Nanostructured Fe-N-C as Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrochemical Characterization
2.1.1. Catalytic Activity towards ORR
2.1.2. Catalytic Activity towards HER
3. Materials and Methods
3.1. Materials
Carbon Support and Catalyst Preparation
3.2. Methods
3.2.1. Electrochemical Characterization
3.2.2. Cyclic Voltammetry (CV)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mecheri, B.; Gokhale, R.R.; Santoro, C.; De Oliveira, M.A.C.; D’Epifanio, A.; Licoccia, S.; Serov, A.; Artyushkova, K.; Atanassov, P. Oxygen Reduction Reaction Electrocatalysts Derived from Iron Salt and Benzimidazole and Aminobenzimidazole Precursors and Their Application in Microbial Fuel Cell Cathodes. ACS Appl. Energy Mater. 2018, 1, 5755–5765. [Google Scholar] [CrossRef]
- De Oliveira, M.A.C.; Mecheri, B.; D’Epifanio, A.; Zurlo, F.; Licoccia, S. Optimization of PGM-free cathodes for oxygen reduction in microbial fuel cells. Electrochim. Acta 2020, 334, 135650. [Google Scholar] [CrossRef]
- Santoro, C.; Gokhale, R.; Mecheri, B.; D’Epifanio, A.; Licoccia, S.; Serov, A.; Artyushkova, K.; Atanassov, P. Design of Iron(II) Phthalocyanine-Derived Oxygen Reduction Electrocatalysts for High-Power-Density Microbial Fuel Cells. ChemSusChem 2017, 10, 3243–3251. [Google Scholar] [CrossRef] [Green Version]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.-L.; Zhao, J.; Gu, L.; Peng, Z.-J.; Bao, Z.-L.; Sun, X.-L.; Guan, M.-Y. Highly active Co–N-doped graphene as an efficient bifunctional electrocatalyst (ORR/HER) for flexible all-solid-state zinc–air batteries. Sustain. Energy Fuels 2020, 4, 6165–6173. [Google Scholar] [CrossRef]
- Ding, J.; Ji, S.; Wang, H.; Pollet, B.G.; Wang, R. Mesoporous CoS/N-doped Carbon as HER and ORR Bifunctional Electrocatalyst for Water Electrolyzers and Zinc-Air Batteries. ChemCatChem 2018, 11, 1026–1032. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-Noble Metal-based Carbon Composites in Hydrogen Evolution Reaction: Fundamentals to Applications. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Sgarbi, R.; Kumar, K.; Jaouen, F.; Zitolo, A.; Ticianelli, E.A.; Maillard, F. Oxygen reduction reaction mechanism and kinetics on M-NxCy and M@N-C active sites present in model M-N-C catalysts under alkaline and acidic conditions. J. Solid State Electrochem. 2019, 25, 45–56. [Google Scholar] [CrossRef]
- Yan, X.; Li, X.; Fu, C.; Lin, C.; Hu, H.; Shen, S.; Wei, G.; Zhang, J. Large specific surface area S-doped Fe–N–C electrocatalysts derived from Metal–Organic frameworks for oxygen reduction reaction. Prog. Nat. Sci. 2020, 30, 896–904. [Google Scholar] [CrossRef]
- Jin, L.; Zhu, B.; Wang, X.; Zhang, L.; Song, D.; Guo, J.; Tao, H. Facile Synthesis of the Amorphous Carbon Coated Fe-N-C Nanocatalyst with Efficient Activity for Oxygen Reduction Reaction in Acidic and Alkaline Media. Materials 2020, 13, 4551. [Google Scholar] [CrossRef]
- Ketpang, K.; Boonkitkoson, A.; Pitipuech, N.; Poompipatpong, C.; Sanetuntikul, J.; Shanmugam, S. Highly Active and Durable Transition Metal-Coordinated Nitrogen Doped Carbon Electrocatalyst for Oxygen Reduction Reaction in Neutral Media. E3S Web Conf. 2020, 141, 1005. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J.; Yang, J.; Xiao, K.; Liang, S.; Xu, J.; Liu, B.; Hou, H. Fe and N co-doped carbon derived from melamine resin capsuled biomass as efficient oxygen reduction catalyst for air-cathode microbial fuel cells. Int. J. Hydrogen Energy 2019, 45, 3163–3175. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Yue, X.; Huang, S. Hydrogen evolution reaction in full pH range on nickel doped tungsten carbide nanocubes as efficient and durable non-precious metal electrocatalysts. Int. J. Hydrogen Energy 2020, 45, 8695–8702. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Artyushkova, K.; Atanassov, P. Platinum group metal-free oxygen reduction electrocatalysts used in neutral electrolytes for bioelectrochemical reactor applications. Curr. Opin. Electrochem. 2020, 23, 106–113. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent Progresses in Oxygen Reduction Reaction Electrocatalysts for Electrochemical Energy Applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.H.; Kwon, H.C.; Yook, S.; Shin, H.; Kim, H.; Choi, M. Hydrogen Peroxide Synthesis via Enhanced Two-Electron Oxygen Reduction Pathway on Carbon-Coated Pt Surface. J. Phys. Chem. C 2014, 118, 30063–30070. [Google Scholar] [CrossRef]
- Alsudairi, A.; Li, J.; Ramaswamy, N.; Mukerjee, S.; Abraham, K.M.; Jia, Q. Resolving the Iron Phthalocyanine Redox Transitions for ORR Catalysis in Aqueous Media. J. Phys. Chem. Lett. 2017, 8, 2881–2886. [Google Scholar] [CrossRef]
- Harnisch, F.; Savastenko, N.A.; Zhao, F.; Steffen, H.; Brüser, V.; Schröder, U. Comparative study on the performance of pyrolyzed and plasma-treated iron(II) phthalocyanine-based catalysts for oxygen reduction in pH neutral electrolyte solutions. J. Power Sources 2009, 193, 86–92. [Google Scholar] [CrossRef]
- Iannaci, A.; Mecheri, B.; D’Epifanio, A.; Elorri, M.J.L.; Licoccia, S. Iron–nitrogen-functionalized carbon as efficient oxygen reduction reaction electrocatalyst in microbial fuel cells. Int. J. Hydrogen Energy 2016, 41, 19637–19644. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Mecheri, B.; Iannaci, A.; D’Epifanio, A.; Licoccia, S. Iron/Polyindole-based Electrocatalysts to Enhance Oxygen Reduction in Microbial Fuel Cells. Electrochim. Acta 2016, 190, 388–395. [Google Scholar] [CrossRef]
- De Oliveira, M.A.C.; Mecheri, B.; D’Epifanio, A.; Placidi, E.; Arciprete, F.; Valentini, F.; Perandini, A.; Valentini, V.; Licoccia, S. Graphene oxide nanoplatforms to enhance catalytic performance of iron phthalocyanine for oxygen reduction reaction in bioelectrochemical systems. J. Power Sources 2017, 356, 381–388. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Fundamental Mechanistic Understanding of Electrocatalysis of Oxygen Reduction on Pt and Non-Pt Surfaces: Acid versus Alkaline Media. Adv. Phys. Chem. 2012, 2012, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Osmieri, L. Transition Metal–Nitrogen–Carbon (M–N–C) Catalysts for Oxygen Reduction Reaction. Insights on Synthesis and Performance in Polymer Electrolyte Fuel Cells. ChemEngineering 2019, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.; Wang, D.-Y.; Chen, C.-C.; Hwang, B.-J.; Dai, H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2015, 9, 28–46. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2017, 5, 1700464. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, M.; Long, A.; Xue, Y.; Liao, H.; Wei, C.; Xu, Z.J. Heterostructured Electrocatalysts for Hydrogen Evolution Reaction Under Alkaline Conditions. Nano-Micro Lett. 2018, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Fan, X.; Kong, F.; Kong, A.; Chen, A.; Zhou, Z.; Shan, Y. Covalent Porphyrin Framework-Derived Fe2P@Fe4N-Coupled Nanoparticles Embedded in N-Doped Carbons as Efficient Trifunctional Electrocatalysts. ACS Appl. Mater. Interfaces 2017, 9, 32840–32850. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.C.; Gomes, V.G.; Dinh, K.N. Ni- and P-doped carbon from waste biomass: A sustainable multifunctional electrode for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Electrochim. Acta 2019, 314, 49–60. [Google Scholar] [CrossRef]
- Ying, L.; Sun, S.; Liu, W.; Zhu, H.; Zhu, Z.; Liu, A.; Yang, L.; Lu, S.; Duan, F.; Yang, C.; et al. Heterointerface engineering in bimetal alloy/metal carbide for superior hydrogen evolution reaction. Renew. Energy 2020, 161, 1036–1045. [Google Scholar] [CrossRef]
- Kamali, S.; Zhiani, M.; Tavakol, H. Synergism effect of first row transition metals in experimental and theoretical activity of NiM/rGO alloys at hydrogen evolution reaction in alkaline electrolyzer. Renew. Energy 2020, 154, 1122–1131. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, F.; He, X.; Chen, B.; Li, G. Enhancing hydrogen evolution reaction activity on cobalt oxide in alkaline electrolyte by doping inactive rare-earth metal. Electrochim. Acta 2020, 363, 137230. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Xiao, W.; Yan, C. Electrodeposition of graded Ni-S film for hydrogen evolution reaction. Mater. Lett. 2017, 193, 77–80. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Kong, D.; Sun, J.; Hymel, T.M.; Cui, Y. Electrochemical Tuning of MoS 2 Nanoparticles on Three-Dimensional Substrate for Efficient Hydrogen. ACS Nano 2014, 8, 4940–4947. [Google Scholar] [CrossRef]
- Paul, S.C.; Dey, S.C.; Molla, A.I.; Islam, S.; Debnath, S.; Miah, M.Y.; Ashaduzzaman, M.; Sarker, M. Nanomaterials as electrocatalyst for hydrogen and oxygen evolution reaction: Exploitation of challenges and current progressions. Polyhedron 2020, 193, 114871. [Google Scholar] [CrossRef]
- Yuan, Q.; Yu, Y.; Sherrell, P.C.; Chen, J.; Bi, X. Fe/Co-based Bimetallic MOF-derived Co 3 Fe 7 @NCNTFs Bifunctional Electrocatalyst for High-Efficiency Overall Water Splitting. Chem. Asian J. 2020, 15, 1728–1735. [Google Scholar] [CrossRef]
- Fang, W.; Wang, J.; Hu, Y.; Cui, X.; Zhu, R.; Zhang, Y.; Yue, C.; Dang, J.; Cui, W.; Zhao, H.; et al. Metal-organic framework derived Fe-Co-CN/reduced graphene oxide for efficient HER and OER. Electrochim. Acta 2020, 365, 137384. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Su, D.; Wei, Z.; Pang, Z.; Wang, Y. In situ Cobalt–Cobalt Oxide/N-Doped Carbon Hybrids as Superior Bifunctional Electrocatalysts for Hydrogen and Oxygen Evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Pi, C.; Huang, C.; Yanga, Y.; Songa, H.; Zhanga, X.; Zheng, Y.; Gaoab, B.; Fua, J.; Chu, P.K.; Huoa, K. In situ formation of N-doped carbon-coated porous MoP nanowires: A highly efficient electrocatalyst for hydrogen evolution reaction in a wide pH range. Appl. Catal. B Environ. 2019, 263, 118358. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Z.-Y.; Lai, Y.-J.; You, Y.; Liu, J.-G.; Wu, X.-L.; Terefe, E.; Chen, C.; Song, L.; Rauf, M.; et al. Phenylenediamine-Based FeNx/C Catalyst with High Activity for Oxygen Reduction in Acid Medium and Its Active-Site Probing. J. Am. Chem. Soc. 2014, 136, 10882–10885. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Li, J.; Jaouen, F. Structure and activity of metal-centered coordination sites in pyrolyzed metal-nitrogen-carbon catalysts for the electrochemical reduction of O2. Curr. Opin. Electrochem. 2018, 9, 198–206. [Google Scholar] [CrossRef]
- Patel, A. Functionalization of Keggin-type nickel substituted phosphotungstate by imidazole: Synthesis, characterization, and catalytic activity. J. Mater. Sci. 2016, 52, 4689–4699. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Costa, C.; Madeira, L.M.; Mata, G.; Vicente, M.A.; Cervantes, M.L.R.; Lopez-Peinado, A.J.; Martin-Aranda, R.M. Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl. Catal. B Environ. 2007, 71, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Han, J.; Bao, H.; Wang, J.-Q.; Zheng, L.; Sun, S.; Wang, Z.L.; Sun, C. 3D N-doped ordered mesoporous carbon supported single-atom Fe-N-C catalysts with superior performance for oxygen reduction reaction and zinc-air battery. Appl. Catal. B Environ. 2020, 280, 119411. [Google Scholar] [CrossRef]
- Yin, P.; Wang, W.; Rao, H.; Wang, G.; Sun, M.; Jiang, Y.; Wang, Y.; Zou, P.; Wang, X.; Zhao, Q.; et al. One-step prepared prussian blue/porous carbon composite derives highly efficient Fe–N–C catalyst for oxygen reduction. Int. J. Hydrogen Energy 2020, 45, 15100–15111. [Google Scholar] [CrossRef]
- Tuo, J.; Lin, Y.; Zhu, Y.; Jiang, H.; Li, Y.; Cheng, L.; Pang, R.; Shen, J.; Song, L.; Li, C. Local structure tuning in Fe-N-C catalysts through support effect for boosting CO2 electroreduction. Appl. Catal. B Environ. 2020, 272. [Google Scholar] [CrossRef]
- Osmieri, L.; Escudero-Cid, R.; Armandi, M.; Videla, A.M.; Fierro, J.L.G.; Ocón, P.; Specchia, S. Fe-N/C catalysts for oxygen reduction reaction supported on different carbonaceous materials. Performance in acidic and alkaline direct alcohol fuel cells. Appl. Catal. B Environ. 2017, 205, 637–653. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Vollebregt, S.; Ishihara, R.; Tichelaar, F.; Hou, Y.; Beenakker, C. Influence of the growth temperature on the first and second-order Raman band ratios and widths of carbon nanotubes and fibers. Carbon 2012, 50, 3542–3554. [Google Scholar] [CrossRef]
- Gambou-Bosca, A.; Belanger, D. Chemical Mapping and Electrochemical Performance of Manganese Dioxide/Activated Carbon Based Composite Electrode for Asymmetric Electrochemical Capacitor. J. Electrochem. Soc. 2015, 162, A5115–A5123. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Larouche, N.; Lefèvre, M.; Jaouen, F.; Stansfield, B.; Dodelet, J.-P. Iron porphyrin-based cathode catalysts for polymer electrolyte membrane fuel cells: Effect of NH3 and Ar mixtures as pyrolysis gases on catalytic activity and stability. Electrochim. Acta 2010, 55, 6450–6461. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serov, A.; Rojas-Carbonell, S.; Atanassov, P. Chemistry of Multitudinous Active Sites for Oxygen Reduction Reaction in Transition Metal-Nitrogen-Carbon Electrocatalysts. J. Phys. Chem. C 2015, 119, 25917–25928. [Google Scholar] [CrossRef]
- Gokhale, R.; Chen, Y.; Serov, A.; Artyushkova, K.; Atanassov, P. Direct synthesis of platinum group metal-free Fe-N-C catalyst for oxygen reduction reaction in alkaline media. Electrochem. Commun. 2016, 72, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Castegnaro, M.; Paschoalino, W.J.; Fernandes, M.R.; Balke, B.; Alves, M.D.C.; Ticianelli, E.A.; Morais, J. Pd–M/C (M = Pd, Cu, Pt) Electrocatalysts for Oxygen Reduction Reaction in Alkaline Medium: Correlating the Electronic Structure with Activity. Langmuir 2017, 33, 2734–2743. [Google Scholar] [CrossRef]
- Guidelli, R.; Compton, R.G.; Feliu, J.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Defining the transfer coefficient in electrochemistry: An assessment (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Guidelli, R.; Compton, R.G.; Feliu, J.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Definition of the transfer coefficient in electrochemistry (IUPAC Recommendations 2014). Pure Appl. Chem. 2014, 86, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Holewinski, A.; Linic, S. Elementary Mechanisms in Electrocatalysis: Revisiting the ORR Tafel Slope. J. Electrochem. Soc. 2012, 159, H864–H870. [Google Scholar] [CrossRef]
- Perez, J.; Gonzalez, E.; Ticianelli, E. Oxygen electrocatalysis on thin porous coating rotating platinum electrodes. Electrochim. Acta 1998, 44, 1329–1339. [Google Scholar] [CrossRef]
- Freitas, W.D.S.; D’Epifanio, A.; Ficca, V.C.; Placidi, E.; Arciprete, F.; Mecheri, B. Tailoring active sites of iron-nitrogen-carbon catalysts for oxygen reduction in alkaline environment: Effect of nitrogen-based organic precursor and pyrolysis atmosphere. Electrochim. Acta 2021, 391, 138899. [Google Scholar] [CrossRef]
- Osmieri, L.; Videla, A.H.A.M.; Specchia, S. The use of different types of reduced graphene oxide in the preparation of Fe-N-C electrocatalysts: Capacitive behavior and oxygen reduction reaction activity in alkaline medium. J. Solid State Electrochem. 2016, 20, 3507–3523. [Google Scholar] [CrossRef]
- Jaouen, F. O2 Reduction Mechanism on Non-Noble Metal Catalysts for PEM Fuel Cells. Part II: A Porous-Electrode Model to Predict the Quantity of H2O2 Detected by Rotating Ring-Disk Electrode. J. Phys. Chem. C 2009, 113, 15433–15443. [Google Scholar] [CrossRef]
- Farahani, F.S.; Mecheri, B.; Majidi, M.R.; de Oliveira, M.A.C.; D’Epifanio, A.; Zurlo, F.; Placidi, E.; Arciprete, F.; Licoccia, S. MnOx-based electrocatalysts for enhanced oxygen reduction in microbial fuel cell air cathodes. J. Power Sources 2018, 390, 45–53. [Google Scholar] [CrossRef]
- Boillot, M.; Didierjean, S.; Lapicque, F. Impedance of a rotating disc electrode with a reversible reaction. J. Appl. Electrochem. 2004, 34, 1191–1197. [Google Scholar] [CrossRef]
- Fu, Y.; Poizeau, S.; Bertei, A.; Qi, C.; Mohanram, A.; Pietras, J.; Bazant, M. Heterogeneous electrocatalysis in porous cathodes of solid oxide fuel cells. Electrochim. Acta 2015, 159, 71–80. [Google Scholar] [CrossRef]
- Sheng, W.; Gasteiger, H.; Shao-Horn, Y. Hydrogen Oxidation and Evolution Reaction Kinetics on Platinum: Acid vs Alkaline Electrolytes. J. Electrochem. Soc. 2010, 157, B1529. [Google Scholar] [CrossRef]
- Roldán, C.A.C.; González-Huerta, R.G.; Alonso-Vante, N. Experimental Protocol for HOR and ORR in Alkaline Electrochemical Measurements. J. Electrochem. Soc. 2018, 165, J3001–J3007. [Google Scholar] [CrossRef]
- Zadick, A.; Dubau, L.; Sergent, N.; Berthomé, G.; Chatenet, M. Huge Instability of Pt/C Catalysts in Alkaline Medium. ACS Catal. 2015, 5, 4819–4824. [Google Scholar] [CrossRef]
- Sebastián, D.; Serov, A.; Artyushkova, K.; Atanassov, P.; Aricò, A.S.; Baglio, V. Performance, methanol tolerance and stability of Fe-aminobenzimidazole derived catalyst for direct methanol fuel cells. J. Power Sources 2016, 319, 235–246. [Google Scholar] [CrossRef]
- Yang, X.; Xia, D.; Kang, Y.; Du, H.; Kang, F.; Gan, L.; Li, J. Unveiling the Axial Hydroxyl Ligand on Fe-N4-C Electrocatalysts and Its Impact on the pH-Dependent Oxygen Reduction Activities and Poisoning Kinetics. Adv. Sci. 2020, 7, 2000176. [Google Scholar] [CrossRef]
- Svane, K.L.; Reda, M.; Vegge, T.; Hansen, H.A. Improving the Activity of M−N 4 Catalysts for the Oxygen Reduction Reaction by Electrolyte Adsorption. ChemSusChem 2019, 12, 5133–5141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, M.; Liu, Y.; Zhang, Q.; Ning, G.; Fan, X.; Wang, H.; Lu, H.; Zhang, Y.; Wang, H. Fe2O3 and Co bimetallic decorated nitrogen doped graphene nanomaterial for effective electrochemical water split hydrogen evolution reaction. J. Electroanal. Chem. 2019, 849. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, Q.; Sun, Y.; Ning, G.; Fan, X.; Wang, H.; Lu, H.; Zhang, Y.; Wang, H. Ni–Fe nanocubes embedded with Pt nanoparticles for hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 20832–20842. [Google Scholar] [CrossRef]
- Song, C.; Wu, S.; Shen, X.; Miao, X.; Ji, Z.; Yuan, A.; Xu, K.; Liu, M.; Xie, X.; Kong, L.; et al. Metal-organic framework derived Fe/Fe3C@N-doped-carbon porous hierarchical polyhedrons as bifunctional electrocatalysts for hydrogen evolution and oxygen-reduction reactions. J. Colloid Interface Sci. 2018, 524, 93–101. [Google Scholar] [CrossRef]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Single site porphyrine-like structures advantages over metals for selective electrochemical CO2 reduction. Catal. Today 2017, 288, 74–78. [Google Scholar] [CrossRef]
- Zhou, Q.-S.; Peng, X.-W.; Zhong, L.-X.; Sun, R.-C. CoSe2 nanobelt coupled with CoMoO4 nanosheet as efficient electrocatalysts for hydrogen and oxygen evolution reaction. Environ. Sci. Ecotechnol. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.; Cong, H.; Tang, J.; Sun, Y.; Hu, X.; Wang, L.; Han, S.; Lin, H. Highly efficient hydrogen evolution reaction of Co3O4 supports on N-doped carbon nanotubes in an alkaline solution. Ionics 2020, 26, 3437–3446. [Google Scholar] [CrossRef]
- Zhang, Z.; Cong, L.; Yu, Z.; Qu, L.; Huang, W. Facile synthesis of Fe–Ni bimetallic N-doped carbon framework for efficient electrochemical hydrogen evolution reaction. Mater. Today Energy 2020, 16, 100387. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, J.; Li, Q.; Zhang, P.-F.; Chen, C.; Yan, D.; Mai, Y. Mesoporous Mo2C/Carbon Hybrid Nanotubes Synthesized by a Dual-Template Self-Assembly Approach for an Efficient Hydrogen Production Electrocatalyst. Langmuir 2018, 34, 10924–10931. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-W.; Brüller, S.; Dong, R.; Zhang, J.; Feng, X.; Müllen, K. Molecular metal–Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 2015, 6, 7992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rheinländer, P.J.; Herranz, J.; Durst, J.; Gasteiger, H. Kinetics of the Hydrogen Oxidation/Evolution Reaction on Polycrystalline Platinum in Alkaline Electrolyte Reaction Order with Respect to Hydrogen Pressure. J. Electrochem. Soc. 2014, 161, F1448–F1457. [Google Scholar] [CrossRef]
- Chao, S.; Xia, Q.; Wang, G.; Zhang, X. Fe2O3 nanoparticles immobilized on N and S codoped C as an efficient multifunctional catalyst for oxygen reduction reaction and overall water electrolysis. Int. J. Hydrogen Energy 2019, 44, 4707–4715. [Google Scholar] [CrossRef]
- Lin, T.-W.; Liu, C.-J.; Dai, C.-S. Ni3S2/carbon nanotube nanocomposite as electrode material for hydrogen evolution reaction in alkaline electrolyte and enzyme-free glucose detection. Appl. Catal. B Environ. 2014, 154-155, 213–220. [Google Scholar] [CrossRef]
- Sun, H.; Nie, M.; Xue, Z.; Luo, J.; Tang, Y.; Li, Q.; Teng, L.; Gao, T.; Xu, K. Study on the simple synthesis and hydrogen evolution reaction of nanosized ZnO coated MoS2. Mater. Chem. Phys. 2021, 262, 124279. [Google Scholar] [CrossRef]
- Jaouen, F.; Marcotte, S.; Dodelet, J.-P.; Lindbergh, G. Oxygen Reduction Catalysts for Polymer Electrolyte Fuel Cells from the Pyrolysis of Iron Acetate Adsorbed on Various Carbon Supports. J. Phys. Chem. B 2003, 107, 1376–1386. [Google Scholar] [CrossRef]
- Jaouen, F.; Lefèvre, M.; Dodelet, J.-P.; Cai, M. Heat-Treated Fe/N/C Catalysts for O2 Electroreduction: Are Active Sites Hosted in Micropores? J. Phys. Chem. B 2006, 110, 5553–5558. [Google Scholar] [CrossRef]
- Morozan, A.; Goellner, V.; Nedellec, Y.; Hannauer, J.; Jaouen, F. Effect of the Transition Metal on Metal–Nitrogen–Carbon Catalysts for the Hydrogen Evolution Reaction. J. Electrochem. Soc. 2015, 162, H719–H726. [Google Scholar] [CrossRef]
- Barman, B.K.; Nanda, K.K. CoFe Nanoalloys Encapsulated in N-Doped Graphene Layers as a Pt-Free Multifunctional Robust Catalyst: Elucidating the Role of Co-Alloying and N-Doping. ACS Sustain. Chem. Eng. 2018, 6, 12736–12745. [Google Scholar] [CrossRef]
- Paulus, U.A.; Schmidt, T.J.; Gasteiger, H.A.; Behm, R.J. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disk electrode study Oxygen reduction on a high-surface area Pt/Vulcan carbon catalysts: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem. 2014, 495, 134–145. [Google Scholar] [CrossRef]
- Ohma, A.; Shinohara, K.; Iiyama, A.; Yoshida, T.; Daimaru, A. Membrane and Catalyst Performance Targets for Automotive Fuel Cells by FCCJ Membrane, Catalyst, MEA WG. ECS Trans. 2011, 41, 775–784. [Google Scholar] [CrossRef]
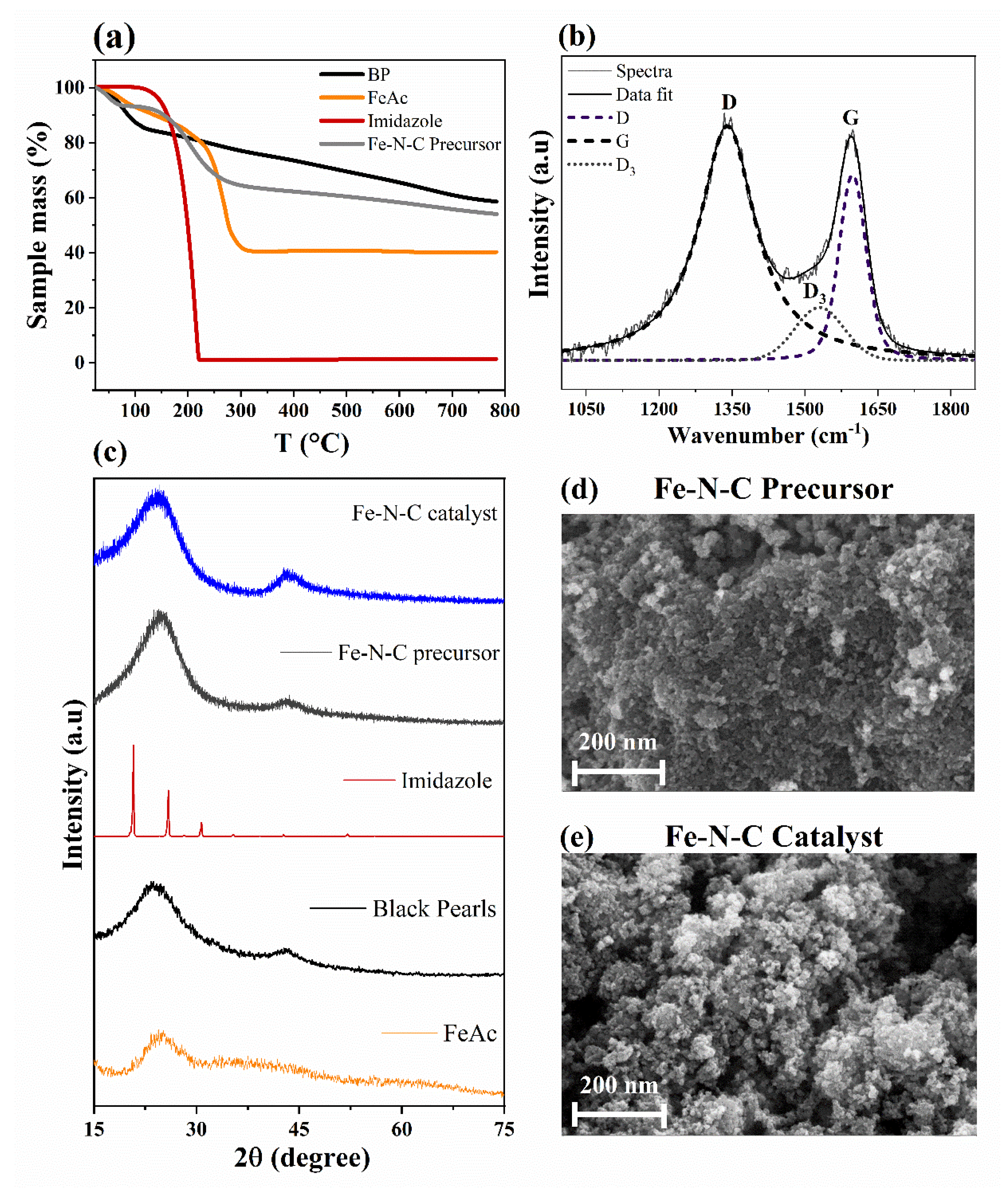
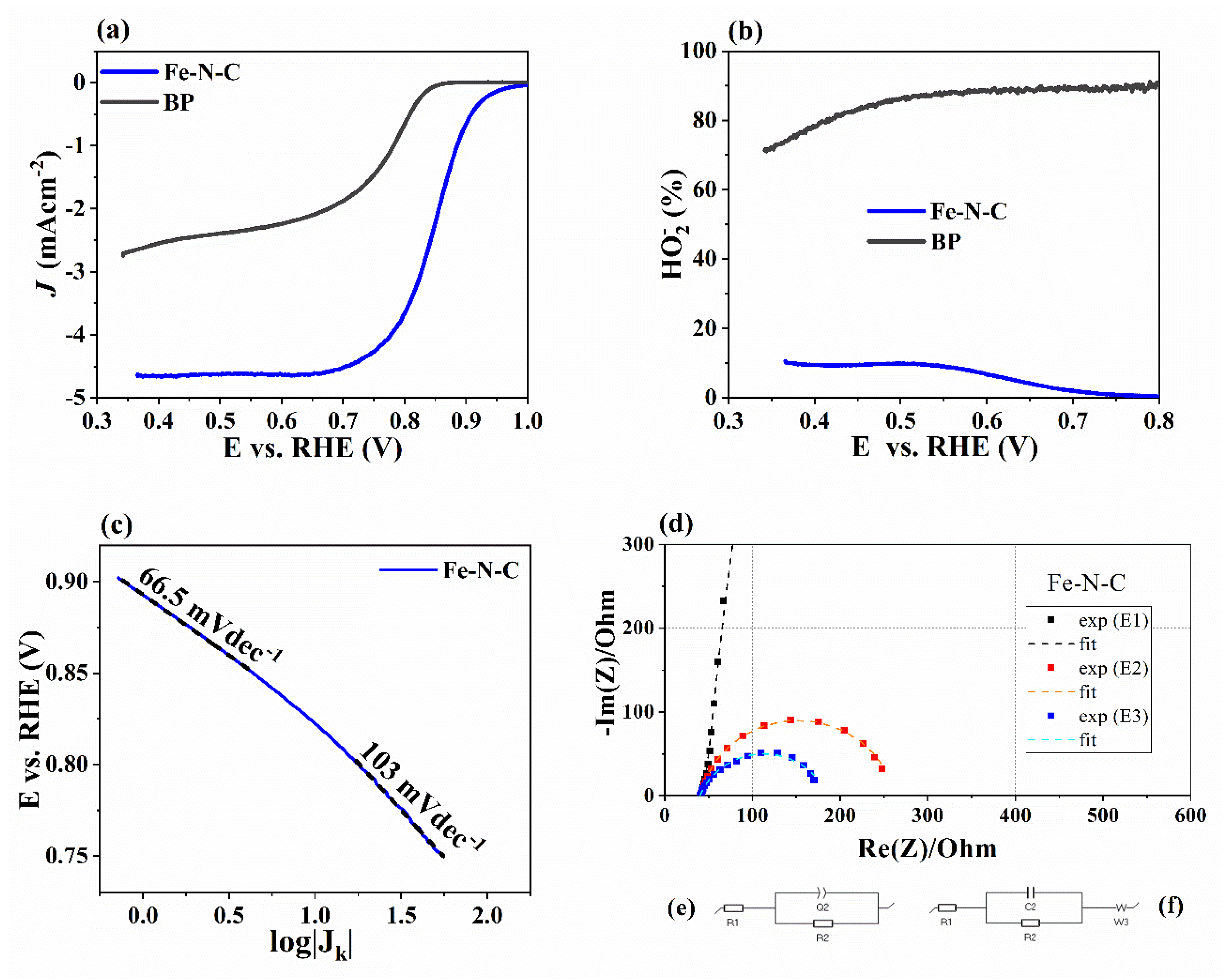
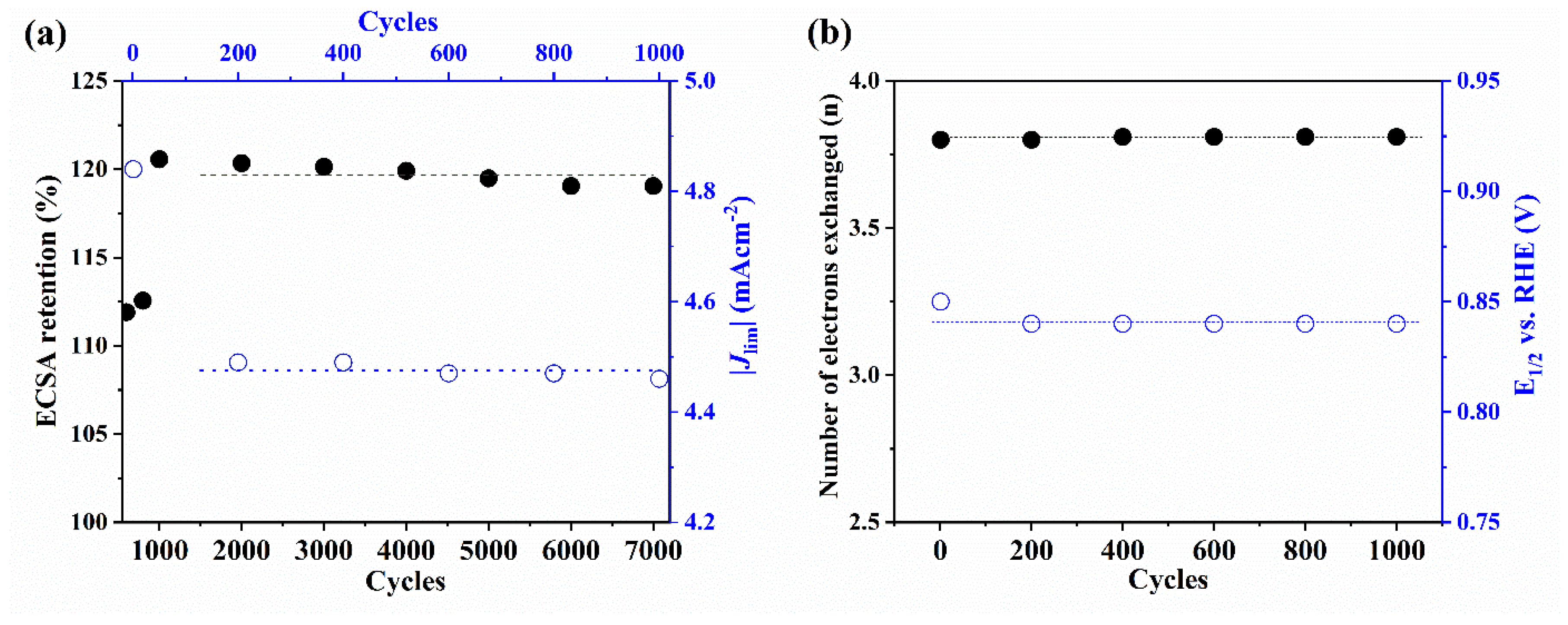

| Parameter | Imidazole | FeAc | BP | Fe-N-C |
|---|---|---|---|---|
| Mass loss I | ||||
| Trange (°C) | 147–224 | 68–135 | 44–117 | 33–75 |
| Mass (wt.%) | 98.6 | 8.2 | 15 | 6.5 |
| Mass loss II | - | |||
| TRange (°C) | - | 204–258 | 136–223 | 138–316 |
| Mass (wt.%) | - | 7.2 | 8 | 29.6 |
| Mass loss III | - | |||
| TRange (°C) | - | 261–369 | 324–744 | 324–755 |
| Mass (wt.%) | - | 38 | 17 | 9.30 |
| Residual mass (wt.%) | 1.4 | 39.2 | 59 | 55 |
| Catalysts | Eonset (mV) | η10 (mV) | Tafel Slope (mVdec−1) | Ref. |
|---|---|---|---|---|
| Fe-N-C | 350 | 478 | 100 | this work |
| Fe2+Ni@NCF | - | 484 | 148 | [80] |
| Fe2+Ni-PBA | - | 526 | 213 | [80] |
| Fe/Fe3C@N-C-2 | - | 560 | 113 | [76] |
| 0.3 Ni-Fe-Pt NCs | 476 | 490 | 89 | [75] |
| Fe2O3/NS-C-800 | 110 | 270 | 165 | [84] |
| Fe2O3(1)-Co(1) NPs-N-GR | 178 | 410 | 77 | [74] |
| Ni3S2/MWCNT-NC | - | 480 | 102 | [85] |
| MoS2–ZnO | - | 490 | 171 | [86] |
| Pt/C | 11 | 76 | 106 | this work |
| Pt/C | 30 | 101 | 64 | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Freitas, W.; Machado Pico, P.P.; D’Epifanio, A.; Mecheri, B. Nanostructured Fe-N-C as Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution. Catalysts 2021, 11, 1525. https://doi.org/10.3390/catal11121525
da Silva Freitas W, Machado Pico PP, D’Epifanio A, Mecheri B. Nanostructured Fe-N-C as Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution. Catalysts. 2021; 11(12):1525. https://doi.org/10.3390/catal11121525
Chicago/Turabian Styleda Silva Freitas, Williane, Pedro Pablo Machado Pico, Alessandra D’Epifanio, and Barbara Mecheri. 2021. "Nanostructured Fe-N-C as Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution" Catalysts 11, no. 12: 1525. https://doi.org/10.3390/catal11121525
APA Styleda Silva Freitas, W., Machado Pico, P. P., D’Epifanio, A., & Mecheri, B. (2021). Nanostructured Fe-N-C as Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution. Catalysts, 11(12), 1525. https://doi.org/10.3390/catal11121525








