J.; Jaroniec, M.; Qiao, S.-Z. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: Achievements, challenges, and opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.; Huang, W.; Taghipour, F. Recent progress and perspectives in the photocatalytic CO2 reduction of Ti-oxide-based nanomaterials. Appl. Surf. Sci. 2017, 396, 1696–1711. [Google Scholar] [CrossRef]
- Sun, W.; Meng, X.; Xu, C.; Yang, J.; Liang, X.; Dong, Y.; Dong, C.; Ding, Y. Amorphous CoOx coupled carbon dots as a spongy porous bifunctional catalyst for efficient photocatalytic water oxidation and CO2 reduction. Chin. J. Catal. 2020, 41, 1826–1836. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, B.; Cheng, B.; Zhang, J.; Zhang, L.; Yu, J. In-situ preparation of TiO2/N-doped graphene hollow sphere photocatalyst with enhanced photocatalytic CO2 reduction performance. Chin. J. Catal. 2021, 42, 1648–1658. [Google Scholar] [CrossRef]
- Thompson, W.A.; Sanchez Fernandez, E.; Maroto-Valer, M.M. Review and analysis of CO2 photoreduction kinetics. ACS Sustain. Chem. Eng. 2020, 8, 4677–4692. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Niu, Y.; He, X.; Qi, J.; Li, X. Photocatalytic Reduction of CO2 Using TiO2-Graphene Nanocomposites. J. Nanomater. 2016, 2016, 6012896. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Lo, A.-Y.; Taghipour, F. Review and prospects of microporous zeolite catalysts for CO2 photoreduction. Appl. Mater. Today 2021, 23, 101042. [Google Scholar] [CrossRef]
- Mikrut, P.; Kobielusz, M.; Macyk, W. Spectroelectrochemical characterization of euhedral anatase TiO2 crystals—Implications for photoelectrochemical and photocatalytic properties of {001} {100} and {101} facets. Electrochim. Acta 2019, 310, 256–265. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Mamiyev, Z.; Dillert, R.; Zheng, N.; Bahnemann, D.W. Rh/TiO2-Photocatalyzed Acceptorless Dehydrogenation of N-Heterocycles upon Visible-Light Illumination. ACS Catal. 2020, 10, 5542–5553. [Google Scholar] [CrossRef]
- Lo, A.-Y.; Taghipour, F. Ordered Mesoporous Photocatalysts for CO2 Photoreduction. J. Mater. Chem. A 2021, 9, 26430–26453. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Gao, M.; Wang, X. B–N co-doped black TiO2 synthesized via magnesiothermic reduction for enhanced photocatalytic hydrogen production. Int. J. Hydrogen Energy 2019, 44, 28629–28637. [Google Scholar] [CrossRef]
- Barkhade, T.; Banerjee, I. Optical Properties of Fe doped TiO2 Nanocomposites Synthesized by Sol-Gel Technique. Mater. Today: Proc. 2019, 18, 1204–1209. [Google Scholar] [CrossRef]
- Wu, T.; Zhen, C.; Wu, J.; Jia, C.; Haider, M.; Wang, L.; Liu, G.; Cheng, H.-M. Chlorine capped SnO2 quantum-dots modified TiO2 electron selective layer to enhance the performance of planar perovskite solar cells. Sci. Bull. 2019, 64, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.H.; Tran, M.L.; Tran, T.T.V.; Juang, R.-S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar] [CrossRef]
- Diaz-Angulo, J.; Arce-Sarria, A.; Mueses, M.; Hernandez-Ramirez, A.; Machuca-Martinez, F. Analysis of two dye-sensitized methods for improving the sunlight absorption of TiO2 using CPC photoreactor at pilot scale. Mater. Sci. Semicond. Process. 2019, 103, 104640. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Goh, B.T.; Mohamed, A.R. Visible-light-active oxygen-rich TiO2 decorated 2D graphene oxide with enhanced photocatalytic activity toward carbon dioxide reduction. Appl. Catal. B 2015, 179, 160–170. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, F.; Li, Y. A review on the effects of TiO2 surface point defects on CO2 photoreduction with H2O. J. Mater. 2017, 3, 17–32. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; Garcia, H. Photocatalytic CO2 Reduction using Non-Titanium Metal Oxides and Sulfides. ChemSusChem 2013, 6, 562–577. [Google Scholar] [CrossRef]
- Li, K.; An, X.; Park, K.H.; Khraisheh, M.; Tang, J. A critical review of CO2 photoconversion: Catalysts and reactors. Catal. Today 2014, 224, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xuan, X.; Wang, Y.; Li, X.; Huang, H.; Zhang, X.; Du, X. Nano-Au-modified TiO2 grown on dendritic porous silica particles for enhanced CO2 photoreduction. Microporous Mesoporous Mater. 2021, 310, 110635. [Google Scholar] [CrossRef]
- Wang, T.; Meng, X.; Liu, G.; Chang, K.; Li, P.; Kang, Q.; Liu, L.; Li, M.; Ouyang, S.; Ye, J. In situ synthesis of ordered mesoporous Co-doped TiO2 and its enhanced photocatalytic activity and selectivity for the reduction of CO2. J. Mater. Chem. A 2015, 3, 9491–9501. [Google Scholar] [CrossRef]
- Xue, H.; Wang, T.; Gong, H.; Guo, H.; Fan, X.; Gao, B.; Feng, Y.; Meng, X.; Huang, X.; He, J. Constructing Ordered Three-Dimensional TiO2 Channels for Enhanced Visible-Light Photocatalytic Performance in CO2 Conversion Induced by Au Nanoparticles. Chem. Asian J. 2018, 13, 577–583. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Photocatalytic CO2 conversion over Au/TiO2 nanostructures for dynamic production of clean fuels in a monolith photoreactor. Clean Technol. Environ. Policy 2016, 18, 2147–2160. [Google Scholar] [CrossRef]
- Štengl, V.; Popelková, D.; Vláčil, P. TiO2–Graphene Nanocomposite as High Performace Photocatalysts. J. Phys. Chem. C 2011, 115, 25209–25218. [Google Scholar] [CrossRef]
- Lee, J.S.; You, K.H.; Park, C.B. Highly Photoactive, Low Bandgap TiO2 Nanoparticles Wrapped by Graphene. Adv. Mater. 2012, 24, 1084–1088. [Google Scholar] [CrossRef]
- Akhavan, O.; Abdolahad, M.; Esfandiar, A.; Mohatashamifar, M. Photodegradation of Graphene Oxide Sheets by TiO2 Nanoparticles after a Photocatalytic Reduction. J. Phys. Chem. C 2010, 114, 12955–12959. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Jiang, X.; Tang, X.; Tang, L.; Zhang, B.; Mao, H. Synthesis and formation mechanism of amorphous silica particles via sol–gel process with tetraethylorthosilicate. Ceram. Int. 2019, 45, 7673–7680. [Google Scholar] [CrossRef]
- Leshuk, T.; Linley, S.; Baxter, G.; Gu, F. Mesoporous Hollow Sphere Titanium Dioxide Photocatalysts through Hydrothermal Silica Etching. ACS Appl. Mater. Interfaces 2012, 4, 6062–6070. [Google Scholar] [CrossRef]



| Designation | Parameter | ||
|---|---|---|---|
| SiO2 Microsphere Diameter (x; nm) | Hydrothermal pH Value (y) | Graphene Mass Fraction (z; wt%) | |
| P25 * | -- | -- | -- |
| hTSx-12G0 * | 100, 200, 300, 400 | 12 | 0 |
| hTS200-yG0 * | 200 | 8, 10, 12, 14 | 0 |
| hTS200-12Gz * | 200 | 12 | 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 60, 70, 80, 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.-C.; Xie, P.-J.; Lai, Y.-W.; Lo, A.-Y. Hollow TiO2 Microsphere/Graphene Composite Photocatalyst for CO2 Photoreduction. Catalysts 2021, 11, 1532. https://doi.org/10.3390/catal11121532
Chung Y-C, Xie P-J, Lai Y-W, Lo A-Y. Hollow TiO2 Microsphere/Graphene Composite Photocatalyst for CO2 Photoreduction. Catalysts. 2021; 11(12):1532. https://doi.org/10.3390/catal11121532
Chicago/Turabian StyleChung, Yi-Chen, Pei-Jie Xie, Yi-Wei Lai, and An-Ya Lo. 2021. "Hollow TiO2 Microsphere/Graphene Composite Photocatalyst for CO2 Photoreduction" Catalysts 11, no. 12: 1532. https://doi.org/10.3390/catal11121532
APA StyleChung, Y.-C., Xie, P.-J., Lai, Y.-W., & Lo, A.-Y. (2021). Hollow TiO2 Microsphere/Graphene Composite Photocatalyst for CO2 Photoreduction. Catalysts, 11(12), 1532. https://doi.org/10.3390/catal11121532







