1. Introduction
Rising concern about global warming in combination with the necessity of limitation emission of greenhouse gasses (GHG) combined with the policy of reducing environmental pollution is leading to the exploration of new energy generation methods. These also include new and more environmentally friendly fuels usage [
1].
Nowadays, the technology of generating energy from gaseous fuels, such methane, hydrogen, or hydrogen-containing fuels obtained from biomass or coal gasification, is receiving widespread attention from researchers [
2]. In principle, fumes after combusting those fuels should only contain carbon dioxide and water vapors, and in combination with carbon dioxide sequestration, it enables the creation of near zero emission energy generation technology. However, in practice, the major concern during the combustion of the mentioned fuels is their high adiabatic flame temperature which is leading to thermal NO
x emissions [
2].
Chemical Looping Combustion (CLC) is a new, promising technology that allows one to burn fuels without direct contact with atmospheric air. During the CLC process, oxygen for the reaction is provided by a material called the oxygen carrier (OC). Oxygen could be released during direct reaction between the OC particle and the fuel, or it could be released spontaneously, due to change of partial oxygen pressure in the reactor, during a process called chemical looping with oxygen uncoupling (CLOU) [
3,
4,
5]. The oxygen carrier is usually a metal oxide that could be either synthetic or natural, when considering the raw material used for OC production. As a result, a variety of materials may be applied, for example, perovskite type OCs [
6] or CaSO
4, or natural ores [
5]. During the CLC process, usually a system of two reactors is used. In the fuel reactor, combustion of fuel takes place, by evolving oxygen from the OC structure. While in the oxidation reactor (air reactor), the oxygen carrier is oxidized back with the application of atmospheric air, and then is transported into the fuel reactor. Lack of direct contact between fuel and atmospheric nitrogen limits the creation of thermal NO
x; therefore, the fumes coming from this process contain mostly H
2O and CO
2 [
5]. CLC technology could be applied for the combustion of a large variety of fuels such as solids (hard coal [
7], lignite, biomass [
8,
9,
10], liquid [
11] or gaseous (syngas, natural gas, methane or hydrogen etc.) [
12,
13,
14].
Suitable materials for the oxygen carrier should be characterized with certain properties such as high oxygen transport capacity and low production cost. The main issue that affects the cost of applying OCs in the fuel combustion process is their chemical and physical stability during muliple redox cycles. According to the National Energy Technology Laboratory (NETL), the make-up cost of OC for practical application in the CLC unit used for power generation should not exceed USD 5 per 1MW
th-hr of energy generated in such an installation [
15]. To remedy this different approach, different solutions are used like applying natural ores, using industrial wastes as OCs or designing new synthetic OCs.
Naturally occurring ores usually exhibit poor mechanical durability and also show agglomeration tendency [
16]. Although it is possible to use single metal oxides such as Fe
2O
3 or Mn
2O
3 as an oxygen carrier, a widely used solution is to apply mixed oxide systems due to their improved characteristics. While single active phase OCs are relatively simple in application, they have numerous disadvantages that could be eliminated using two or more active phases [
17]. For example, iron oxide, despite being relatively cheap, exhibits a decent reactivity towards methane and a tendency to agglomerate, which shortens its lifetime in CLC reactors. Moreover, Fe
2O
3 does not exhibit CLOU properties that could be particularly valuable during the combustion of solid fuels [
18]. In comparison, single phase manganese OCs have CLOU properties (evolving of O
2 from oxide structure due to high temperature) but their reoxidation is undergone with relatively slow rates. They also exhibit poor reactivity toward numerous gaseous fuels [
4,
19]. Forming combined systems with two or more active phases may be a solution to those problems. Potentially, it may eliminate such disadvantages and make emerge new properties due to the synergy effect [
20,
21].
For example, in the case of the Fe-Mn OC system, the formation of bixbyite (Fe
xMn
1-x)
2O
3 has been reported. Bixbyite phase tends to transition into (Fe
xMn
1-x)
3O
4 spinel with the releasing of gaseous oxygen. Moreover, spinel could be further reduced to so-called manganowüstite (Fe
xMn
1-x)O. Those reactions are occurring in specific conditions, depending on temperature and partial oxygen pressure [
9,
19]. The bixbyite and spinel phases are characterized by the reactivity greater than the reactivity observed for pure manganese oxide. They also exhibit CLOU properties in contrast to pure Fe
2O
3 [
10,
21,
22].
However, mixed oxide carriers could undergo deactivation due to the formation of less reactive phases such as manganese-rich spinels of Fe-Mn systems [
23]. Thus, a vital part of designing new OCs is upgrading their stability. It could be achieved, for example, by mixing the active phases of OC with an inert material (support). Supporting phases are responsible for the task of improving mechanical and chemical stability. Furthermore, the support phase could be inert or can react with the active phases of OCs, occasionally leading to improving or aggravating their properties [
21].
Mn-Fe systems have previously attracted considerable attention from scientists, because of promising CLC and CLOU properties [
19,
24,
25,
26]. Both manganese and iron oxides are characterized by low cost, and they are also relatively safe for the environment. However, some studies showed that they exhibit fairly low stability while being used as OC carriers during continuous operation in fluidized bed CLC reactors [
19]. Other studies showed a common practice to improve those OCs characteristics by, for example, an application of additional inert phases that could positively affect their mechanical and chemical stability [
21,
26].
Titanium oxide, due to its relatively low price, seems to be a promising support material. Although it tends to react with both manganese and iron, some reaction products, such as an ilmenite (FeTiO
3), could profitably exhibit promising CLC capability with both gaseous and solid fuels. It could be also obtained by oxidation of iron (III) titanate (Fe
2TiO
5) [
27,
28]. Moreover, the appearance of ilmenite phase could strengthen the mechanical durability of OC. Some papers discussed the presence of MnTiO
3, i.e., a product of manganese and titanium oxide reaction showing a poor reactivity [
29]. On the contrary, it has been also reported that titanium oxide can improve releasing oxygen from, for example, manganese-rich bixbyite and spinel phases by forming MnTiO
3 and low content manganese spinel phases [
30]. That effect is particularly interesting since it showed that manganese-rich spinels are characterized by poor reactivity and CLOU properties [
7,
20]; thus, the addition of titanium oxide into manganese-rich oxygen carries could further improve their characteristics.
Due to the promising properties such as stability during multiple working cycles and resistance to the influence of ash coming from combustion products, mixed Fe-Mn-Ti systems were recently studied as promising materials for CLC and as efficient materials for biomass and solid fuel combustion. They also have been reported to have ferromagnetic properties, which could be useful for future industrial applications as an option for recovery reacted OC particles from ash [
5,
29,
31].
In this work, Fe-Mn OCs (containing Fe
2O
3 20, 30, 35, and 50 wt.% and MnO
2 of 65, 55, 50, 35, and 85 wt.%) with addition of 15% wt. TiO
2 were synthesized by mechanical mixing and calcination. Their CLC properties were tested with the application of model gaseous fuel 4% H
2 and thermogravimetric analyzer period, The aim of this work is to broaden our knowledge of bimetallic Fe-Mn OCs and natural continuation of our research on supported low cost oxygen carriers with CLC and CLOU properties [
14]. Hydrogen is an environmentally friendly energy carrier derived from biomass or coal [
32]. Hydrogen is also a part of the synthesis gas, which can be used as a gaseous fuel for CLC for power generation [
13]. Thus, it was applied in this work to examine the reactivity of synthesized Fe-Mn based OCs. Furthermore, H
2 was used to explore the oxygen transfer capacity of the materials under severe reducing conditions [
6,
13]. This study aims to evaluate the redox reactivity in the function of temperature of the Fe-Mn-Ti oxide family.
The results of this study will greatly contribute to chemical looping combustion bimetallic oxygen carriers knowledge.
2. Results
2.1. Phase Composition of the Ceramics
Analysis of the obtained diffractograms, shown in
Figure 1, indicates that the ceramics were well crystalized materials. The samples F20M65–F50M35 synthetized with both iron (III) oxide and manganese (IV) oxide showed the presence of bixbyite and mixed iron-manganese titanate phases.
Manganese (IV) oxide has been reported to undergo reduction into Mn
2O
3 in temperatures above 500 °C, and Mn
3O
4 in temperatures over 900 °C; while Mn
2O
3 is considered the most thermodynamically stable at lower temperatures, although reoxidation of Mn
3O
4 in temperatures above 800 °C occurs very slowly [
33].
Mixed iron manganese oxides were present in all bimetallic OCs; however, they differ in terms of manganese content. For example, for the F20M65 sample, it was specifically (Fe0.2Mn0.8)2O3 detected with ICSD 264710, and for samples less rich in Mn samples, from F30M55 to F50M35, the iron manganese oxide of (Fe0.42Mn0.58)2O3 type was detected. Furthermore, in the F50M35 sample containing 35 wt.% of MnO2, the iron manganese trioxide of (Fe0.42Mn0.58)2O3 type, iron titanate Fe2TiO5, mixed iron (II) manganese (II) titanate Fe0.6Mn0.4TiO3, and iron oxide Fe2O3 were detected. Although the mixed iron–manganese titanate (Fe0.6Mn0.4)TiO3 in XRD diffractogram peaks partially cover iron (III) titanate and bixbyite peaks, the presence of that phase in the fresh F50M35 sample and reacted samples was confirmed by using the EDS method.
The diffraction peaks clearly corresponded with those oxides, with the ICSD card number of 154347, 51225, 247551 and 33643, respectively. In the case of fresh F20M65 sample, unreacted rutile was detected; meanwhile, in the reacted samples, instead of iron titanate detected in the more abundant in iron samples, manganese titanate was formed (
Table 1). Formation of manganese titanate instead of iron titanate in rich manganese Fe-Mn-Ti systems has been previously reported [
30]. The reference ceramics M85 showed the presence of two forms of manganese oxide, i.e., manganese (III) oxide Mn
2O
3, and hausmannite Mn
3O
4, with some perovskite type manganese titanate MnTiO
3 (
Table 1).
Figure 1 shows the XRD patterns obtained at RT for the five oxygen carriers of the Fe-Mn-Ti-O family. Furthermore, phase identification was also carried out for the used materials. Those carriers after reaction with fuel were processed with a stream of air. X-ray powder diffraction patterns recorded for both unreacted and reacted samples over teens of redox cycles are shown below.
As the mechanical strength is one of the key parameters shaping the functional properties of the obtained mixed compounds, for this purpose 30 grains of each composition were subjected to crushing strength tests in this work.
Figure 2 shows the crushing strengths obtained for fresh and reacted OCs samples. The average value obtained for 30 grains of each composition could be arranged in the following row: 0.98 N (F20M65) > 0.94 N (F30M55) > 0.91 N (F35M50) and 0.89 N (F50M35). Those values are still acceptable since they are close to the required 1 N. In the paper [
29], for some Fe-Mn-Ti materials, the mechanical strength values were in the range of 0.7 to 0.9 N; for example, for F17MT51 and F33MT9 it was 0.7 N, while for F46MT9 and F61MT9 it was 0.8, and 0.9 N, respectively. For those samples, both higher (50 and 51 wt.%) and lower (9 wt.%) titania content was used in contrast to this paper. Contrary to what has been reported by [
29], these results indicate different patterns. Although this study also showed Fe-Mn-Ti OCs, there is a significant gap between mechanical strengths trends. For example, a slight increase in crushing strength was observed with the increase of Fe content, even if the amount of Fe was doubled as for F17MT51 and M34MT50 (increase from 0.7 to 0.8 N) as reported; which is opposite to our findings, showing the reverse trend. This can be explained by the utilization of different amounts of inert material 15 wt.%, and by applying higher manganese oxide (IV) addition, which is 65–50 wt.%, leading to substantially different final phase compositions.
Based on the analysis of
Figure 2, where the crushing strengths were obtained for fresh materials, it is clear to see the presence of three extracted groups of mechanical strength, with the maximum at 0.9, 1.2 and 1.5 N. This observation correlates well with the phase composition data summarized in
Table 1. The obtained perovskite structure of iron manganese oxides and iron titanate may be responsible for improved strength. Below those ranges, there are very few grains with low strength of about 0.5 N, and they may be attributed to the presence of iron oxide or rutile rich phases. On the other hand, for the reference sample, there is a group of grains with a strength of 1.3 N and 1.6 N, which correlates well with hausmannite and manganese (III) oxide occurrence. There are also 8 grains showing 1 N strength, which are attributed to the evidence of manganese titanate MnTiO
3.
In general, the highest possible value of the compressive and abrasive forces acting on the OC grains during the CLC process is desirable. Following the experiments for fresh samples, additional measurements were carried out for reused samples. After multicycle CLC tests, the samples were subjected to the compressive forces again to evaluate the complete OCs performance.
Figure 2 shows that the measured strength values decreased slightly for all OCs, both for the mixed Fe-Mn-Ti and for the reference monometallic Mn-Ti sample.
2.2. Examination of the Ability to Transport Oxygen
Reactivity of Fe-Mn-Ti oxides was studied by TG method. This is shown in
Figure 3. A small amount of a sample of ca. 50 mg of each composition was placed in alumina crucible and heated with a 20 K/min rate to 800 °C temperature. As the required process temperature was reached, alternatively, fuel and air were used for the fuel combustion stage and oxygen carrier material regeneration for 3 cycles at each temperature. As the results of the test are presented in
Figure 3, it can be seen that all the obtained oxide materials take part in fuel combustion, following by reduced OCs reoxidation within a wide temperature range, which is 800–1000 °C.
The data were used further for calculation of the reaction rates and for evaluation of the oxygen transport capacity.
A principal factor for OC choice for industrial applications is the oxygen carrier’s ability to transport oxygen needed for fuel combustion. Therefore, the oxygen transport capacity was assessed based on the prediction and experienced from thermogravimetric examinations. Theoretical oxygen transport capacity of as-synthesized materials was calculated based on the amount of active compounds such as manganese and iron oxide constructing the OCs. Those theoretical reflections are considered, depending on the process conditions, different final reduction states, and are shown in
Table 2. For bimetallic MnO
2-Fe
2O
3 oxides, both CLC and CLOU properties may be expected. This is because the existence of manganese oxide may contribute to both CLOU and CLC capacity; while the presence of iron oxide in OC contributes to CLC capacity.
The produced samples contain different rations of Fe:Mn oxide, which is 20–50 wt.% of Fe2O3, and a parallel 65–15 wt.% of MnO2, for the F20M65–F50M15 family. Hypothetically, if reduced to the metallic form, the ceramics should evolve from 27.9 up to 29.9 wt.% of free oxygen. Due to the wide range of concentration, both metal oxides can significantly contribute to the capacity. In a case where both oxides are partially reduced, in this scenario, 4.9–25.9 mass % of oxygen can be transferred. The reference sample, containing only one active metal oxide, i.e., MnO2, can release from 7.8 to 31.3 wt.%.
These theoretical maximum extents of mass reduction data will be used and compared further with the experimentally observed capacities.
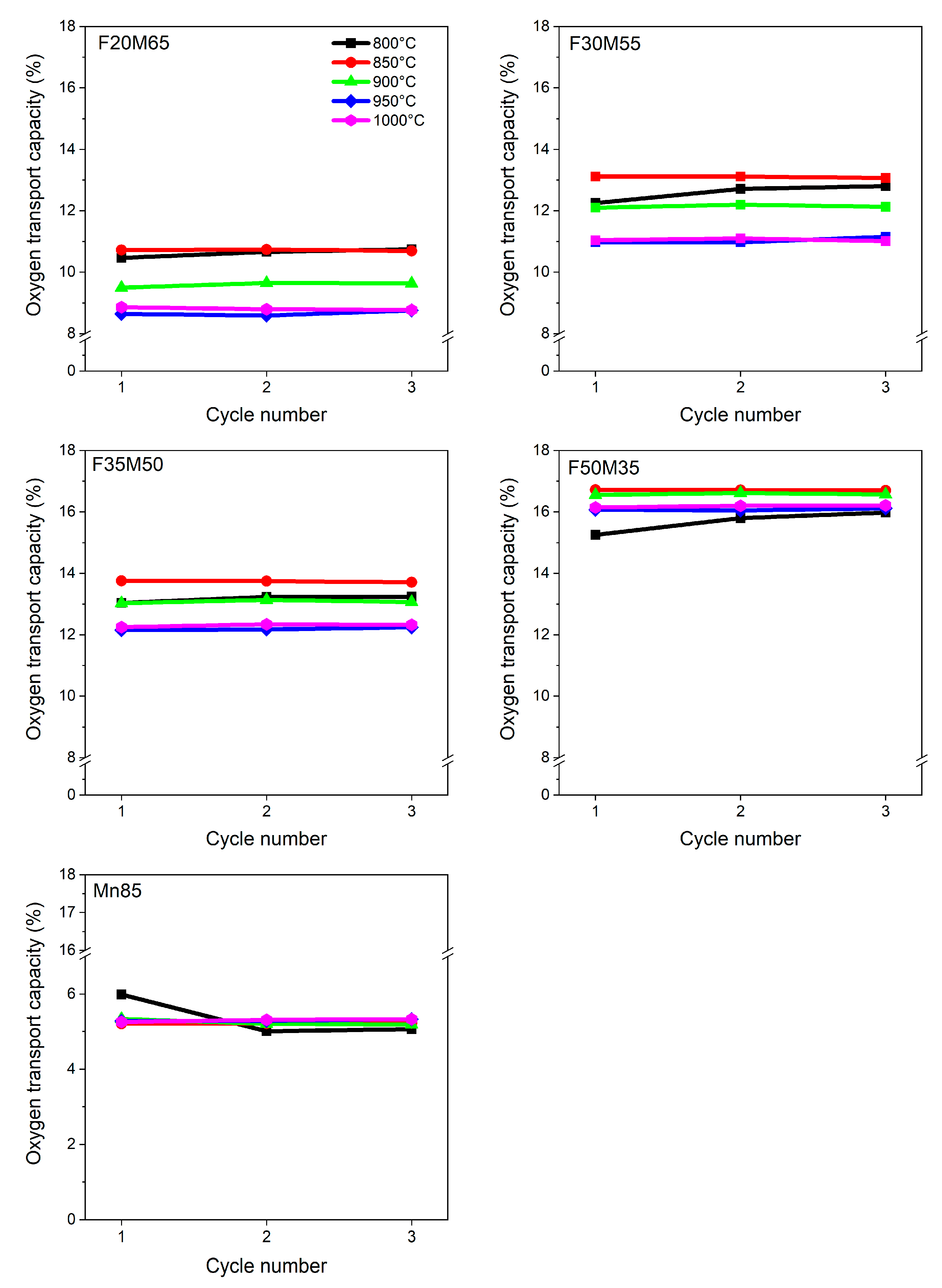
Figure 4 shows the experimental oxygen capacities obtained for Fe-Mn-Ti oxide family members. They were observed in the 800–1000 °C temperature range. The obtained data for iron–manganese materials containing 20–50 wt.% of Fe
2O
3 showed that the experimental oxygen transport capacities determined via TGA were lower than the predicted maximal values (
Table 2). At a temperature of 800 °C, the F20M65 sample can deliver 10.5 wt.% of O
2. With an increased amount of iron oxide, this resulted in an increase of capacity to 12.8, 13.2, 15.9 for F30M55, F35M50, F50M15 samples, respectively. Additionally, the positive maintaining capacities through cycling were detected. On the other hand, the monometallic sample shows the lowest capacity which is estimated at ca. 5.0 wt.%, which is far from the predicted values (
Table 2).
When increasing temperature, the stable oxygen evolving from the oxides structure to the fuel was observed and maintained as stable with the cycling number. This behavior supports the beneficial use of bimetals instead of one. However, further increase of temperature leads, first, to a small increase of capacities for manganese-rich samples with formal composition F20M65, F30M55 and F35M50, reaching maximal values at 850 °C, and at that point, slightly decreasing. Additionally, the F50M15 sample shows different behavior. As a sample is capable to transport more oxygen than the other bimetallic carriers, here, the capacity reaches a maximum at 850 and 900 °C temperatures, which was experimentally proven to be ca. 16.7 wt.%.
Interestingly, for all mixed iron manganese oxides, stable performance is observed within increasing cycling number (1–3 cycles). Moreover, some activation between the 1st and 2nd cycle is observed for ternary compounds at the very beginning of cycling at 800 °C. This behavior may be due to the variety of phase compositions. For F20M65, F30M55 and F35M50, OC active form of oxides are both (Fe
0.42Mn
0.58)
2O
3 (bixbyite) and Fe
2TiO
5 (iron (III) titanate); while the different redox behavior enabling more oxygen to release detected for F50M15 OC may be explained by the presence of active iron manganese trioxide (FeMnO
3) aside from iron (III) titanate (Fe
2TiO
5). Additionally, the sample contained also mixed iron (II) manganese (II) titanate (Fe
0.6Mn
0.4TiO
3) and finally, some iron oxide (Fe
2O
3). Experimental capacities increased with increase of temperature from 800 °C to 850 °C for all oxygen carriers as predicted. However, the most striking observation to emerge from the analysis was the decrease of capacities for some compositions above 850 °C, while for others, over 900 °C. This is a rather surprising result. In the first group, compositions F20M65, F30M55 and F35M50 show such behavior; while the F50M15 sample shows a decrease of oxygen capacities above a 900 °C temperature. These findings also confirm those of earlier studies for other group of FeMn oxides [
7,
20]. The decreases observed in this work are certainly due to the multiplex phase compositions of obtained samples (
Table 1). One possible explanation for such an observation is that both bixbyite, iron (II) manganese (II) titanate reactivity increases with an increase of temperature. The different behavior of the F50M15 sample is due to the additional presence of Fe
2O
3, enabling higher capacities to be reached; while a decrease of capacities may be due to the unfavorable presence of high-manganese spinels owning some restrictions. Similar trends have been reported by Ksepko et al. in their work related to the FeMn/Zr carriers [
7]. It was proven in the work that high-manganese content spinel may initially show partial and finally, poor reactivity during CLOU tests.
For pure MnO2/TiO2 OC, some decrease in cycling number (1–2) is observed at 800 °C. For M85 at 800–1000 °C, the most potential reduction stage according to the experimentally proven 5.0 wt.% and compared to the predicted capacity 7.8 wt.% is the partial reduction of MnO2 to Mn2O3. For mixed metal OCs, observed capacities were well suited for Fe2O3 + MnO2/FeO + Mn3O4 reduction for F20M65 material; while observed maximal extents of reduction fit well with Fe2O3 + MnO2/FeO + MnO and Fe2O3 + MnO2/Fe3O4 + MnO reactions for F30M55, and Fe2O3 + MnO2/FeO + MnO for F35M50. Samples containing the highest amount of Fe, i.e., F50M15 well match with Fe2O3 + MnO2/FeO + Mn reduction.
The relatively high oxygen capacities obtained and proven via TGA in this work are advantages. Primarily, they enable a lower bed inventory to be used. Subsequently, they allow one to decrease OC circulation rates in industrial CLC applications.
2.3. Reactivity with Fuel and Air and Thermal Stability
Evaluation of the reactivity of the synthesized OC ceramics towards fuel and air was carried out through TGA experiments at 800–1000° C temperature suitable for the chemical looping combustion processes. The characterization of the oxygen carriers using thermogravimetric method was performed to learn about:
- i.
the effect of oxygen carrier chemical composition on its reactivity,
- ii.
the effective temperature for efficient gaseous fuel combustion when Fe-Mn-Ti oxides are applied,
- iii.
thermal stability and resistivity of the ceramics.
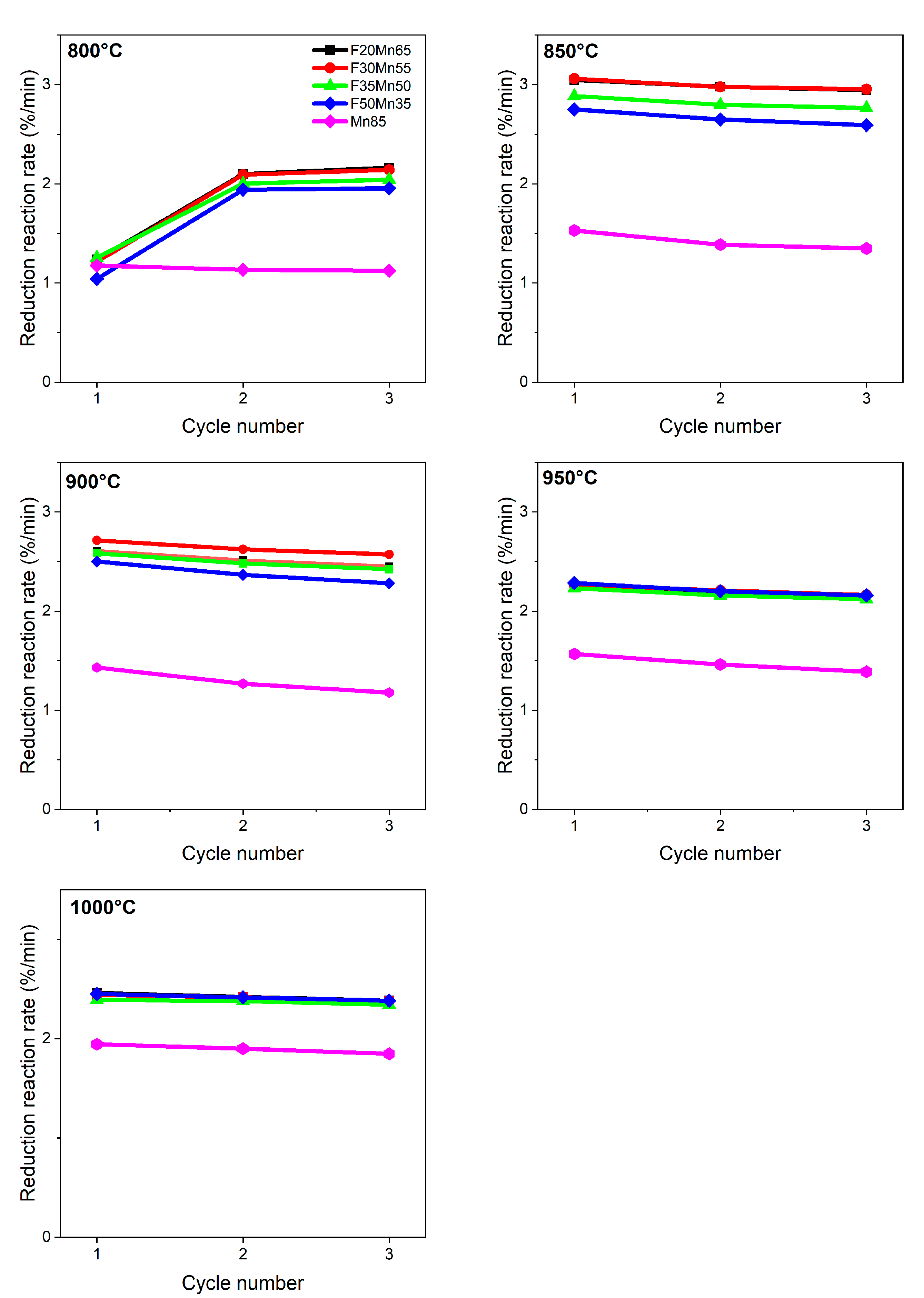
Reduction reaction rates as a function of cycling number for specific OC compositions are shown in
Figure 5, at each temperature. At 800 °C, all doped samples show similar behavior. At the first cycle, the highest reduction rate was observed for F20M65 material, then it slightly increased as the amount of manganese content increased in the sample. Between the first and second cycle, some clear increase in the rate by 68% was observed for all samples. It means some activation occurs at this stage. For the M85 sample, the lowest rate 1.2 %/min was observed and slightly decreased with a cycle number. When the chemical looping hydrogen combustion process temperature was increased by 50 °C, a further increase of rate was observed. For example, for the sample with the lowest Fe content, i.e., F20M65, ca. 2.5 times faster combustion processed at 850 °C, while for the sample with the highest Fe content, i.e., F50M35, the rates were 2.6 times faster than that observed at 800 °C. However, further sample heating led slightly to the rates decreasing, and finally, both at 950 °C and 1000 °C, there were no significant differences observed for the doped samples. For the reference material, i.e., M85, in general, the reduction reaction rates were of similar value at 850–1000 °C, and simultaneously, significantly lower values were achieved compared to doped materials. Additionally, it can be concluded that the introduction of the second metal to OC helped and positively influenced the rates. Based on the behavior of the samples, the best temperature range for Fe-Mn-Ti working duty is 850–900 °C.
Regeneration (
Figure 6) of previously reduced in looping process OCs processed significantly faster than the reduction reaction (
Figure 5). At 800 °C, the fastest reactions 5.48%/min were for F50M35 composition and slightly decreased when the amount of Fe decreased in the sample. The slowest oxidation was observed for undoped M85 material. When the regeneration temperature increased to 850 °C, reaction sped up evidently. As a positive result of temperature, 10.21%/min rate was observed for the F50M35 composition, which is almost doubled compared to 850 °C, and those rates maintained unchanged through a wide temperature range. Regeneration reactions were stable at all temperatures tested. The reference M85 showed both the lowest reduction rates and the lowest regeneration rate, which corresponds well with other works [
9].
2.4. Characterization after Redox Tests
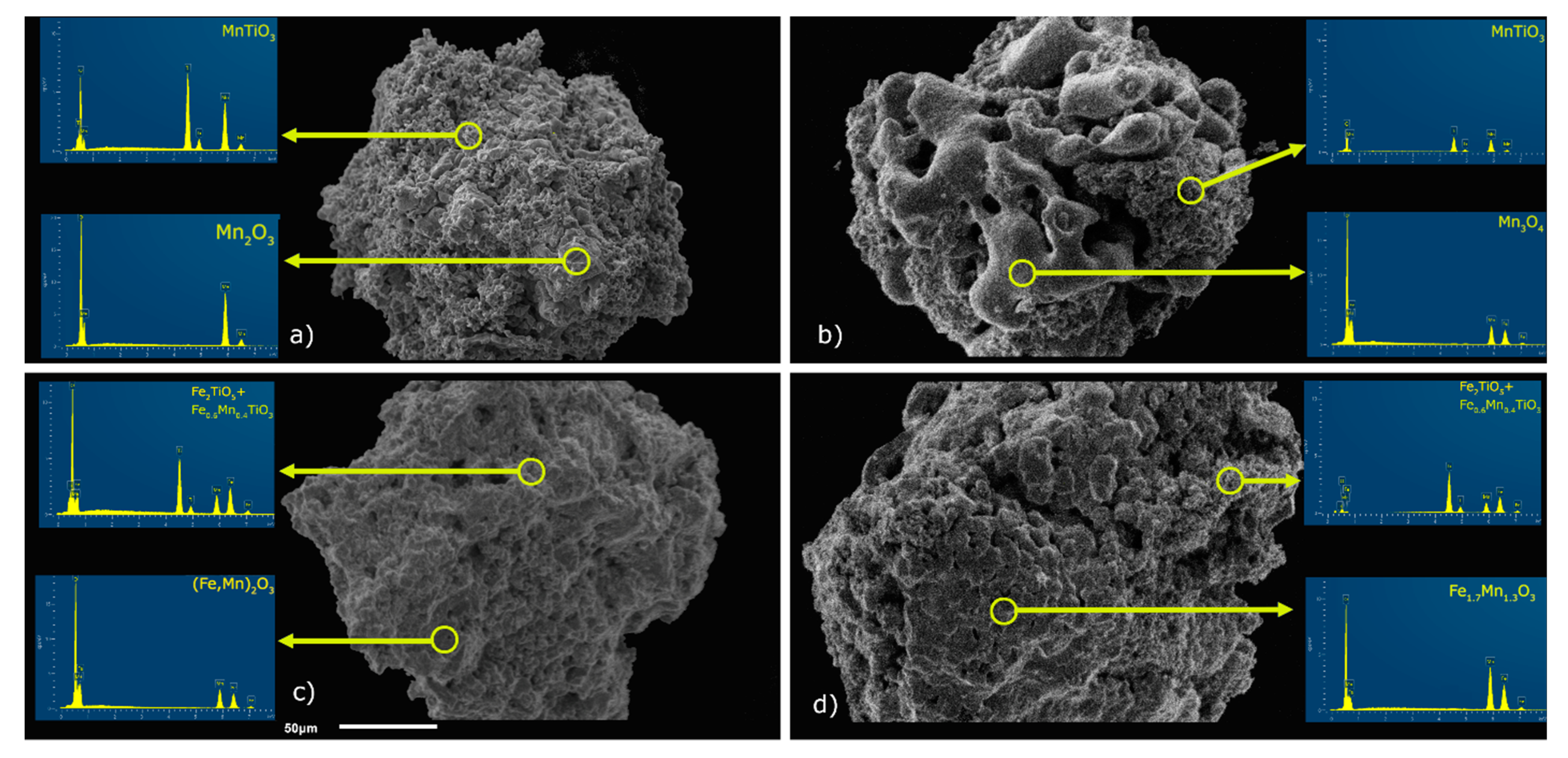
The obtained Fe-Mn-Ti-O samples were subjected to mechanical strength test after cycling testing, as mentioned earlier, which is also shown in
Figure 2. Comparing these results with those for as-synthesized samples, it can be concluded with no doubt as a decrease in mechanical strength. Now, for 30 grains of each composition, the strength is moved to lower values for all of the carrier compositions. Their values oscillated between 0.5 and 0.95 N within the two subgroups. The deeper insight may clarify the reasons of such behavior. Some new phases were formed during cycling redox reactions in OC samples (see
Figure 1 and
Figure 7). EDS analysis exhibited the existence of mixed Fe-Mn titanate fuzzy structures on the surface of OC grains (
Figure 7). Meanwhile, bixbyite and spinel-like phases were apparent as compact crystalline forms. In the case of fresh samples, they were partially covered by titanate. or had the form of compact blocks, clearly visible on the surface of the reacted samples (
Figure 7 and
Figure 8). Spinel and perovskite oxides, specifically iwakiite (Fe
1.3Mn
1.7O
4) and manganese titanate (MnTiO
3), are the new phases that occurred in the samples, as shown in X-ray diffractograms of the reacted samples. Moreover, some pure iron oxide forms have appeared in F20M65, F30M55 and F50M35 samples. In F20M65 manganese-rich samples, magnetite (Fe
3O
4) was confirmed, while for manganese-poor F50M35 ceramics, it was precisely the iron oxide (Fe
2O
3) detected. For the reference sample, the X-ray diffraction peaks can be attributed to the presence of hausmannite (Mn
3O
4) and manganese titanate (MnTiO
3), since the manganese (III) oxide, observed in the fresh samples, completely transformed into hausmannite. The presence of iwakiite in manganese-rich samples and bixbyite to spinel transition in reacted Fe-Mn OC systems were also reported in our previous works [
7,
14].
In the case of the M85 sample, a complete conversion of Mn
2O
3 to hausmannite (Mn
3O
4) was observed. Although Mn
2O
3 is considered to be the most thermodynamically stable, manganese oxide reoxidation of hausmannite occurs very slowly at high temperatures. It has been experimentally proven that after 2 h of continuous oxidation in 800 °C, the obtaining of Mn
2O
3 was not possible [
33]. Moreover, continuous reduction oxidation cycling could lead to increased crystallinity of the sample [
33], which was also reported during the current research (
Figure 7 and
Figure 8 j). As such, the poorer mechanical strength of the used samples is a result of phase transformation mainly to iwakiite (Fe
1.3Mn
1.7O
4) and iron oxides which are now the dominant phases in iron–manganese doped samples (
Figure 1). Furthermore, the reacted samples exhibited a partially molten surface with visible big pores (
Figure 8). The appearance of those pores could be partially responsible for the lowering of OCs mechanical durability presented in
Figure 2.
As specified in previous work [
14], the high content of iron is positively affecting the OC reactivity. However, when comparing the redox behavior of Fe-Mn OCs doped with two different inerts such as ZrO
2 (published works) and TiO
2 (this work), those carriers with titanium dioxide addition show better higher temperature stability. Correspondingly, in this study, the lesser amount of spinel-type phase and substantially more of bixbyite was detected in the reacted samples (
Figure 1). An addition of titanium oxide, that is able to react with both of the active phases such as MnO
2 and Fe
2O
3 of the examined OCs [
5,
21,
34], could positively activate manganese-rich spinels. Similar trends have been reported by [
30] in their work. As a consequence of TiO
2 addition to Fe-Mn oxides, fruitful regeneration of Fe-rich spinels to bixbyite structure may be gained [
7,
23,
35].
3. Materials and Methods
3.1. Synthesis and Materials Quality Examination
Fe
2O
3-MnO
2 supported on 15 wt.% TiO
2 samples with different chemical compositions OCs were prepared by using mechanical mixing method (
Table 3). High quality powders of Fe
2O
3 and MnO
2 (<99%, Sigma Aldrich, Steinheim, Gemany) were mixed roughly with inert TiO
2, and then deionized water was added to obtain a paste. The paste was dried at 115 °C overnight, crushed and calcined at 1050 °C in air for 10 h. Ten wt.% of graphite was added to the mixture to improve the porosity/surface area of the samples. The graphite is oxidized to CO
2 during high temperature calcination in air and is well known to be a porosity increasing agent. After cooling, the procedure was repeated. In other words, the sample was crushed and thoroughly mixed with water and graphite addition again. It was then calcined at 1050 °C in air for 10 h. Calcined samples were subjected to sieving to isolate the desired fraction of 125–180 µm. As a result, five different oxygen carriers were obtained (
Table 3), The symbol F20M65 refers to 20 wt.% Fe
2O
3–65wt.% MnO
2 and 15wt.% TiO
2 samples, etc. To examine the effect of manganese addition, different concentrations of Mn oxides were prepared and finally, the M85 sample containing only MnO
2 and TiO
2 was obtained.
3.2. Phase Composition
XRD patterns of “fresh” Fe2O3-MnO2/TiO2 oxygen carriers and “used” after reaction with hydrogen and then regenerated with air were collected at room temperature to the X-ray powder diffractometer MiniFlex600 by RIGAKU (Tokyo, Japan). The experiments were carried out at the following conditions: 40 kV, 15 mA, and with filtered CuKα radiation of λ = 1.54056 Å. The diffraction patterns were obtained with 20–80° 2Θ with a step of 0.02°. Phase analysis of the obtained XRD diffractograms was performed using SmartLab Studio II software (ver. 4.3.147.0 (2014) by Rigaku Corporation, Tokyo, Japan) and crystallographic data provided by ICSD (Inorganic Crystal Structure Database) (Karlsruhe, Germany).
3.3. Microstructure
The microstructure of the material surface was examined with the application of a scanning electron microscope instrument, JEOL JSM–6610 LV (Tokyo, Japan). The surface morphology was studied by gluing carbon tape onto the samples; carbon tape was not used in the chemical analysis of the samples. The study was conducted using a low vacuum detector at an accelerating voltage of 15 kV and different magnification of image 50-1000x.
An energy dispersion X-ray spectrometer (EDS) was applied in this study for chemical microanalysis evaluation. Therefore, Oxford Aztec Energy (Abingdon, Great Britain), with an Si (Li) X-ray detector was used. Both the composition of the entire grain surface together with a point analysis of selected grain parts to examine the composition and possible presence of the expected structures was analyzed. EDS analysis was performed using Aztec software (ver 2.1 (2013), by Oxford Instruments, Abingdon, Great Britain).
3.4. Thermogravimetric Analysis (TG)
Thermogravimetric experiments were conducted using a thermal analyzer (STA 409 F5 Jupiter by Netzsch Selb, Germany) which was coupled to a quadrupole mass spectrometer QMS 403 Aëolos Quadro (Netzsch Selb, Germany). The OCs’ mass changes were measured isothermally. First, the sample was heated in Ar atmosphere with a heating rate of 20 K/min to 800 °C. When the desired temperature was reached, the sample was held for about 10 min. and flushed with synthetic air, then the experiment was started with repeated introduction of reducing and oxidizing gases. As soon as the sample was stabilized, the OCs’ particles were exposed to three consecutive redox cycles, conducted at atmospheric pressure to determine their reactivity.
In the CLC measurements, for each test, a sample weighing ca. 50 mg was placed in an alumina made crucible. A mixture of 4% H2/Ar was used for the reduction, while 20% O2/N2 was used for the oxidation reactions. The reduction time was 45 min. and the oxidation time 20 min. The reaction gas flow rates were 100 mL/min. Between the fuel combustion stage and OC regeneration stage, the TGA chamber was flushed with Ar for 10 min. This was proceeded to ensure avoiding the reduction gases mixing with air. Prior to the main testing, additional examinations were carried out to ensure the quality of the experiments were achieved. The OCs’ mass and gas flow rates, used in the reactivity experiments, were chosen to avoid limitations in the external film mass transfer and inter-particle diffusion. To determine the stability of the carriers, the redox cycles were conducted at atmospheric pressure. To understand the effect of temperature, a reactivity test was carried out at 800–1000 °C. The reaction rates were calculated by differentiating the mass data with respect to time.
3.5. Mechanical Strength
Mechanical strength test was carried out to assess both the strength and durability of the synthesized OC. The Digital Force Gauge Shimpo FG-5000A, by Shimpo Instruments (Kyoto, Japan), model was used. The instrument provides solid force testing within a capacity of 5.0 kg and a resolution of 0.001 kg. For the testing, 30 particles of each composition of the Fe-Mn-Ti-O materials have been analyzed. Both as-synthesized and used materials were subjected to mechanical strength testing.