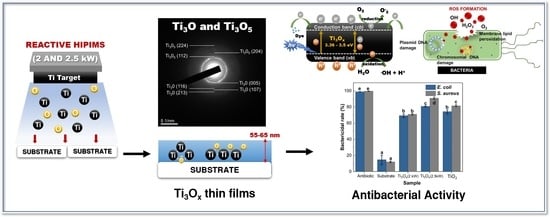
A Study on the Characteristic and Antibacterial Activity of Ti3Ox Thin Films
Abstract
:1. Introduction
2. Results and Discussion
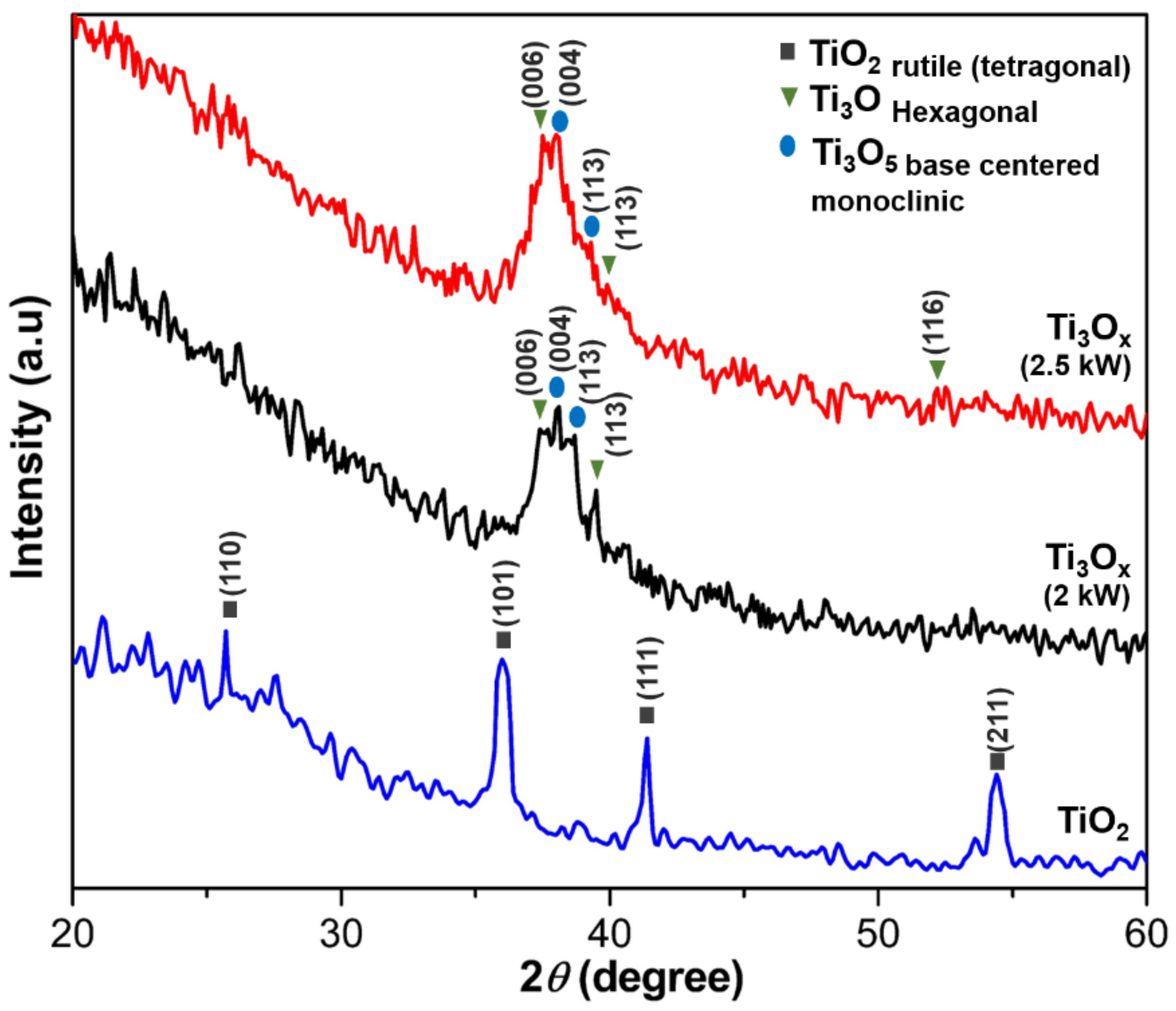
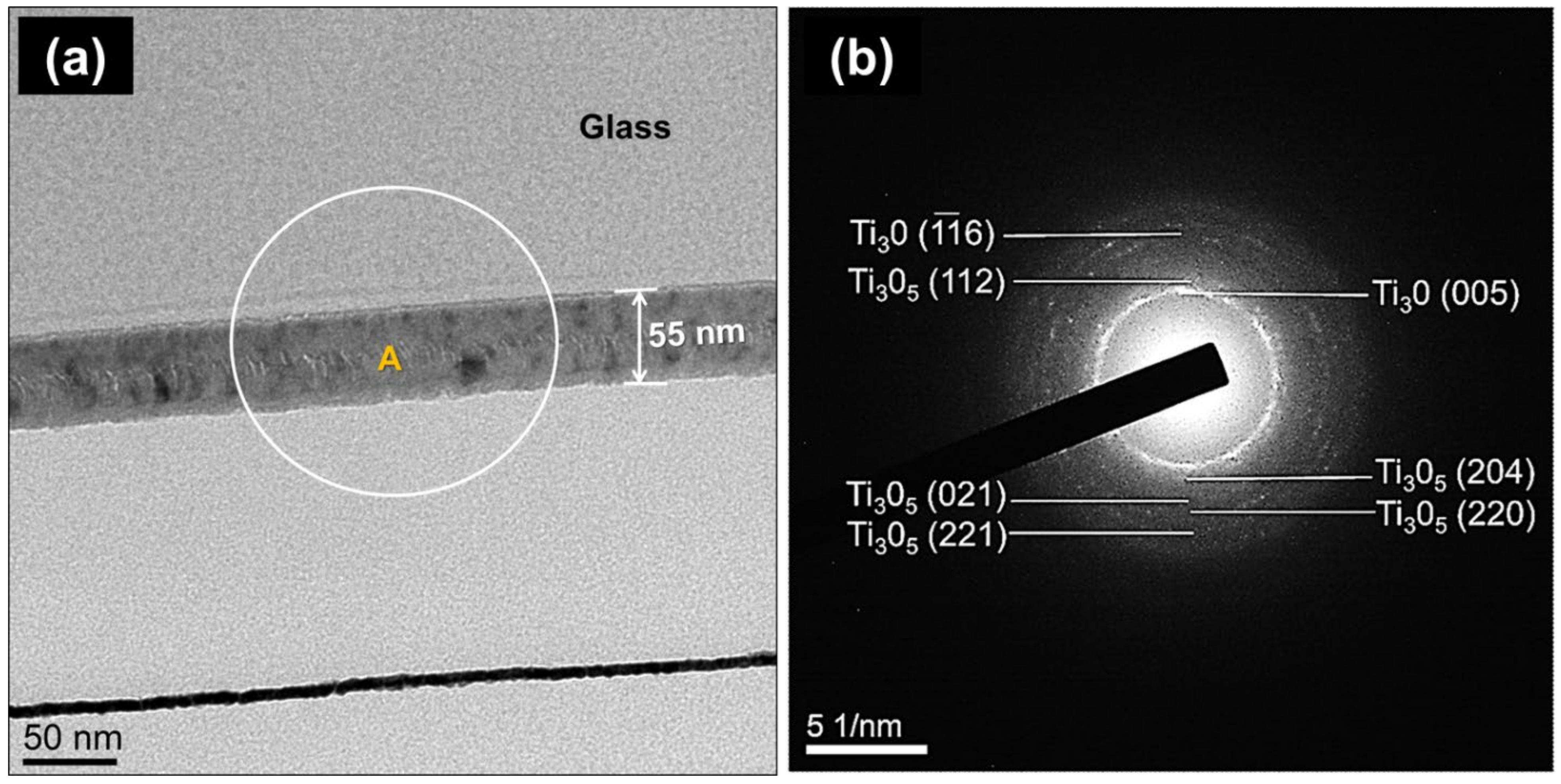
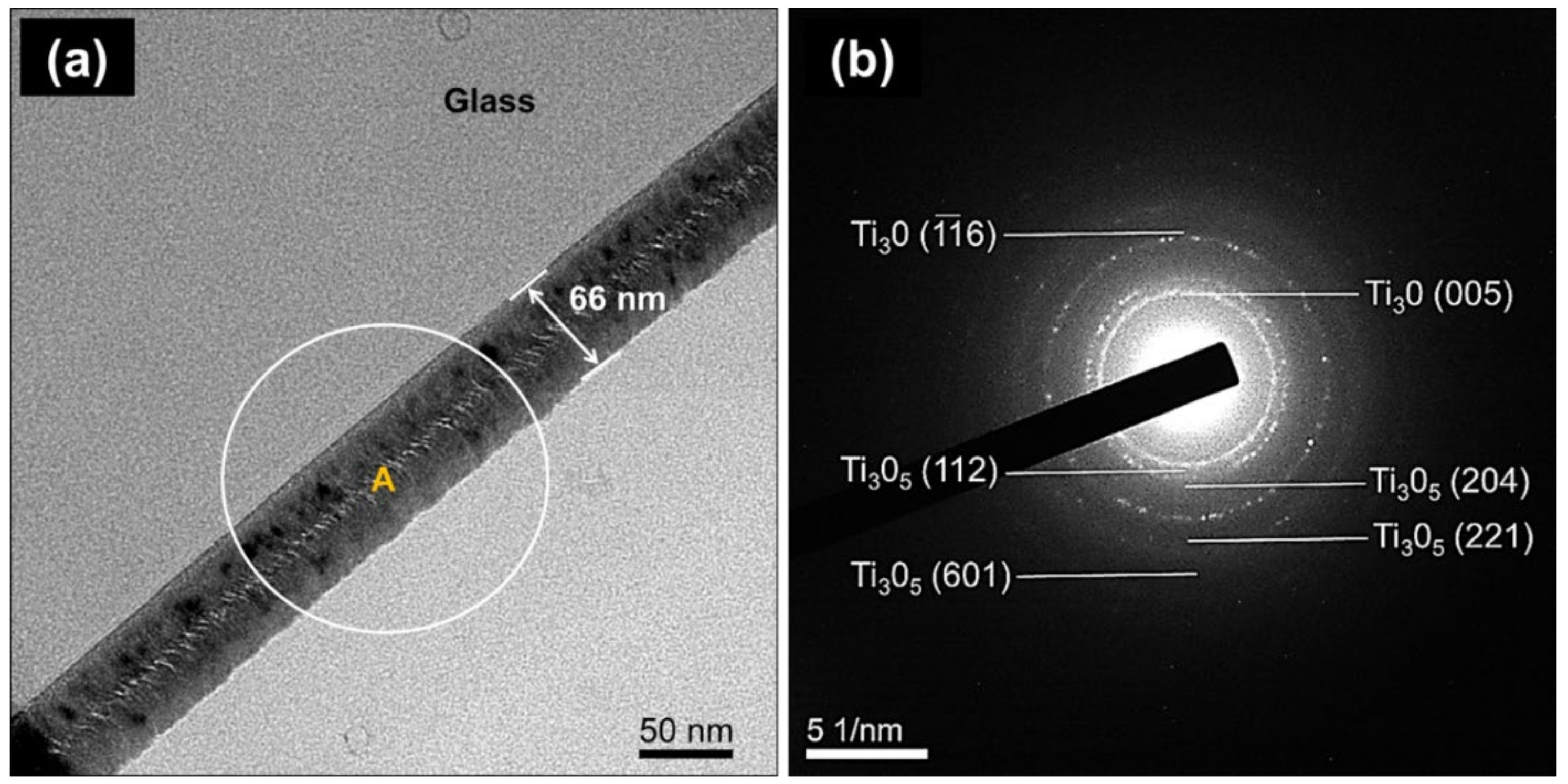
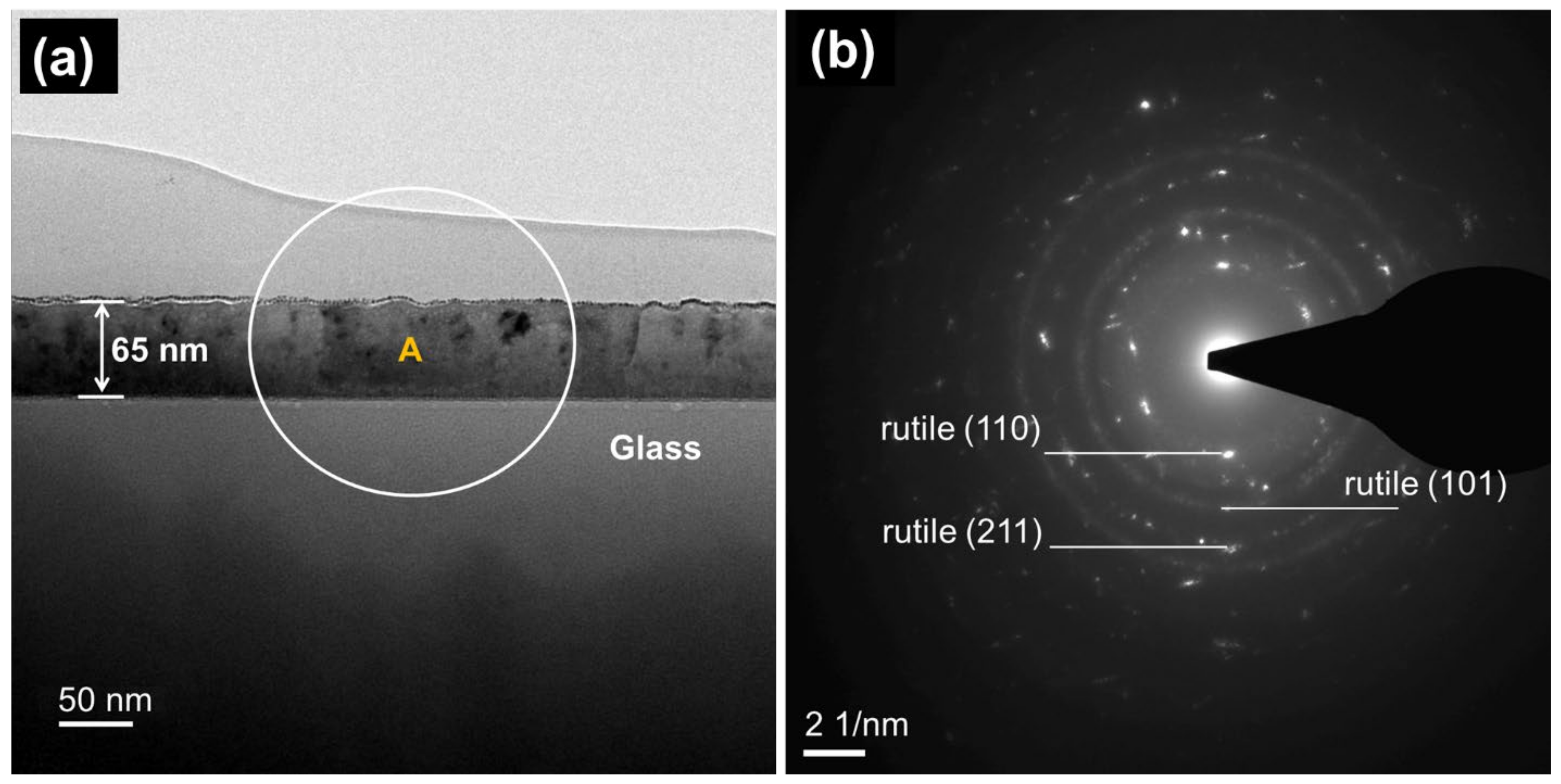
2.1. Phase Structure and Microstructure of Ti3Ox Films
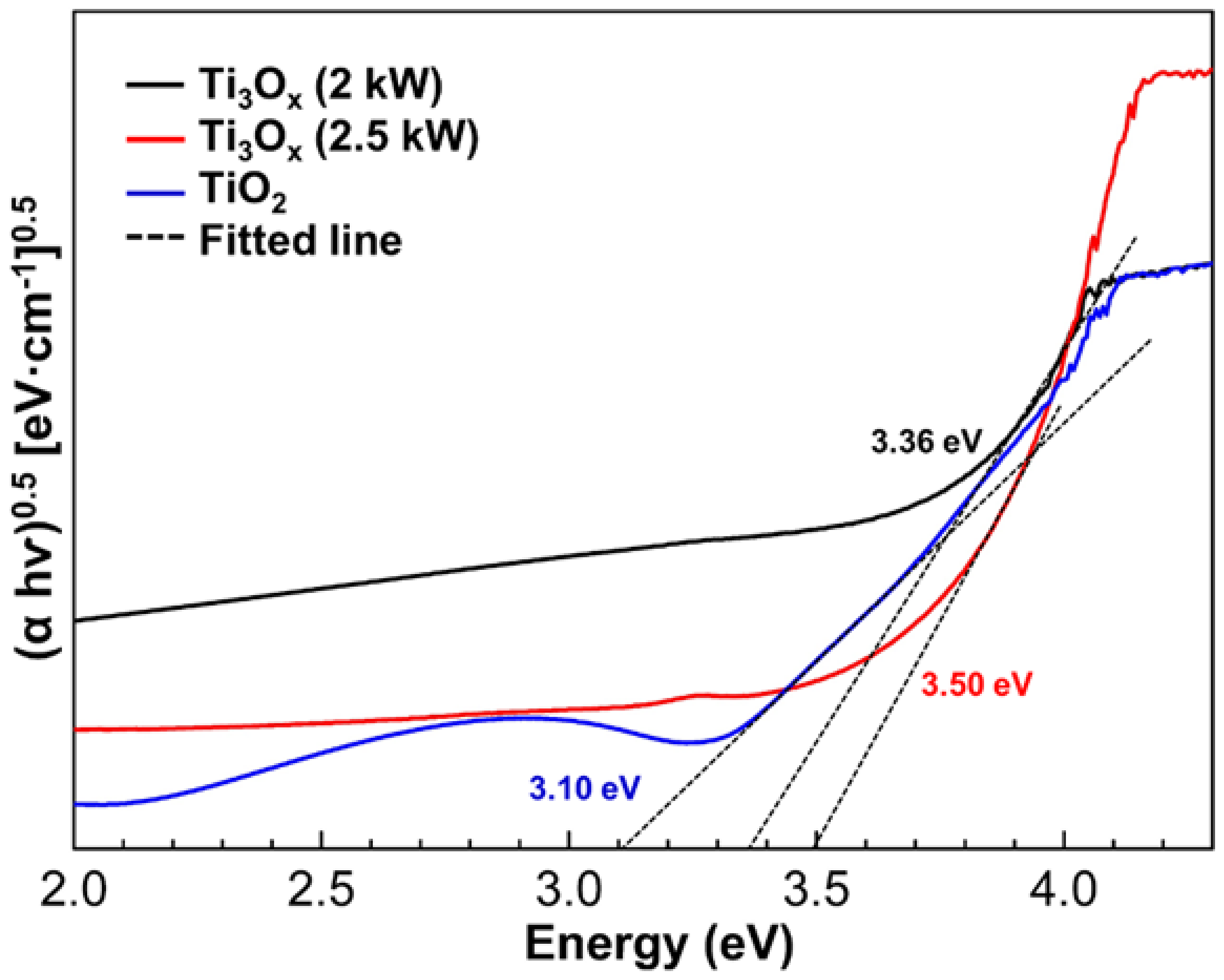
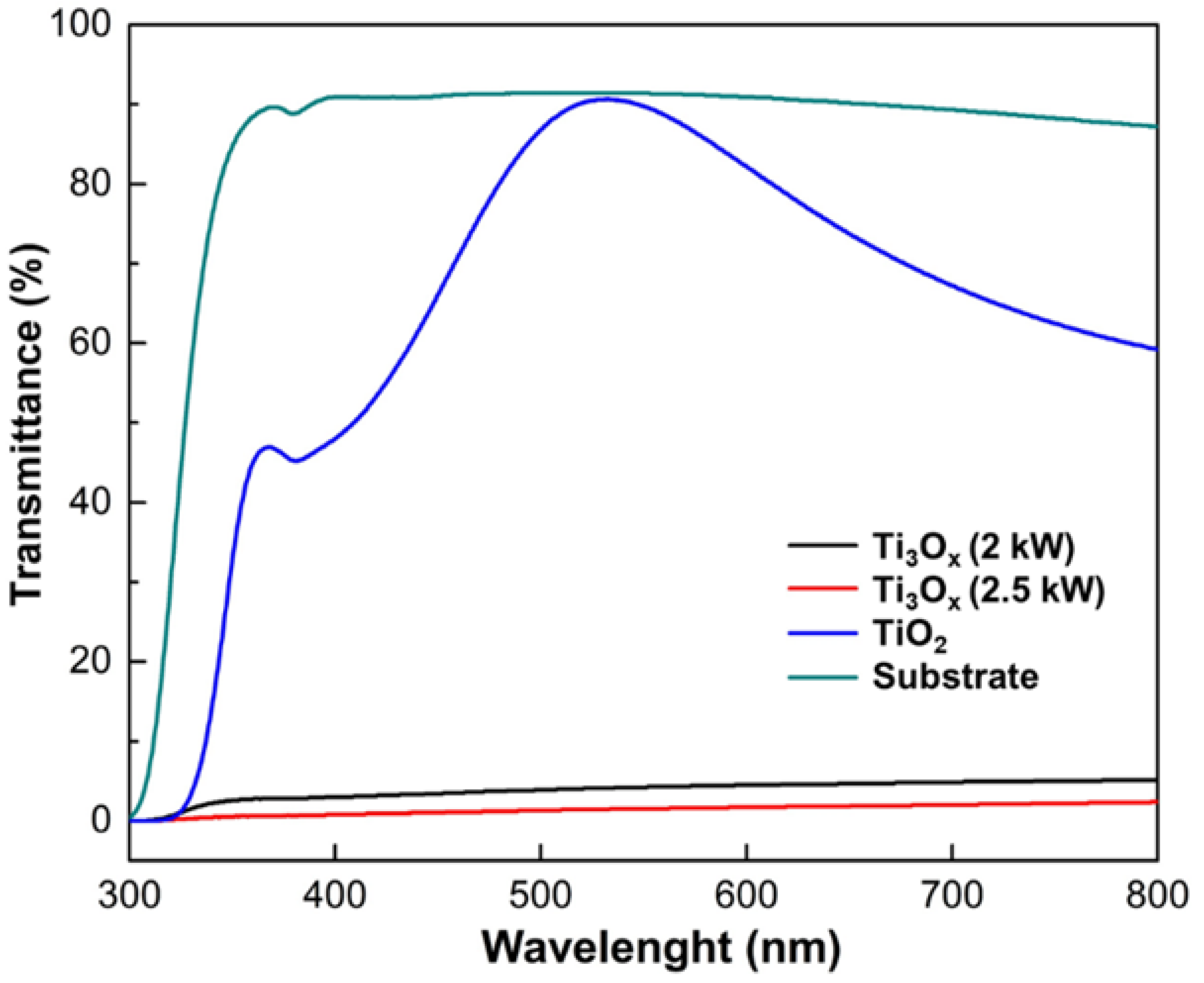
2.2. Optical Properties and Photocatalytic Activity of Ti3Ox Films
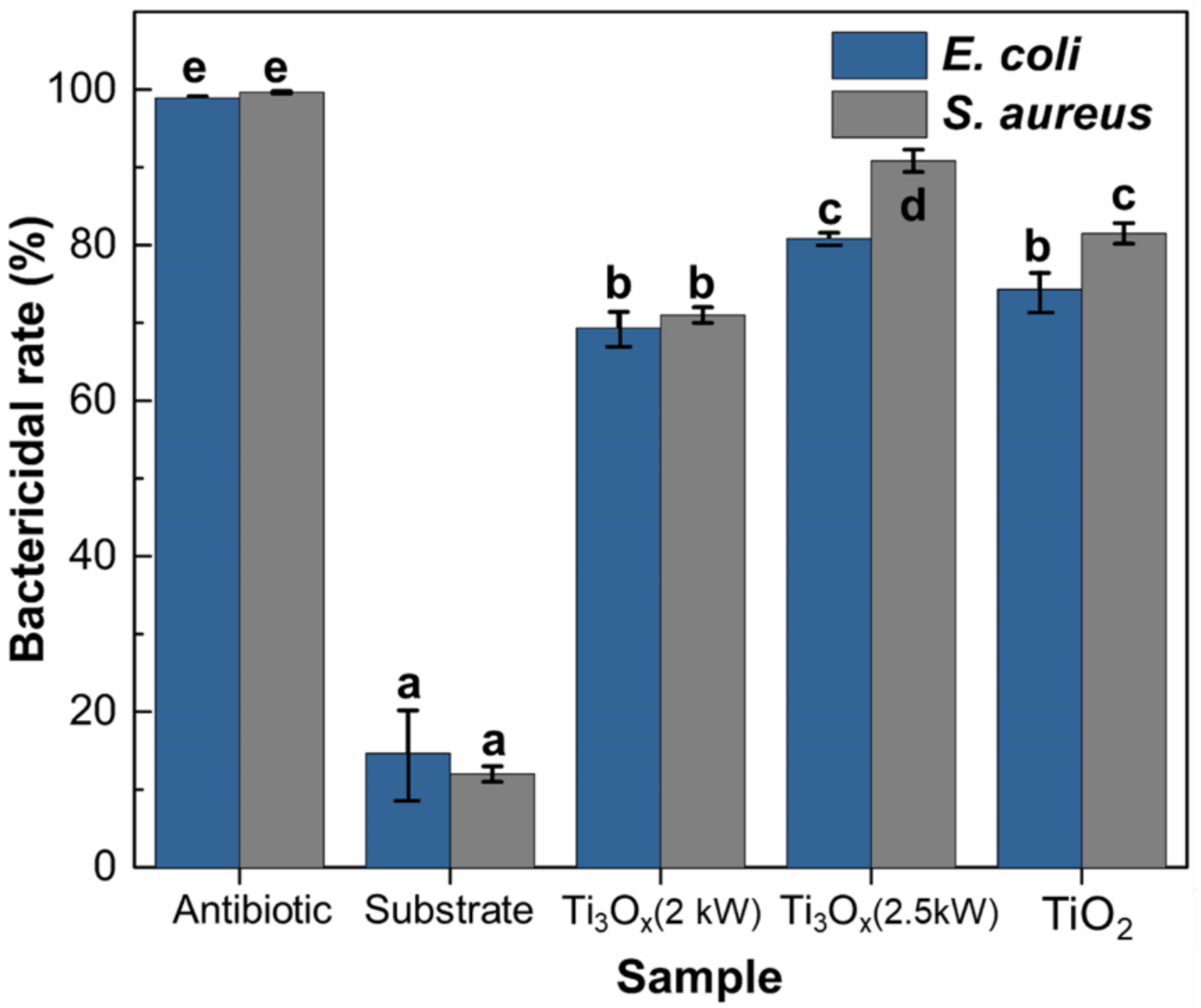
2.3. Antibacterial Activity of Ti3Ox Films
3. Materials and Methods
3.1. Preparation of Ti3Ox Thin Films
3.2. Sample Characterization
3.3. Optical Properties Characterization
3.4. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.Y.; Selvaraj, P.; Senguttuvan, G.; Hsu, C.J. Electro-optical and dielectric properties of TiO2 nanoparticles in nematic liquid crystals with high dielectric anisotropy. J. Mol. Liq. 2019, 286, 110902. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Kathirvel, S.; Pedaballi, S.; Su, C.; Chen, B.-R.; Li, W.-R. Morphological control of TiO2 nanocrystals by solvothermal synthesis for dye-sensitized solar cell applications. Appl. Surf. Sci. 2020, 519, 146082. [Google Scholar] [CrossRef]
- Lee, P.-Y.; Widyastuti, E.; Lin, T.-C.; Chiu, C.-T.; Xu, F.-Y.; Tseng, Y.-T.; Lee, Y.-C. The phase evolution and photocatalytic properties of a Ti-TiO2 bilayer thin film prepared using thermal oxidation. Coatings 2021, 11, 808. [Google Scholar] [CrossRef]
- Lu, Y.; Matsuda, Y.; Sagara, K.; Hao, L.; Otomitsu, T.; Yoshida, H. Fabrication and Thermoelectric Properties of Magneli Phases by Adding Ti into TiO2. In Proceedings of the Advanced Materials Research; Trans Tech Publications Ltd: Zurich, Switzerland, 2012; Volume 415, pp. 1291–1296. [Google Scholar]
- Huang, S.-S.; Lin, Y.-H.; Chuang, W.; Shao, P.-S.; Chuang, C.-H.; Lee, J.-F.; Lu, M.-L.; Weng, Y.-T.; Wu, N.-L. Synthesis of high-performance titanium sub-oxides for electrochemical applications using combination of sol–gel and vacuum-carbothermic processes. ACS Sustain. Chem. Eng. 2018, 6, 3162–3168. [Google Scholar] [CrossRef]
- Xu, B.; Sohn, H.Y.; Mohassab, Y.; Lan, Y. Structures, preparation and applications of titanium suboxides. RSC Adv. 2016, 6, 79706–79722. [Google Scholar] [CrossRef]
- Pabón, B.M.; Beltrán, J.I.; Sánchez-Santolino, G.; Palacio, I.; López-Sánchez, J.; Rubio-Zuazo, J.; Rojo, J.M.; Ferrer, P.; Mascaraque, A.; Muñoz, M.C. Formation of titanium monoxide (001) single-crystalline thin film induced by ion bombardment of titanium dioxide (110). Nat. Commun. 2015, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Fusi, M.; Maccallini, E.; Caruso, T.; Casari, C.S.; Bassi, A.L.; Bottani, C.E.; Rudolf, P.; Prince, K.C.; Agostino, R.G. Surface electronic and structural properties of nanostructured titanium oxide grown by pulsed laser deposition. Surf. Sci. 2011, 605, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Lackner, J.M.; Waldhauser, W.; Ebner, R.; Major, B.; Schöberl, T. Pulsed laser deposition of titanium oxide coatings at room temperature—Structural, mechanical and tribological properties. Surf. Coatings Technol. 2004, 180, 585–590. [Google Scholar] [CrossRef]
- Farstad, M.H.; Ragazzon, D.; Grönbeck, H.; Strømsheim, M.D.; Stavrakas, C.; Gustafson, J.; Sandell, A.; Borg, A. TiOx thin films grown on Pd (100) and Pd (111) by chemical vapor deposition. Surf. Sci. 2016, 649, 80–89. [Google Scholar] [CrossRef]
- Greczynski, G.; Petrov, I.; Greene, J.E.; Hultman, L. Paradigm shift in thin-film growth by magnetron sputtering: From gas-ion to metal-ion irradiation of the growing film. J. Vac. Sci. Technol. A 2019, 37, 60801. [Google Scholar] [CrossRef] [Green Version]
- Horprathum, M.; Eiamchai, P.; Chindaudom, P.; Pokaipisit, A.; Limsuwan, P. Oxygen partial pressure dependence of the properties of TiO2 thin films deposited by DC reactive magnetron sputtering. Procedia Eng. 2012, 32, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Okimura, K. Low temperature growth of rutile TiO2 films in modified rf magnetron sputtering. Surf. Coatings Technol. 2001, 135, 286–290. [Google Scholar] [CrossRef]
- Gouttebaron, R.; Cornelissen, D.; Snyders, R.; Dauchot, J.P.; Wautelet, M.; Hecq, M. XPS study of TiOx thin films prepared by dc magnetron sputtering in Ar–O2 gas mixtures. Surf. Interface Anal. 2000, 30, 527–530. [Google Scholar] [CrossRef]
- Godfroid, T.; Gouttebaron, R.; Dauchot, J.P.; Leclere, P.; Lazzaroni, R.; Hecq, M. Growth of ultrathin Ti films deposited on SnO2 by magnetron sputtering. Thin Solid Films 2003, 437, 57–62. [Google Scholar] [CrossRef]
- Tiron, V.; Velicu, I.-L.; Dobromir, M.; Demeter, A.; Samoila, F.; Ursu, C.; Sirghi, L. Reactive multi-pulse HiPIMS deposition of oxygen-deficient TiOx thin films. Thin Solid Films 2016, 603, 255–261. [Google Scholar] [CrossRef]
- Greczynski, G.; Mráz, S.; Hans, M.; Primetzhofer, D.; Lu, J.; Hultman, L.; Schneider, J.M. Unprecedented Al supersaturation in single-phase rock salt structure VAlN films by Al+ subplantation. J. Appl. Phys. 2017, 121, 171907. [Google Scholar] [CrossRef] [Green Version]
- Zauner, L.; Ertelthaler, P.; Wojcik, T.; Bolvardi, H.; Kolozsvári, S.; Mayrhofer, P.H.; Riedl, H. Reactive HiPIMS deposition of Ti-Al-N: Influence of the deposition parameters on the cubic to hexagonal phase transition. Surf. Coatings Technol. 2020, 382, 125007. [Google Scholar] [CrossRef]
- Mareš, P.; Dubau, M.; Polášek, J.; Mates, T.; Kozák, T.; Vyskočil, J. High deposition rate films prepared by reactive HiPIMS. Vacuum 2021, 191, 110329. [Google Scholar] [CrossRef]
- Depla, D.; Heirwegh, S.; Mahieu, S.; De Gryse, R. Towards a more complete model for reactive magnetron sputtering. J. Phys. D Appl. Phys. 2007, 40, 1957. [Google Scholar] [CrossRef]
- Lou, B.-S.; Yang, Y.-C.; Qiu, Y.-X.; Diyatmika, W.; Lee, J.-W. Hybrid high power impulse and radio frequency magnetron sputtering system for TiCrSiN thin film depositions: Plasma characteristics and film properties. Surf. Coatings Technol. 2018, 350, 762–772. [Google Scholar] [CrossRef]
- Pansila, P.; Witit-Anun, N.; Chaiyakun, S. Influence of sputtering power on structure and photocatalyst properties of DC magnetron sputtered TiO2 thin film. Procedia Eng. 2012, 32, 862–867. [Google Scholar] [CrossRef] [Green Version]
- Kamble, S.S.; Radhakrishnan, J.K. Influence of O2 flow rate on the characteristics of TiO2 thin films deposited by RF reactive sputtering. Mater. Today Proc. 2020, 33, 709–715. [Google Scholar] [CrossRef]
- Sério, S.; Jorge, M.E.M.; Maneira, M.J.P.; Nunes, Y. Influence of O2 partial pressure on the growth of nanostructured anatase phase TiO2 thin films prepared by DC reactive magnetron sputtering. Mater. Chem. Phys. 2011, 126, 73–81. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, C.; Liu, X.; Lin, Y.; Gao, G.; Ma, C.; Yin, Y.; Li, X. Structure and transport properties of titanium oxide (Ti2O, TiO1+ δ, and Ti3O5) thin films. J. Alloys Compd. 2019, 786, 607–613. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Tsunobuchi, Y.; Matsuda, T.; Hashimoto, K.; Namai, A.; Hakoe, F.; Tokoro, H. Synthesis of a metal oxide with a room-temperature photoreversible phase transition. Nat. Chem. 2010, 2, 539. [Google Scholar] [CrossRef]
- Shang, J.T.; Chen, C.M.; Cheng, T.C.; Lee, Y.C. Influences of annealing temperature on microstructure and properties for TiO2 films deposited by DC magnetron sputtering. Jpn. J. Appl. Phys. 2015, 54, 125501. [Google Scholar] [CrossRef]
- Starbova, K.; Yordanova, V.; Nihtianova, D.; Hintz, W.; Tomas, J.; Starbov, N. Excimer laser processing as a tool for photocatalytic design of sol–gel TiO2 thin films. Appl. Surf. Sci. 2008, 254, 4044–4051. [Google Scholar] [CrossRef]
- Domaschke, M.; Zhou, X.; Wergen, L.; Romeis, S.; Miehlich, M.E.; Meyer, K.; Peukert, W.; Schmuki, P. Magnéli-phases in anatase strongly promote cocatalyst-free photocatalytic hydrogen evolution. ACS Catal. 2019, 9, 3627–3632. [Google Scholar] [CrossRef] [Green Version]
- Morakul, S.; Otsuka, Y.; Ohnuma, K.; Tagaya, M.; Motozuka, S.; Miyashita, Y.; Mutoh, Y. Enhancement effect on antibacterial property of gray titania coating by plasma-sprayed hydroxyapatite-amino acid complexes during irradiation with visible light. Heliyon 2019, 5, e02207. [Google Scholar] [CrossRef] [Green Version]
- Abir, M.M.M.; Otsuka, Y.; Ohnuma, K.; Miyashita, Y. Effects of composition of hydroxyapatite/gray titania coating fabricated by suspension plasma spraying on mechanical and antibacterial properties. J. Mech. Behav. Biomed. Mater. 2021, 125, 104888. [Google Scholar] [CrossRef] [PubMed]
- Matsuya, T.; Morakul, S.; Otsuka, Y.; Ohnuma, K.; Tagaya, M.; Motozuka, S.; Miyashita, Y.; Mutoh, Y. Visible light-induced antibacterial effects of the luminescent complex of hydroxyapatite and 8-hydroxyquinoline with gray titania coating. Appl. Surf. Sci. 2018, 448, 529–538. [Google Scholar] [CrossRef]
- Ould-Hamouda, A.; Tokoro, H.; Ohkoshi, S.-I.; Freysz, E. Single-shot time resolved study of the photo-reversible phase transition induced in flakes of Ti3O5 nanoparticles at room temperature. Chem. Phys. Lett. 2014, 608, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Shang, J.-X.; Wang, F.-H. Electronic, magnetic and optical properties of β-Ti3O5 and λ-Ti3O5: A density functional study. Comput. Mater. Sci. 2014, 81, 158–162. [Google Scholar] [CrossRef]
- Qi, W.; Du, J.; Peng, Y.; Wang, Y.; Xu, Y.; Li, X.; Zhang, K.; Gong, C.; Luo, M.; Peng, H. Self-induced preparation of Ti3O5 nanorods by chemical vapor deposition. Vacuum 2017, 143, 380–385. [Google Scholar] [CrossRef]
- Sun, P.; Hu, X.; Wei, G.; Wang, R.; Wang, Q.; Wang, H.; Wang, X. Ti3O5 nanofilm on carbon nanotubes by pulse laser deposition: Enhanced electrochemical performance. Appl. Surf. Sci. 2021, 548, 149269. [Google Scholar] [CrossRef]
- Nupriyonok, I.S.; Chaplanov, A.M.; Shibko, A.N. Oxidation of titanium films irradiated with weak photon beam in the course of thermal treatment. In Proceedings of the 5th International Conference on Industrial Lasers and Laser Applications’ 95: International Society for Optics and Photonics, Moscow, Russia, 24–26 June 1995; Volume 2713, pp. 321–324. [Google Scholar]
- Zhou, B.; Jiang, X.; Liu, Z.; Shen, R.; Rogachev, A.V. Preparation and characterization of TiO2 thin film by thermal oxidation of sputtered Ti film. Mater. Sci. Semicond. Process. 2013, 16, 513–519. [Google Scholar] [CrossRef]
- Yeh, M.-Y.; Lee, P.-Y.; Shang, J.-T.; Lee, Y.-C. Effect of thermal oxidation temperatures on the phase evolution and photocatalytic property of tungsten-doped TiO2 thin film. Jpn. J. Appl. Phys. 2018, 57, 125801. [Google Scholar] [CrossRef]
- Pouilleau, J.; Devilliers, D.; Garrido, F.; Durand-Vidal, S.; Mahé, E. Structure and composition of passive titanium oxide films. Mater. Sci. Eng. B 1997, 47, 235–243. [Google Scholar] [CrossRef]
- Stegemann, C.; Moraes, R.S.; Duarte, D.A.; Massi, M. Thermal annealing effect on nitrogen-doped TiO2 thin films grown by high power impulse magnetron sputtering plasma power source. Thin Solid Films 2017, 625, 49–55. [Google Scholar] [CrossRef]
- Khan, H.; Bera, S.; Sarkar, S.; Jana, S. Fabrication, structural evaluation, optical and photoelectrochemical properties of soft lithography based 1D/2D surface patterned indium titanium oxide sol-gel thin film. Surf. Coat. Technol. 2017, 328, 410–419. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Sahdan, M.Z.; Nayan, N.; Embong, Z.; Hak, C.R.C.; Adriyanto, F. Neutron beam interaction with rutile TiO2 single crystal (111): Raman and XPS study on Ti3+-oxygen vacancy formation. Mater. Lett. 2020, 263, 127143. [Google Scholar] [CrossRef]
- Nair, P.B.; Justinvictor, V.B.; Daniel, G.P.; Joy, K.; Ramakrishnan, V.; Thomas, P. V Effect of RF power and sputtering pressure on the structural and optical properties of TiO2 thin films prepared by RF magnetron sputtering. Appl. Surf. Sci. 2011, 257, 10869–10875. [Google Scholar] [CrossRef]
- Kunti, A.K.; Sekhar, K.C.; Pereira, M.; Gomes, M.J.M.; Sharma, S.K. Oxygen partial pressure induced effects on the microstructure and the luminescence properties of pulsed laser deposited TiO2 thin films. AIP Adv. 2017, 7, 15021. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yoshikawa, A.S. Fundamental Properties and Modern Photonic and Electronic Devices; Springer: New York, NY, USA, 2007. [Google Scholar]
- Bikondoa, O.; Pang, C.L.; Ithnin, R.; Muryn, C.A.; Onishi, H.; Thornton, G. Direct visualization of defect-mediated dissociation of water on TiO2 (110). Nat. Mater. 2006, 5, 189–192. [Google Scholar] [CrossRef]
- Minato, T.; Sainoo, Y.; Kim, Y.; Kato, H.S.; Aika, K.; Kawai, M.; Zhao, J.; Petek, H.; Huang, T.; He, W. The electronic structure of oxygen atom vacancy and hydroxyl impurity defects on titanium dioxide (110) surface. J. Chem. Phys. 2009, 130, 124502. [Google Scholar] [CrossRef]
- Shi, H.; Liu, Y.-C.; Zhao, Z.-J.; Miao, M.; Wu, T.; Wang, Q. Reactivity of the defective rutile TiO2 (110) surfaces with two bridging-oxygen vacancies: Water molecule as a probe. J. Phys. Chem. C 2014, 118, 20257–20263. [Google Scholar] [CrossRef]
- Obregón, S.; Rodríguez-González, V. Photocatalytic TiO2 thin films and coatings prepared by sol–gel processing: A brief review. J. Sol-Gel Sci. Technol. 2021, 58, 1–17. [Google Scholar] [CrossRef]
- Qutub, N.; Pirzada, B.M.; Umar, K.; Sabir, S. Synthesis of CdS nanoparticles using different sulfide ion precursors: Formation mechanism and photocatalytic degradation of Acid Blue-29. J. Environ. Chem. Eng. 2016, 4, 808–817. [Google Scholar] [CrossRef]
- Yu, J.C.; Zhang, L.; Zheng, Z.; Zhao, J. Synthesis and characterization of phosphated mesoporous titanium dioxide with high photocatalytic activity. Chem. Mater. 2003, 15, 2280–2286. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadić, N.; Radić, N.; Grbić, B.; Vasilić, R. Effect of Tb3+ doping on the photocatalytic activity of TiO2 coatings formed by plasma electrolytic oxidation of titanium. Surf. Coatings Technol. 2018, 337, 279–289. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Dasari, T.P.; Pathakoti, K.; Hwang, H.-M. Determination of the mechanism of photoinduced toxicity of selected metal oxide nanoparticles (ZnO, CuO, Co3O4 and TiO2) to E. coli bacteria. J. Environ. Sci. 2013, 25, 882–888. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; dos Santos, V.A.P.M.; Fernández-García, M. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Van Acker, H.; Coenye, T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017, 25, 456–466. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Recent advances in TiO2 films prepared by sol-gel methods for photocatalytic degradation of organic pollutants and antibacterial activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Phuinthiang, P.; Trinh, D.T.T.; Channei, D.; Ratananikom, K.; Sirilak, S.; Khanitchaidecha, W.; Nakaruk, A. Novel strategy for the development of antibacterial TiO2 thin film onto polymer substrate at room temperature. Nanomaterials 2021, 11, 1493. [Google Scholar] [CrossRef]
- Russell, A.D. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 2003, 52, 750–763. [Google Scholar] [CrossRef] [Green Version]
- Epand, R.M.; Epand, R.F. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim. Biophys. Acta (BBA) Biomembranes 2009, 1788, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Vyas, S.; Tiwary, R.; Shubham, K.; Chakrabarti, P. Study the target effect on the structural, surface and optical properties of TiO2 thin film fabricated by RF sputtering method. Superlattices Microstruct. 2015, 80, 215–221. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Semiconductors. In Optical Properties of Solids; Springer: Berlin/Heidelberg, Germany, 1969; pp. 123–136. [Google Scholar]
- Tayade, R.J.; Natarajan, T.S.; Bajaj, H.C. Photocatalytic degradation of methylene blue dye using ultraviolet light emitting diodes. Ind. Eng. Chem. Res. 2009, 48, 10262–10267. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; de Soares, N.F.F.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]



| Sample | Atomic Concentration (%) | Ti: O Ratio | |
|---|---|---|---|
| Ti | O | ||
| Ti3Ox (2 kW) | 48.86 | 51.14 | 0.95 |
| Ti3Ox (2.5 kW) | 54.67 | 45.33 | 1.20 |
| TiO2 | 29.94 | 70.06 | 0.42 |
| Sputtering Power (kW) | Argon Flow (sccm) | Oxygen Flow (sccm) | Deposition Rate (nm/min) | Thickness (nm) |
|---|---|---|---|---|
| 2 | 200 | 5 | 99 | 55 |
| 2.5 | 200 | 5 | 119 | 66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widyastuti, E.; Xu, F.-Y.; Chiu, C.-T.; Jan, J.-H.; Hsu, J.-L.; Lee, Y.-C. A Study on the Characteristic and Antibacterial Activity of Ti3Ox Thin Films. Catalysts 2021, 11, 1416. https://doi.org/10.3390/catal11111416
Widyastuti E, Xu F-Y, Chiu C-T, Jan J-H, Hsu J-L, Lee Y-C. A Study on the Characteristic and Antibacterial Activity of Ti3Ox Thin Films. Catalysts. 2021; 11(11):1416. https://doi.org/10.3390/catal11111416
Chicago/Turabian StyleWidyastuti, Endrika, Fu-Yang Xu, Chen-Tien Chiu, Jhen-Hau Jan, Jue-Liang Hsu, and Ying-Chieh Lee. 2021. "A Study on the Characteristic and Antibacterial Activity of Ti3Ox Thin Films" Catalysts 11, no. 11: 1416. https://doi.org/10.3390/catal11111416
APA StyleWidyastuti, E., Xu, F.-Y., Chiu, C.-T., Jan, J.-H., Hsu, J.-L., & Lee, Y.-C. (2021). A Study on the Characteristic and Antibacterial Activity of Ti3Ox Thin Films. Catalysts, 11(11), 1416. https://doi.org/10.3390/catal11111416