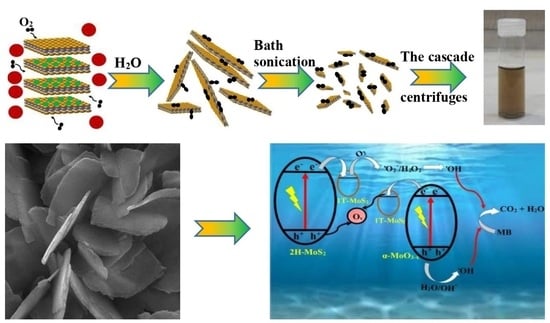
Single-Layer MoS2-MoO3-x Heterojunction Nanosheets with Simultaneous Photoluminescence and Co-Photocatalytic Features
Abstract
:1. Introduction
2. Results and Discussion
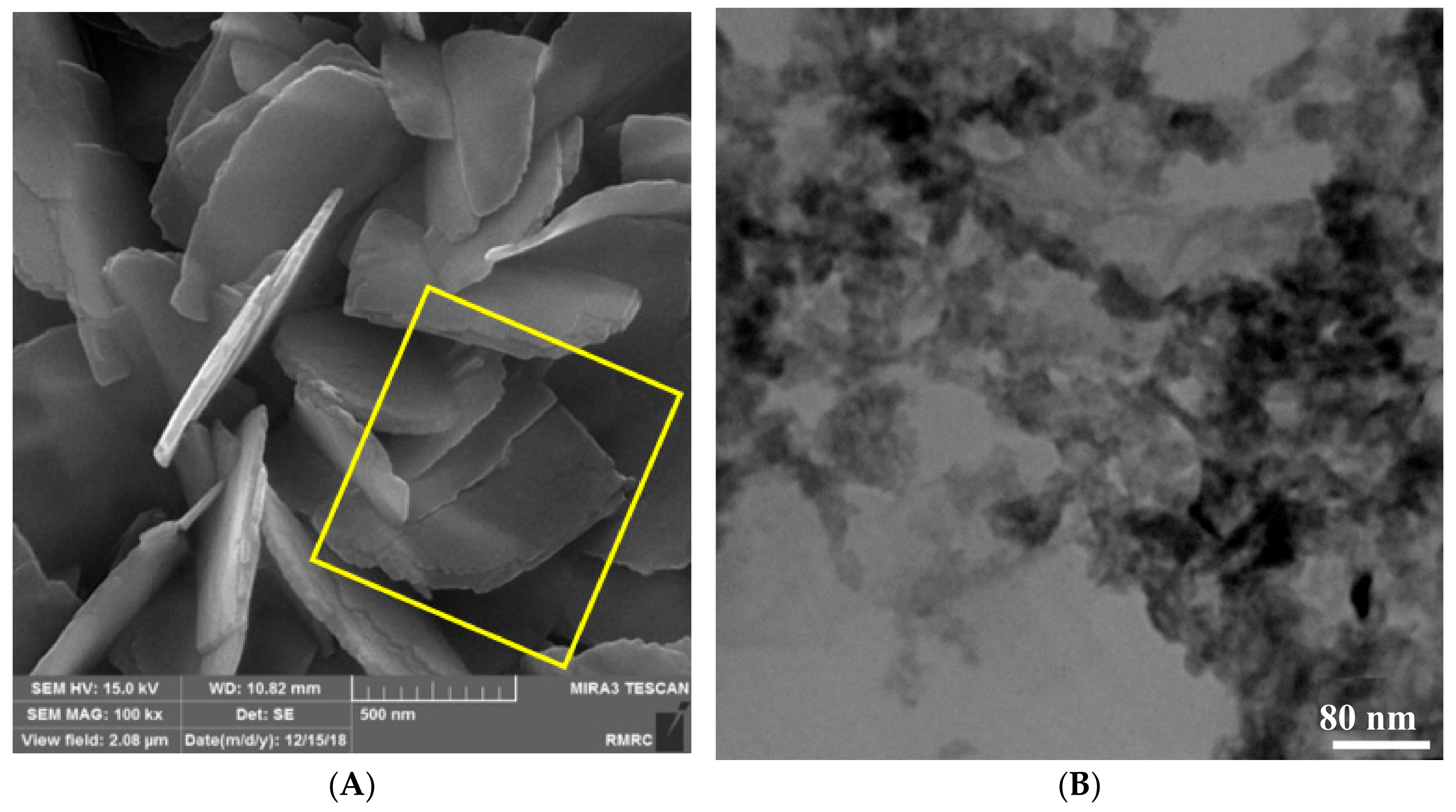
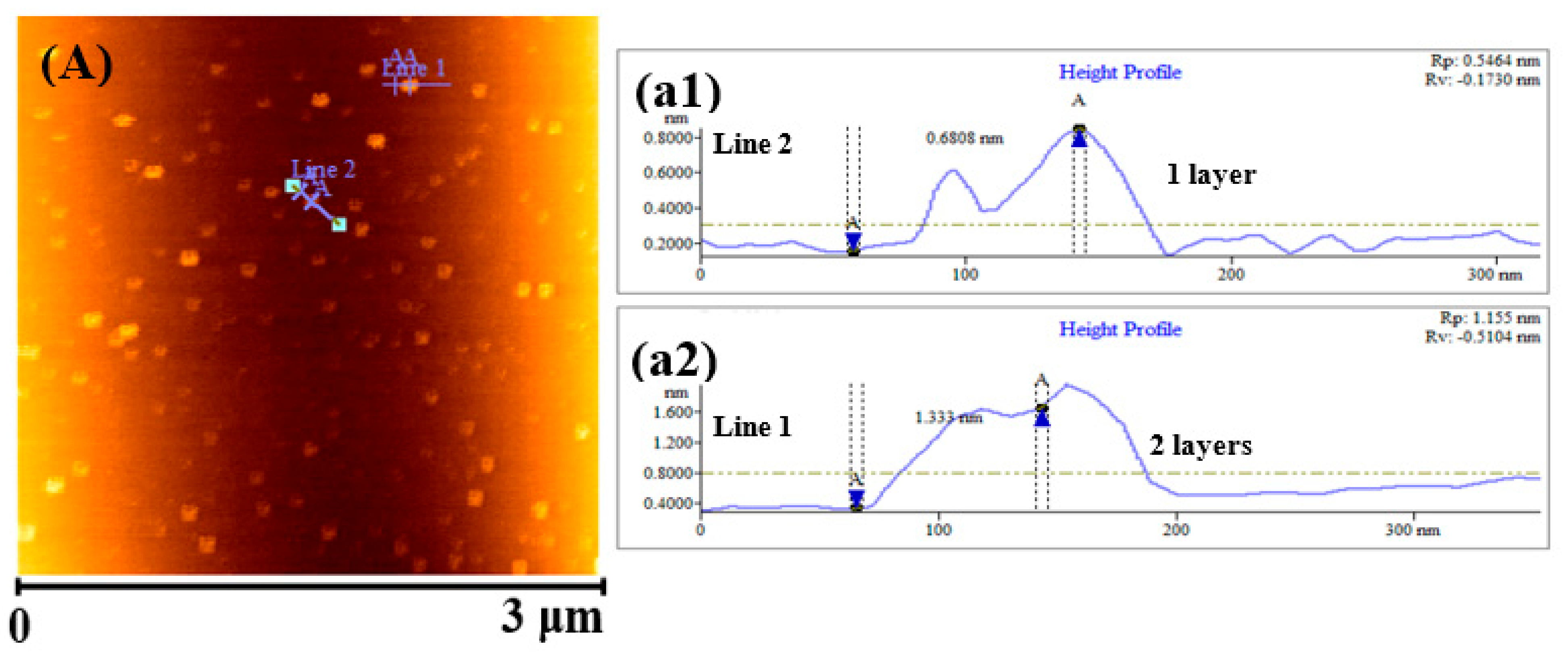
2.1. Structural Analysis
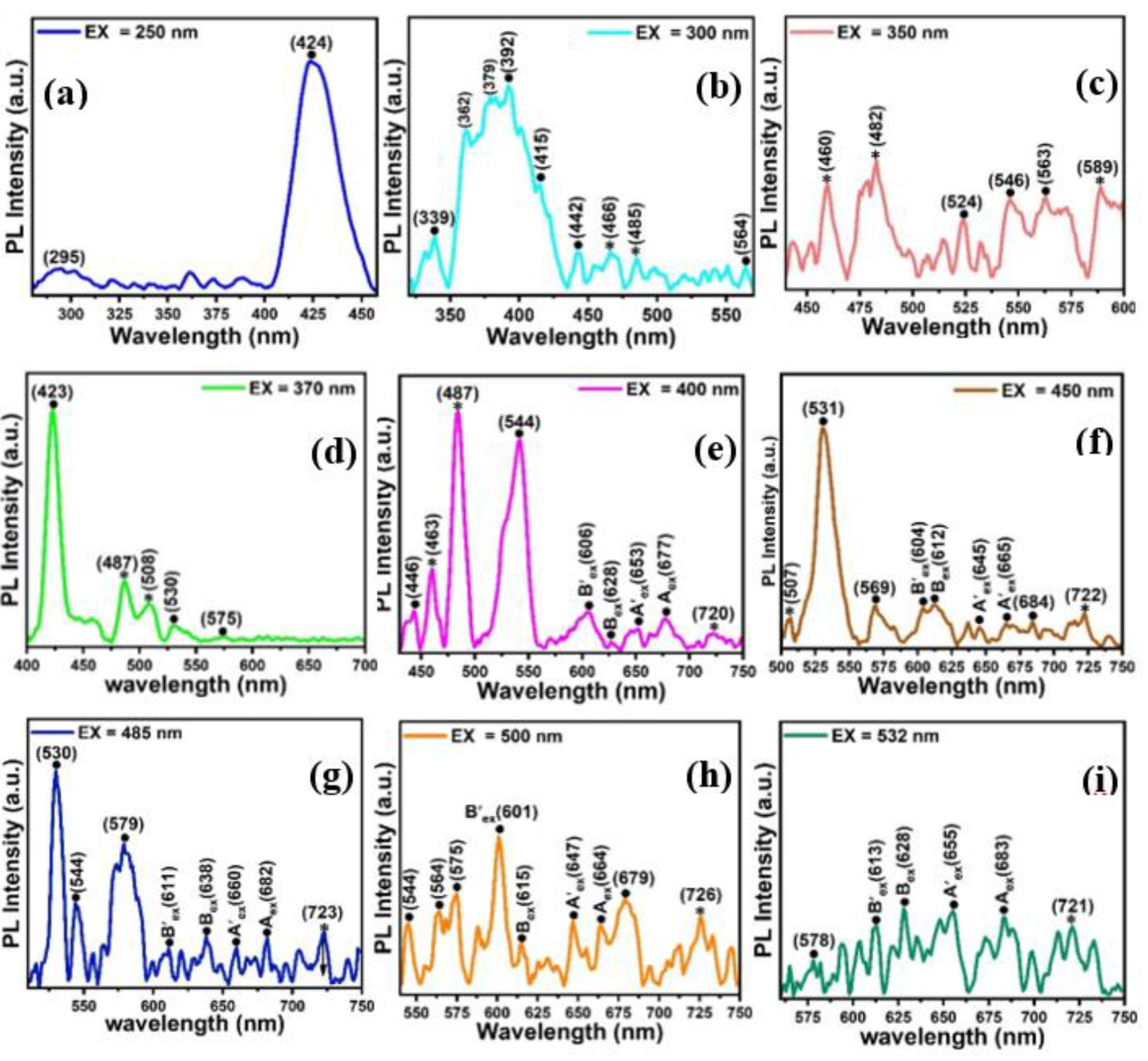
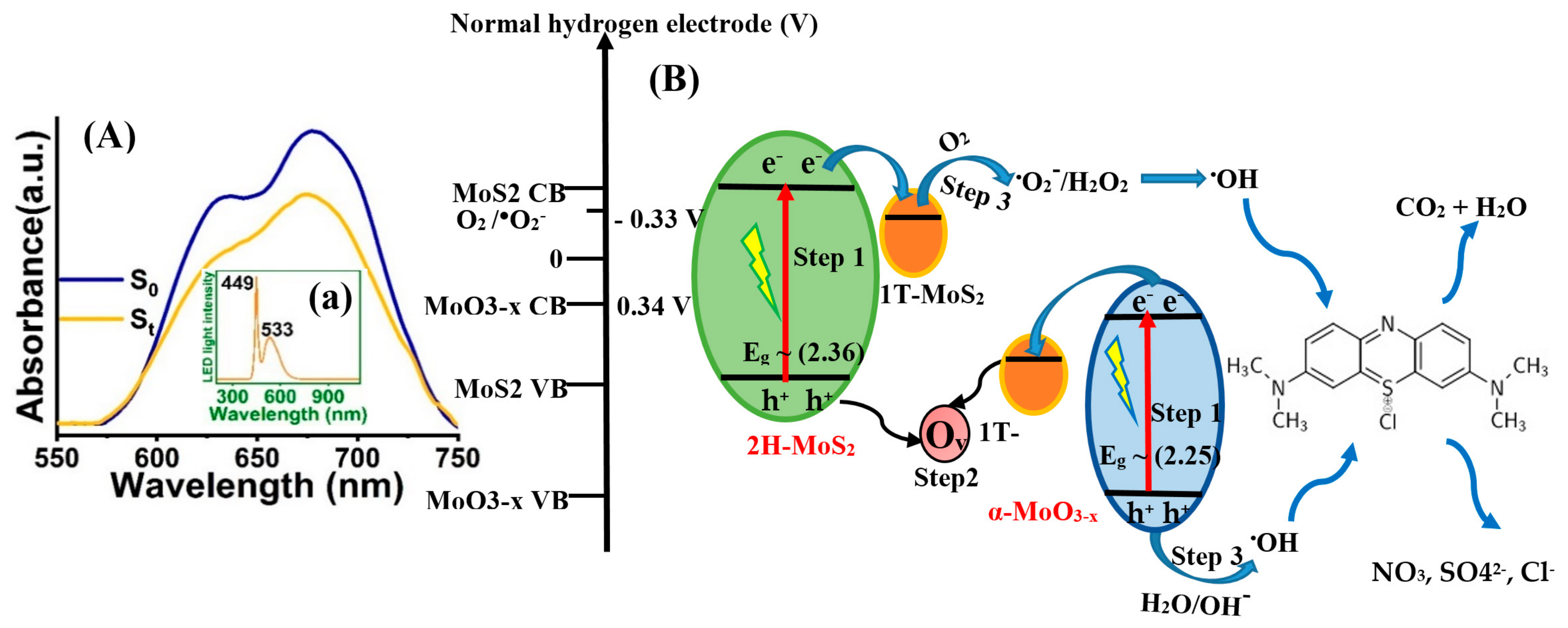
2.2. Optical Analysis
2.3. Photocatalytic Activity Test
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Two-Step Preparation of MoS2-MoO3-x Single-Layer Heterojunctions
4.2.1. Exfoliation of Pristine MoS2 into Single Layers via Sodium Intercalation and Hydration
4.2.2. The Centrifugation Cascades for Extracting MoS2-MoO3-x Single-Layer Heterojunctions
4.2.3. The Application of MoS2-MoO3-x Heterojunctions for MB Photocatalytic Degradation
4.3. Material Characterizations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christopher, P.; Xin, H.; Marimuthu, A.; Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 2012, 11, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Hahn, R.; Schmuki, P. Self-organized, free-standing TiO2 nanotube membrane for flow-through photocatalytic applications. Nano Lett. 2007, 7, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Flash photo stimulation of human neural stem cells on graphene/TiO2 heterojunction for differentiation into neurons. Nanoscale 2013, 5, 10316–10326. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Li, L.; Liu, N.; Wang, D.-J.; Xue, Y.-C.; Tang, L. Molybdenum disulfide (MoS2) as a co-catalyst for photocatalytic degradation of organic contaminants: A review. Process Saf. Environ. Prot. 2018, 118, 40–58. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. Recent development on MoS2-based photocatalysis: A review. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 39–55. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf. Coatings Technol. 2010, 205, 219–223. [Google Scholar] [CrossRef]
- Akhavan, O.; Choobtashani, M.; Ghaderi, E. Protein degradation and RNA efflux of viruses photocatalyzed by graphene-tungsten oxide composite under visible light irradiation. J. Phys. Chem. C 2012, 116, 9653–9659. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Rahimi, K. Adverse effects of graphene incorporated in TiO2 photocatalyst on minuscule animals under solar light irradiation. J. Mater. Chem. 2012, 22, 23260–23266. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Zhang, J.; Chen, K.; Huang, C.; Chen, H.; Qiu, X. Plasmonic MoO3-x nanosheets with tunable oxygen vacancies as efficient visible light responsive photocatalyst. Appl. Surf. Sci. 2019, 490, 395–402. [Google Scholar] [CrossRef]
- Etman, A.S.; Abdelhamid, H.N.; Yuan, Y.; Wang, L.; Zou, X.; Sun, J. Facile water-based strategy for synthesizing MoO3–x nanosheets: Efficient visible light photocatalysts for dye degradation. ACS Omega 2018, 3, 2193–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Zhang, F.; Li, Y.; Wang, H.; Wang, Q.; Wang, C.; Xu, H.; Li, H. Chemical reduction implanted oxygen vacancy on the surface of 1D MoO3-x/g-C3N4 composite for boosted LED light-driven photoactivity. J. Mater. Sci. 2019, 54, 5343–5358. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shirazian, S.A. Near infrared laser stimulation of human neural stem cells into neurons on graphene nanomesh semiconductors. Colloids Surf. B Biointerfaces 2015, 126, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Graphene nanomesh by ZnO nanorod photocatalysts. ACS Nano 2010, 4, 4174–4180. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Lasting antibacterial activities of Ag-TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J. Colloid Interface Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Sim, D.M.; Han, H.J.; Yim, S.; Choi, M.-J.; Jeon, J.; Jung, Y.S. Long-term stable 2H-MoS2 dispersion: Critical role of solvent for simultaneous phase restoration and surface functionalization of liquid-exfoliated MoS2. ACS Omega 2017, 2, 4678–4687. [Google Scholar] [CrossRef] [PubMed]
- Portone, A.; Romano, L.; Fasano, V.; Di Corato, R.; Camposeo, A.; Fabbri, F.; Cardarelli, F.; Pisignano, D.; Persano, L. Low-defectiveness exfoliation of MoS2 nanoparticles and their embedment in hybrid light-emitting polymer nanofibers. Nanoscale 2018, 10, 21748–21754. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, X.; Liu, M.; Luo, H.; Deng, L.; Huang, L.; Wei, S.; Zhou, C.; Xu, Y. Molybdenum disulfide quantum dots prepared by bipolar-electrode electrochemical scissoring. Nanomaterials 2019, 9, 906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Zhang, H.; Dong, S.; Liu, Y.; Nai, C.T.; Shin, H.S.; Jeong, H.Y.; Liu, B.; Loh, K.P. High yield exfoliation of two-dimensional chalcogenides using sodium naphthalenide. Nat. Commun. 2014, 5, 2995. [Google Scholar] [CrossRef]
- Chen, J.; Liao, Y.; Wan, X.; Tie, S.; Zhang, B.; Lan, S.; Gao, X. A high performance MoO3@MoS2 porous nanorods for adsorption and photodegradation of dye. J. Solid State Chem. 2020, 291, 121652. [Google Scholar] [CrossRef]
- Lu, X.; Jin, Y.; Zhang, X.; Xu, G.; Wang, D.; Lv, J.; Zheng, Z.; Wu, Y. Controllable synthesis of graphitic C3N4/ultrathin MoS2 nanosheet hybrid nanostructures with enhanced photocatalytic performance. Dalt. Trans. 2016, 45, 15406–15414. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, D.; Qiao, Q.; Yu, Y.; Peterson, D.; Zafar, A.; Kumar, R.; Curtarolo, S.; Hunte, F.; Shannon, S.; et al. All the catalytic active sites of MoS2 for hydrogen evolution. J. Am. Chem. Soc. 2016, 138, 16632–16638. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Sun, Z.; Hu, Y.H. Synthesis, stabilization and applications of 2-dimensional 1T metallic MoS2. J. Mater. Chem. A 2018, 6, 23932–23977. [Google Scholar] [CrossRef]
- Zak, A.; Feldman, Y.; Lyakhovitskaya, V.; Leitus, G.; Popovitz-Biro, R.; Wachtel, E.; Cohen, H.; Reich, S.; Tenne, R. Alkali metal intercalated fullerene-like MS2 (M = W, Mo) nanoparticles and their propertie. J. Am. Chem. Soc. 2002, 124, 4747–4758. [Google Scholar] [CrossRef]
- Wang, H.; Sofer, Z.; Moo, J.G.S.; Pumera, M. Simultaneous self-exfoliation and autonomous motion of MoS2 particles in water. Chem. Commun. 2015, 51, 9899–9902. [Google Scholar] [CrossRef] [Green Version]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Attanayake, N.H.; Thenuwara, A.C.; Patra, A.; Aulin, Y.V.; Tran, T.M.; Chakraborty, H.; Borguet, E.; Klein, M.L.; Perdew, J.P.; Strongin, D.R. Effect of intercalated metals on the electrocatalytic activity of 1T-MoS2 for the hydrogen evolution reaction. ACS Energy Lett. 2018, 3, 7–13. [Google Scholar] [CrossRef]
- Saber, M.R.; Khabiri, G.; Maarouf, A.A.; Ulbricht, M.; Khalil, A.S.G. A comparative study on the photocatalytic degradation of organic dyes using hybridized 1T/2H, 1T/3R and 2H MoS2 nano-sheets. RSC Adv. 2018, 8, 26364–26370. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Wu, R.; Liu, H.; Yang, X.; Sun, Y.; Chen, C. Synthesis of metal-phase-assisted 1T@2H-MoS2 nanosheet-coated black TiO2 spheres with visible light photocatalytic activities. J. Mater. Sci. 2018, 53, 10302–10312. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Tang, Z.; Fu, H.; Zhu, Z. High photocatalytic performance of a type-II α-MoO3@MoS2 heterojunction: From theory to experiment. Phys. Chem. Chem. Phys. 2016, 18, 14074–14085. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, Y.; Luo, Z.; Pan, C.; Yang, J.; Fang, Y.; Deng, H.; Liu, C.; Tan, Q.; Liu, F.; et al. Nanostructured MoO3 for Efficient Energy and Environmental Catalysis. Molecules 2020, 25, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltrusaitis, J.; Mendoza-Sanchez, B.; Fernandez, V.; Veenstra, R.; Dukstiene, N.; Roberts, A.; Fairley, N. Generalized molybdenum oxide surface chemical state XPS determination via informed amorphous sample model. Appl. Surf. Sci. 2015, 326, 151–161. [Google Scholar] [CrossRef]
- Sreedhara, M.B.; Matte, H.S.S.R.; Govindaraj, A.; Rao, C.N.R. Synthesis, characterization, and properties of few-layer MoO3. Chem. An. Asian J. 2013, 8, 2430–2435. [Google Scholar] [CrossRef]
- Balendhran, S.; Walia, S.; Nili, H.; Ou, J.Z.; Zhuiykov, S.; Kaner, R.B.; Sriram, S.; Bhaskaran, M.; Kalantar-zadeh, K. Two-dimensional molybdenum trioxide and dichalcogenides. Adv. Funct. Mater. 2013, 23, 3952–3970. [Google Scholar] [CrossRef]
- Du, J.; Wang, J.; Deng, Y.; He, Y. Plasmonic hot electron transfer-induced multicolor MoO3-x-based chromogenic system for visual and colorimetric determination of silver(I). Microchim. Acta 2020, 187, 120. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, R.; Yang, F.; Jiao, W.; Liu, W.; Hao, L.; He, X. Preparation of MoO3 QDs through combining intercalation and thermal exfoliation. J. Mater. Chem. C 2016, 4, 6720–6726. [Google Scholar] [CrossRef]
- Tan, X.; Wang, L.; Cheng, C.; Yan, X.; Shen, B.; Zhang, J. Plasmonic MoO3-x@MoO3 nanosheets for highly sensitive SERS detection through nanoshell-isolated electromagnetic enhancement. Chem. Commun. 2016, 52, 2893–2896. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yi, W.; Li, J.; Xi, G.; Li, Y.; Yang, H.; Liu, J. Direct growth of defect-rich MoO3-x ultrathin nanobelts for efficiently catalyzed conversion of isopropyl alcohol to propylene under visible light. J. Mater. Chem. A 2016, 4, 1566–1571. [Google Scholar] [CrossRef]
- Patra, K.K.; Ghosalya, M.K.; Bajpai, H.; Raj, S.; Gopinath, C.S. Oxidative Disproportionation of MoS2/GO to MoS2/MoO3- x/RGO: Integrated and Plasmonic 2D-Multifunctional Nanocomposites for Solar Hydrogen Generation from Near-Infrared and Visible Regions. J. Phys. Chem. C 2019, 123, 21685–21693. [Google Scholar] [CrossRef]
- Datta, R.S.; Haque, F.; Mohiuddin, M.; Carey, B.J.; Syed, N.; Zavabeti, A.; Zhang, B.; Khan, H.; Berean, K.J.; Ou, J.Z.; et al. Highly active two dimensional α-MoO3-X for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 24223–24231. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Mao, X.; Zhou, D.; Feng, S.; Shi, X.; Ma, Y.; Wei, X.; Mao, Y. Bandgap modulation of MoS2 monolayer by thermal annealing and quick cooling. Nanoscale 2016, 8, 18995–19003. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.; Li, M.; Wang, S.; Wang, P.; Papakonstantinou, P. Electrocatalytic Hydrogen evolution reaction on edges of a few layer molybdenum disulfide nanodots. ACS Appl. Mater. Interfaces 2015, 7, 14113–14122. [Google Scholar] [CrossRef] [Green Version]
- Sharma, L.; Botari, T.; Tiwary, C.S.; Halder, A. Hydrogen evolution at the in situ MoO3 /MoS2 heterojunctions created by nonthermal O2 plasma treatment. ACS Appl. Energy Mater. 2020, 3, 5333–5342. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Bao, S.; Zhang, Z.; Fei, H.; Wu, Z. Phase engineering of a multiphasic 1T/2H MoS2 catalyst for highly efficient hydrogen evolution. J. Mater. Chem. A 2017, 5, 2681–2688. [Google Scholar] [CrossRef]
- Kim, C.; Nguyen, T.P.; Van Le, Q.; Jeon, J.M.; Jang, H.W.; Kim, S.Y. Performances of liquid-exfoliated transition metal dichalcogenides as hole injection layers in organic light-emitting diodes. Adv. Funct. Mater. 2015, 25, 4512–4519. [Google Scholar] [CrossRef]
- Chandoul, F.; Boukhachem, A.; Hosni, F.; Moussa, H.; Fayache, M.S.; Amlouk, M.; Schneider, R. Change of the properties of nanostructured MoO3 thin films using gamma-ray irradiation. Ceram. Int. 2018, 44, 12483–12490. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; He, X.; Zhong, W.; Du, Y. Luminescent monolayer MoS2 quantum dots produced by multi-exfoliation based on lithium intercalation. Appl. Surf. Sci. 2015, 359, 130–136. [Google Scholar] [CrossRef]
- Li, B.; Jiang, L.; Li, X.; Ran, P.; Zuo, P.; Wang, A.; Qu, L.; Zhao, Y.; Cheng, Z.; Lu, Y. Preparation of monolayer MoS2 quantum dots using temporally shaped femtosecond laser ablation of bulk MoS2 targets in water. Sci. Rep. 2017, 7, 11182. [Google Scholar] [CrossRef] [PubMed]
- Mouri, S.; Miyauchi, Y.; Matsuda, K. Tunable photoluminescence of monolayer MoS2 via chemical doping. Nano Lett. 2013, 13, 5944–5948. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single and few-layer MoS2. ACS Nano 2010, 4, 6–7. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Deokar, G.; Rajput, N.S.; Vancsó, P.; Ravaux, F.; Jouiad, M.; Vignaud, D.; Cecchet, F.; Colomer, J.F. Large area growth of vertically aligned luminescent MoS2 nanosheets. Nanoscale 2017, 9, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Seguin, L.; Figlarz, M.; Cavagnat, R.; Lassègues, J.-C.C. Infrared and Raman spectra of MoO3 molybdenum trioxides and MoO3 · xH2O molybdenum trioxide hydrates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 1323–1344. [Google Scholar] [CrossRef]
- Dieterle, M.; Weinberg, G.; Mestl, G. Raman spectroscopy of molybdenum oxides-Part I. Structural characterization of oxygen defects in MoO3-x by DR UV/VIS, Raman spectroscopy and X-ray diffraction. Phys. Chem. Chem. Phys. 2002, 4, 812–821. [Google Scholar] [CrossRef]
- Li, B.L.; Chen, L.X.; Zou, H.L.; Lei, J.L.; Luo, H.Q.; Li, N.B. Electrochemically induced Fenton reaction of nanoporous morphology. Nanoscale 2014, 6, 9831–9838. [Google Scholar] [CrossRef] [PubMed]
- Chikan, V.; Kelley, D.F. Size-dependent spectroscopy of MoS2 nanoclusters. J. Phys. Chem. B 2002, 106, 3794–3804. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Y.; Xia, Z.; Wei, W. As-prepared MoS2 quantum dot as a facile fluorescent probe for long-term tracing of live cells. Nanotechnology 2016, 27, 275101. [Google Scholar] [CrossRef]
- Dhakal, K.P.; Duong, D.L.; Lee, J.; Nam, H.; Kim, M.; Kan, M.; Lee, Y.H.; Kim, J. Confocal absorption spectral imaging of MoS2: Optical transitions depending on the atomic thickness of intrinsic and chemically doped MoS2. Nanoscale 2014, 6, 13028–13035. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Lakshmi, K.V.; Huang, L. Eco-friendly synthesis of metal dichalcogenides nanosheets and their environmental remediation potential driven by visible light. Sci. Rep. 2015, 5, 15718. [Google Scholar] [CrossRef] [Green Version]
- Fadil, D.; Hossain, R.F.; Saenz, G.A.; Kaul, A.B. On the chemically-assisted excitonic enhancement in environmentally-friendly solution dispersions of two-dimensional MoS2 and WS2. J. Mater. Chem. C 2017, 5, 5323–5333. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Yang, C.; Yao, Y.; Huang, L.; Li, S.; Wang, J.; Ji, Y. Rapid and selective fluorometric determination of tannic acid using MoO3-x quantum dots. Microchim. Acta 2019, 186, 247. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. Synthesis and characterization of solar photoactive TiO2 nanoparticles with enhanced structural and optical properties. Adv. Powder Technol. 2018, 29, 211–219. [Google Scholar] [CrossRef]
- Nan, H.; Wang, Z.; Wang, W.; Liang, Z.; Lu, Y.; Chen, Q.; He, D.; Tan, P.; Miao, F.; Wang, X.; et al. Strong photoluminescence enhancement of MoS2 through defect engineering and oxygen bonding. ACS Nano 2014, 8, 5738–5745. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 Quantum Dot-Interspersed Exfoliated MoS2. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef]
- Wu, J.-Y.Y.; Lin, M.-N.N.; De Wang, L.-D.; Zhang, T. Photoluminescence of MoS2 prepared by effective grinding-assisted sonication exfoliation. J. Nanomater. 2014, 2014, 852735. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Feng, P.; Wang, Z.; Jiao, X.; Akhtar, F. Novel fabrication and enhanced photocatalytic MB degradation of hierarchical porous monoliths of MoO3 Nanoplates. Sci. Rep. 2017, 7, 1845. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.J.; Zhao, X.J.; Chu, Z.J.; Xu, H.; Liu, G.Q.; Huang, C.Z.; Zhang, L. New off-on sensor for captopril sensing based on photoluminescent MoOx quantum dots. ACS Omega 2017, 2, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Tohidi, H.; Moshfegh, A.Z. Synthesis and electrochromic study of sol-gel cuprous oxide nanoparticles accumulated on silica thin film. Thin Solid Film. 2009, 517, 6700–6706. [Google Scholar] [CrossRef]
- Saha, N.; Sarkar, A.; Ghosh, A.B.; Dutta, A.K.; Bhadu, G.R.; Paul, P.; Adhikary, B. Highly active spherical amorphous MoS2: Facile synthesis and application in photocatalytic degradation of rose bengal dye and hydrogenation of nitroarenes. RSC Adv. 2015, 5, 88848–88856. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Deng, C.; Xu, J.; Zhang, K.; Sun, M. Metastable h-MoO3 and stable α-MoO3 microstructures: Controllable synthesis, growth mechanism and their enhanced photocatalytic activity. J. Exp. Nanosci. 2015, 10, 1336–1346. [Google Scholar] [CrossRef]
- Karade, S.S.; Dubal, D.P.; Sankapal, B.R. MoS2 ultrathin nanoflakes for high performance supercapacitors: Room temperature chemical bath deposition (CBD). RSC Adv. 2016, 6, 39159–39165. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O. Thickness dependent activity of nanostructured TiO2/α-Fe2O3 photocatalyst thin films. Appl. Surf. Sci. 2010, 257, 1724–1728. [Google Scholar] [CrossRef]
- Mondal, S.; De Anda Reyes, M.E.; Pal, U. Plasmon induced enhanced photocatalytic activity of gold loaded hydroxyapatite nanoparticles for methylene blue degradation under visible light. RSC Adv. 2017, 7, 8633–8645. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O.; Saadati, M.; Jannesari, M. Graphene jet nanomotors in remote controllable self-propulsion swimmers in pure water. Nano Lett. 2016, 16, 5619–5630. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Szydłowska, B.M.; Harvey, A.; Yuan, S.; Vega-Mayoral, V.; Davies, B.R.; Zhao, P.; Hanlon, D.; Santos, E.J.G.; Katsnelson, M.I.; et al. Production of Highly Monolayer Enriched Dispersions of Liquid-Exfoliated Nanosheets by Liquid Cascade Centrifugation. ACS Nano 2016, 10, 1589–1601. [Google Scholar] [CrossRef]






| Heterojunction Photocatalyst | Heterojunction Type | Synthesis Method | Light Source | Indirect Band Gap Energy (eV) | Direct Band Gap Energy (eV) | Conductive Domain | LSPR Domain | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| α-MoO3@MoS2 hybrid rod–sphere structure | Type-II | Hydrothermal method | 150 W mercury lamp | α-MoO3: 2.7, MoS2~1.2 | - | - | - | RhB degradation | [30] |
| MoS2/MoO3-x/RGO nanocomposite | Type-II | Hydrothermal method | Sunlight simulator with flux of 100 mW/cm2 | MoS2/MoO3-x/RGO: 1.78 | - | 1T-MoS2 and rGO | @ 750 nm | H2 generation | [39] |
| porous MoO3@MoS2 core–shell nanorod | Type-II | Hydrothermal method | Xe lamp, 300 W, sunlight irradiation | MoO3: 3.50, MoS2: 2.03 | - | 1T-MoS2. | - | RhB degradation | [20] |
| MoS2-MoO3-x single-layer heterojunction | Type-II | Liquid-phase chemical exfoliation | Visible light LED (1 W) | MoO3: 1.95 and 2.25, MoS2: 1.75 | 2.16 and 2.36 | 1T-MoS2. | @731 and 963 nm | MB degradation | This work |
| Centrifuge Number | Dispersions Used for Centrifugation | Revolutions per Minute (rpm) | Relative Centrifugal Force (g) | Runtime (min.) |
|---|---|---|---|---|
| 1 | Initial Dispersion | 2000 | 376 | 10 |
| 2 | Supernatant of 2000 | 4000 | 1503 | 30 |
| 3 | Supernatant of 6000 | 6000 | 3381 | 20 |
| 4 | Supernatant of 6000 | 8000 | 6010 | 30 |
| 5 | Supernatant of 8000 | 110,000 | 9391 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadati, M.; Akhavan, O.; Fazli, H. Single-Layer MoS2-MoO3-x Heterojunction Nanosheets with Simultaneous Photoluminescence and Co-Photocatalytic Features. Catalysts 2021, 11, 1445. https://doi.org/10.3390/catal11121445
Saadati M, Akhavan O, Fazli H. Single-Layer MoS2-MoO3-x Heterojunction Nanosheets with Simultaneous Photoluminescence and Co-Photocatalytic Features. Catalysts. 2021; 11(12):1445. https://doi.org/10.3390/catal11121445
Chicago/Turabian StyleSaadati, Maryam, Omid Akhavan, and Hossein Fazli. 2021. "Single-Layer MoS2-MoO3-x Heterojunction Nanosheets with Simultaneous Photoluminescence and Co-Photocatalytic Features" Catalysts 11, no. 12: 1445. https://doi.org/10.3390/catal11121445
APA StyleSaadati, M., Akhavan, O., & Fazli, H. (2021). Single-Layer MoS2-MoO3-x Heterojunction Nanosheets with Simultaneous Photoluminescence and Co-Photocatalytic Features. Catalysts, 11(12), 1445. https://doi.org/10.3390/catal11121445