Manganese Molybdenum Oxide Micro Rods Adorned Porous Carbon Hybrid Electrocatalyst for Electrochemical Determination of Furazolidone in Environmental Fluids
Abstract
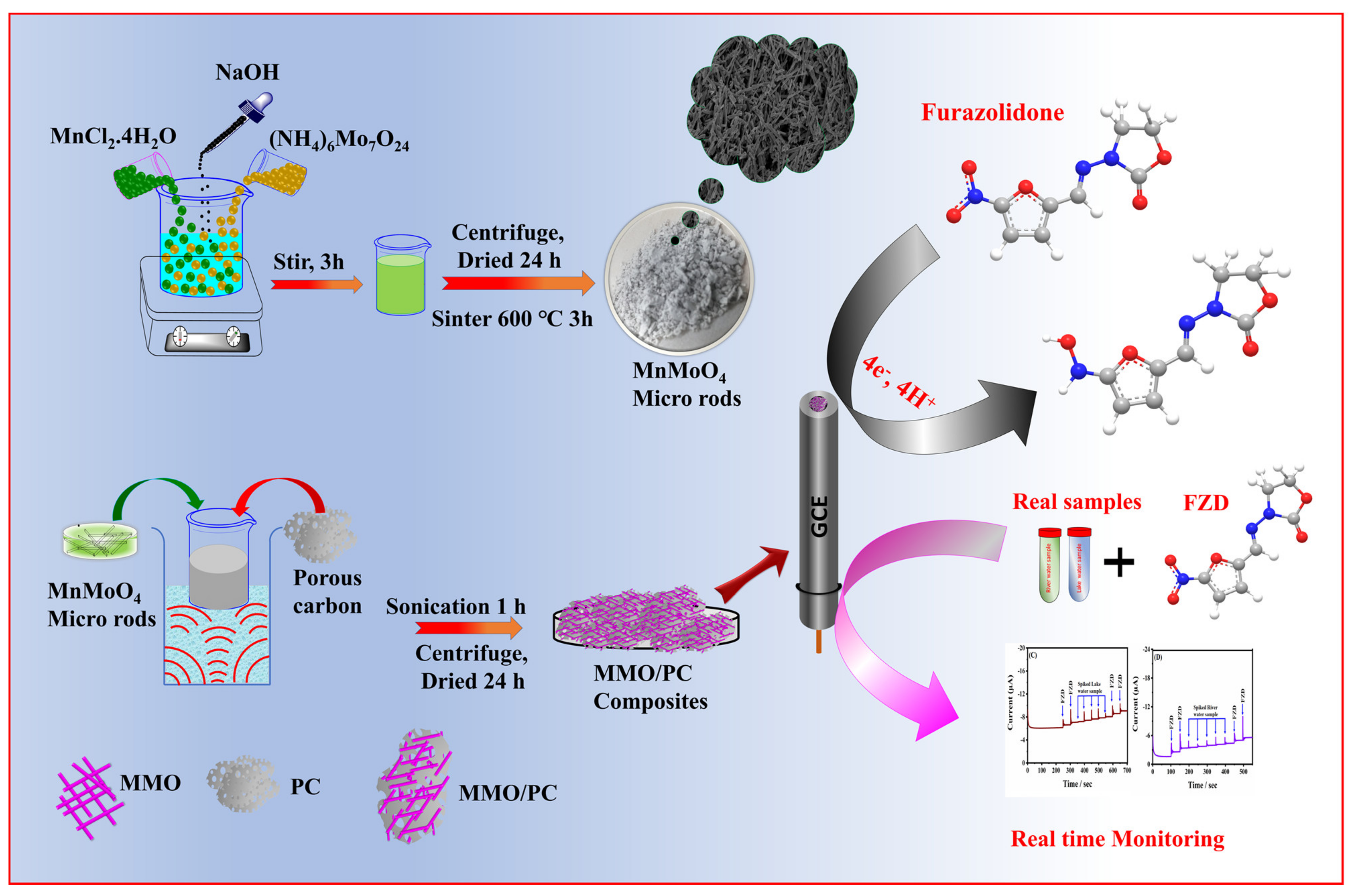
:1. Introduction
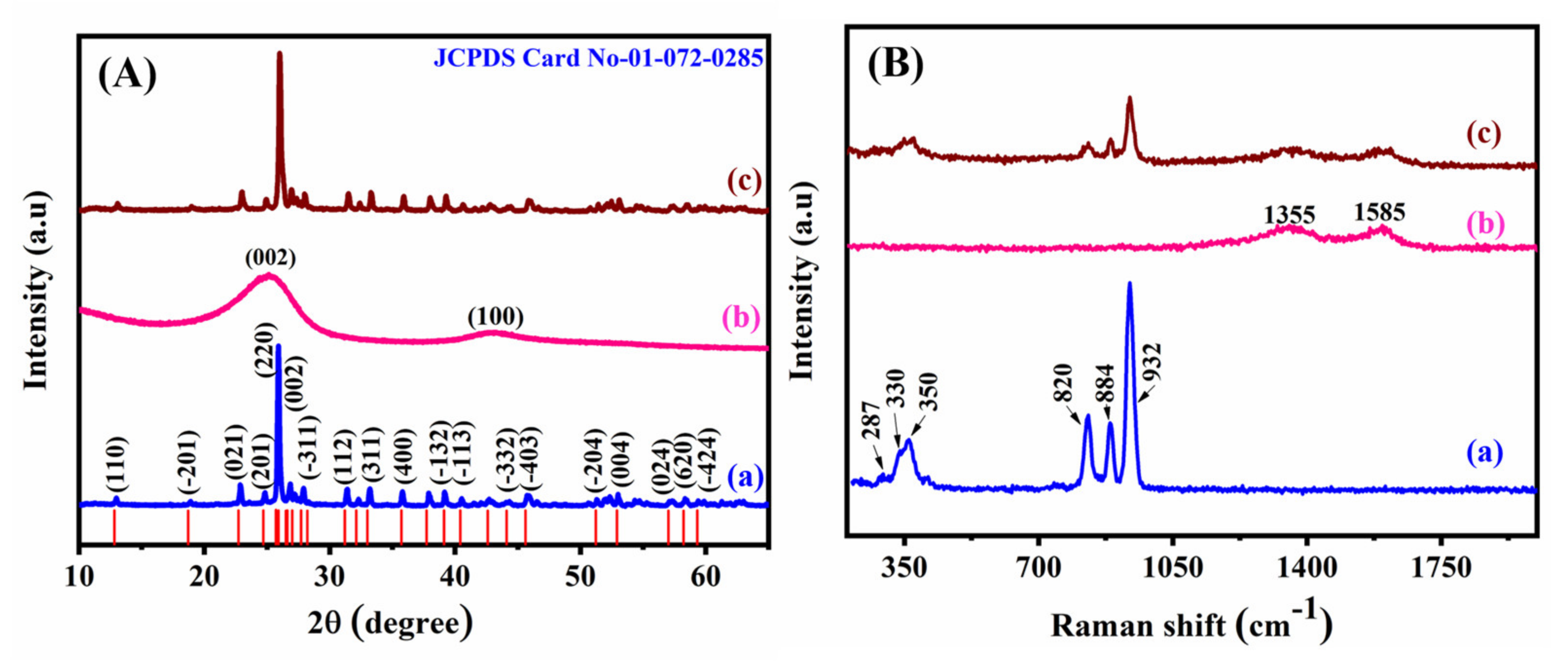
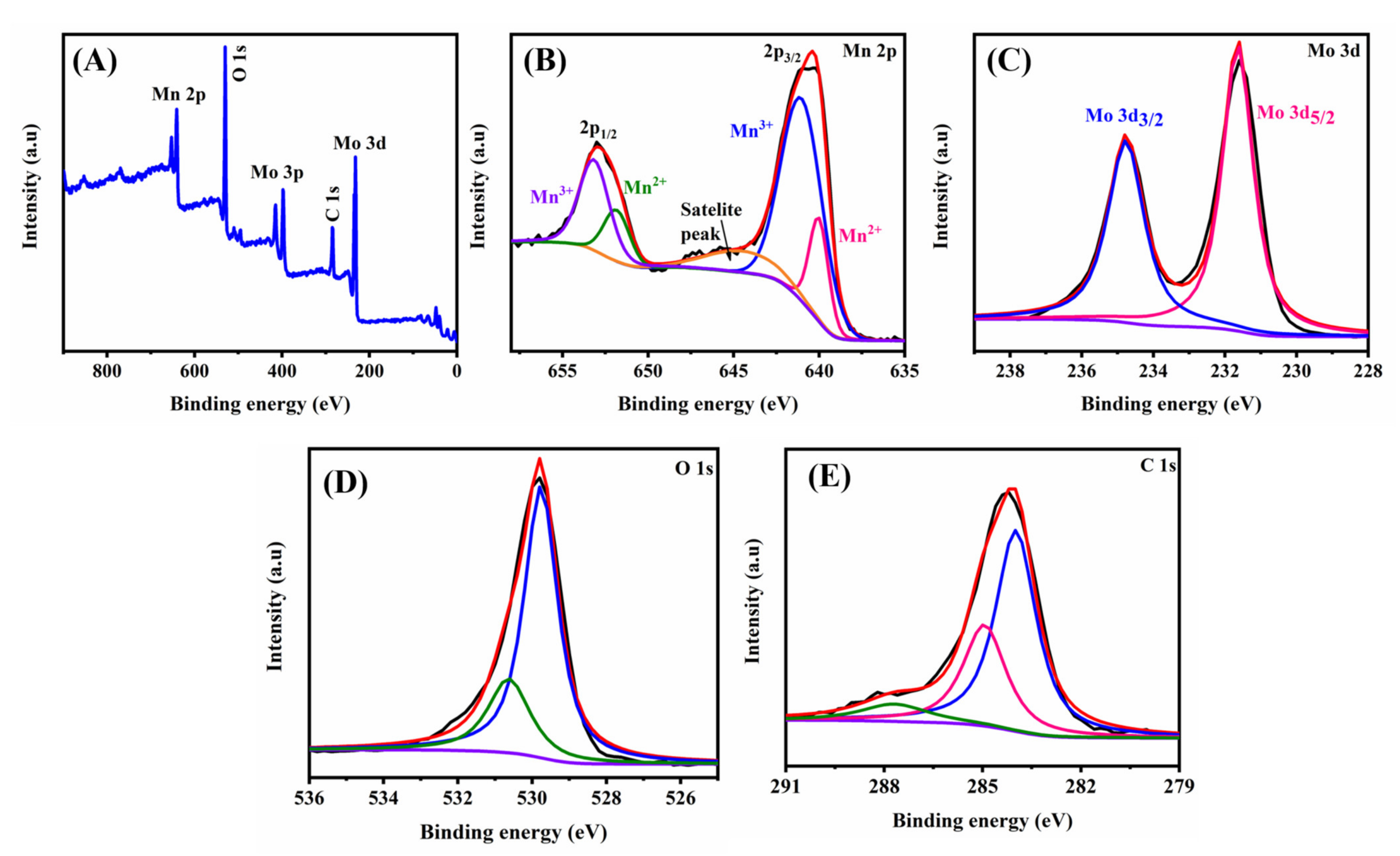
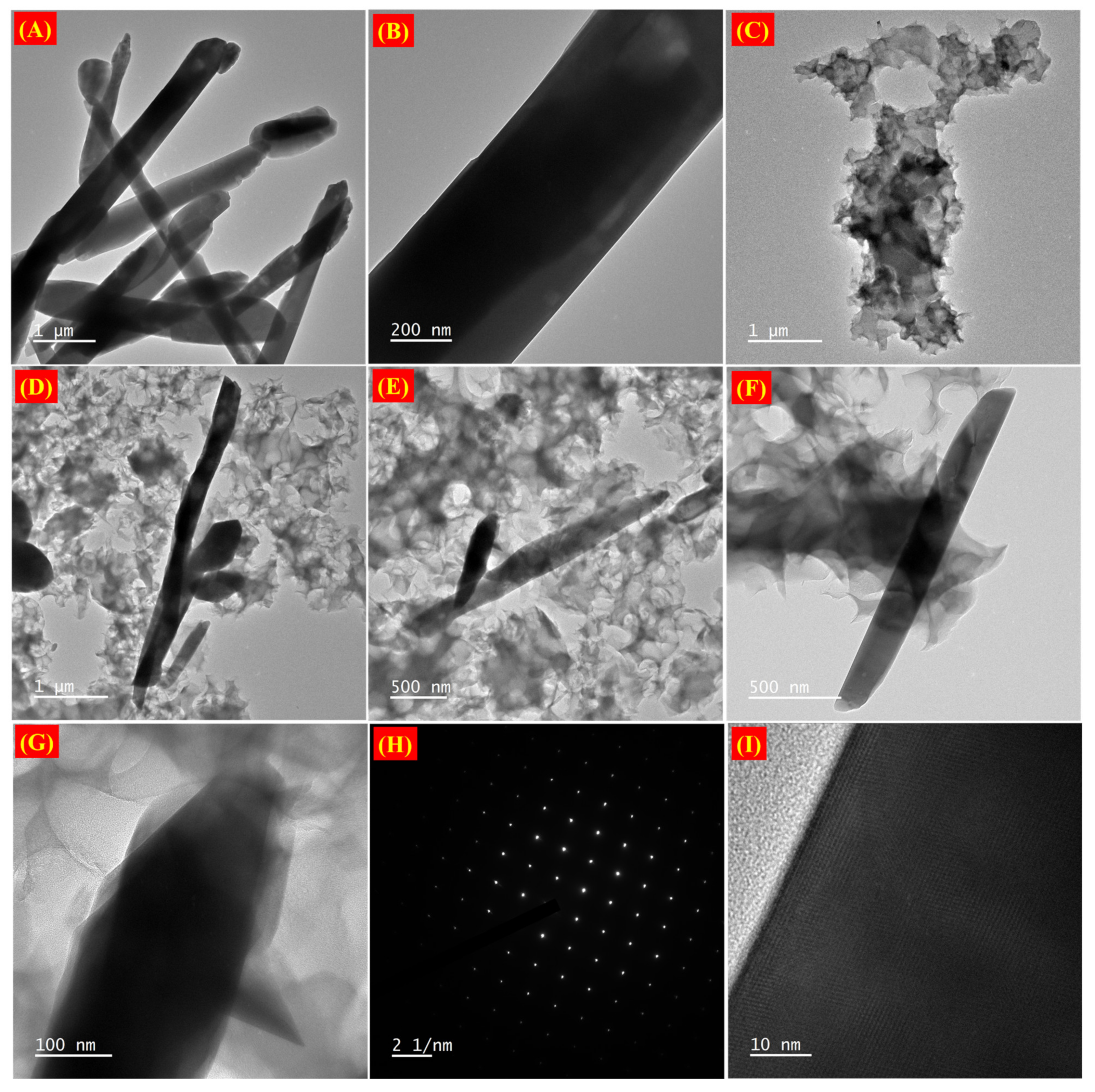
2. Results and Discussion
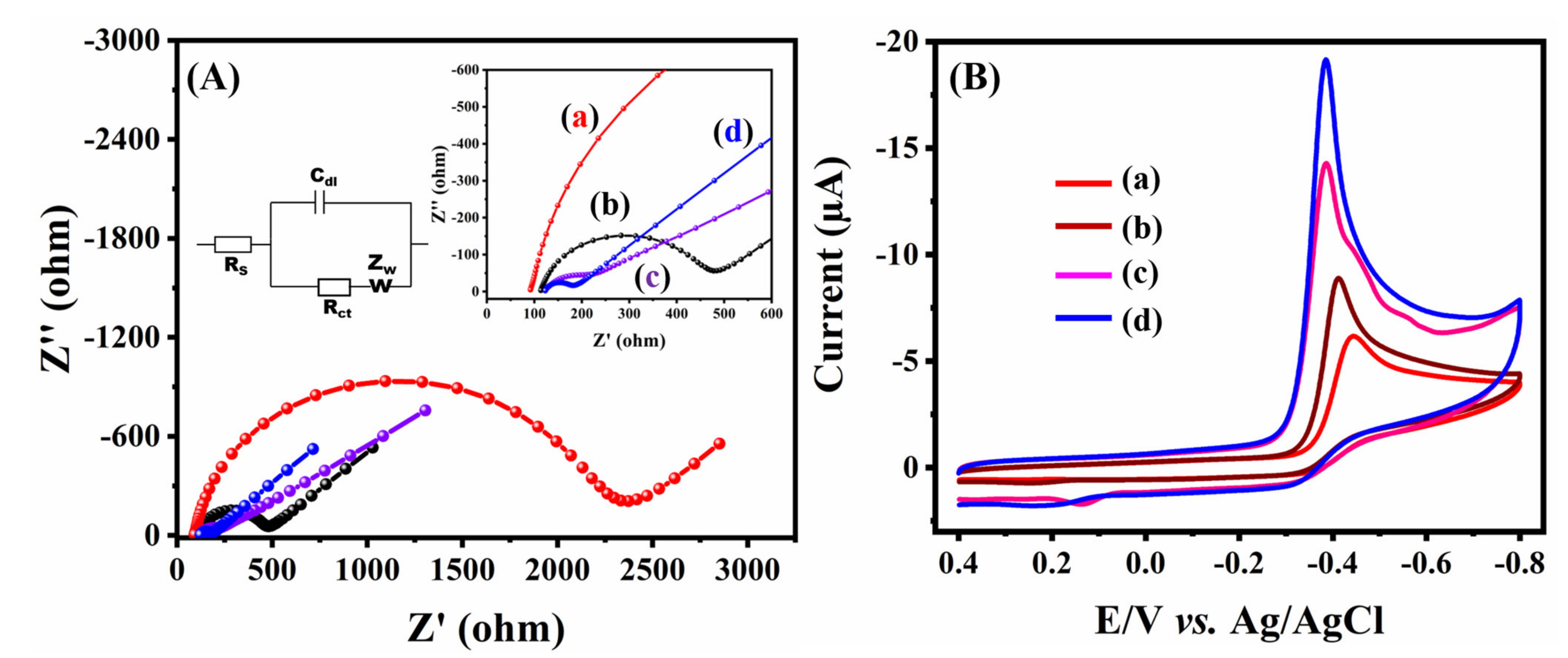
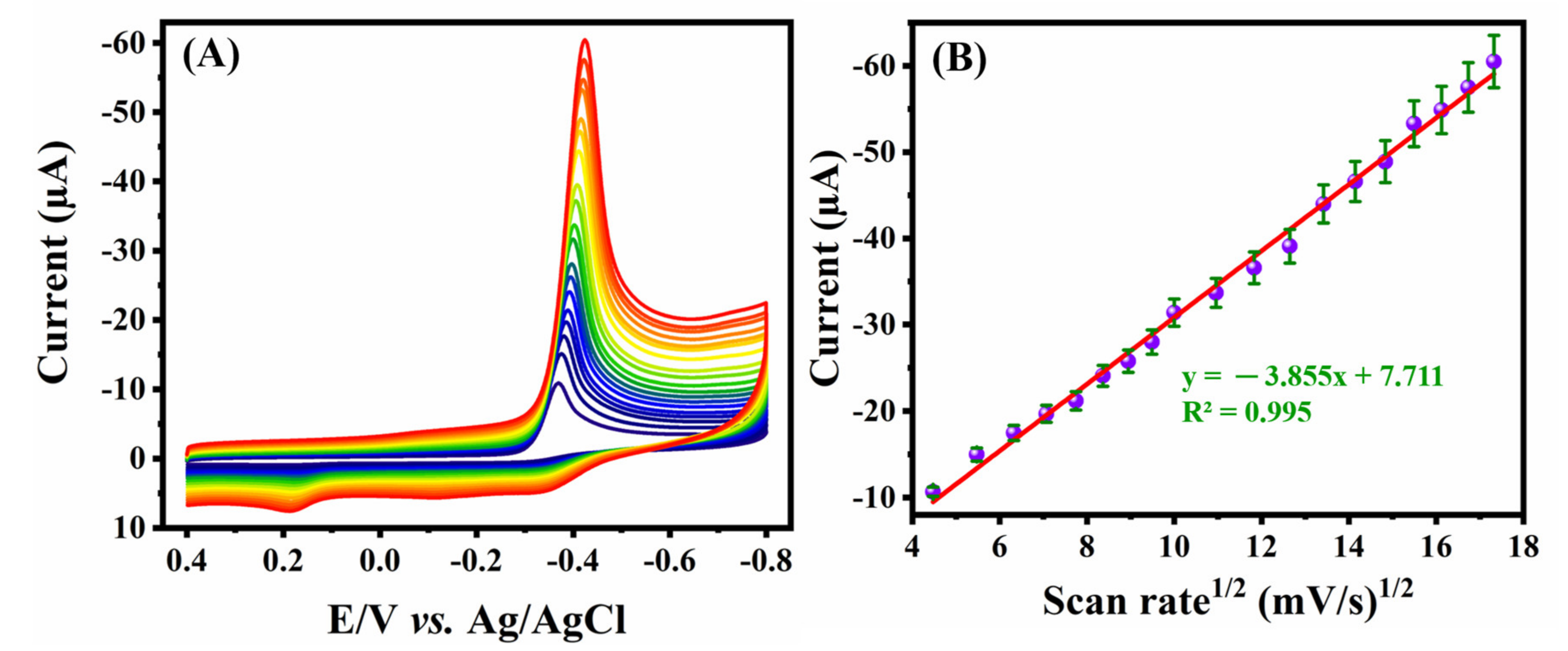
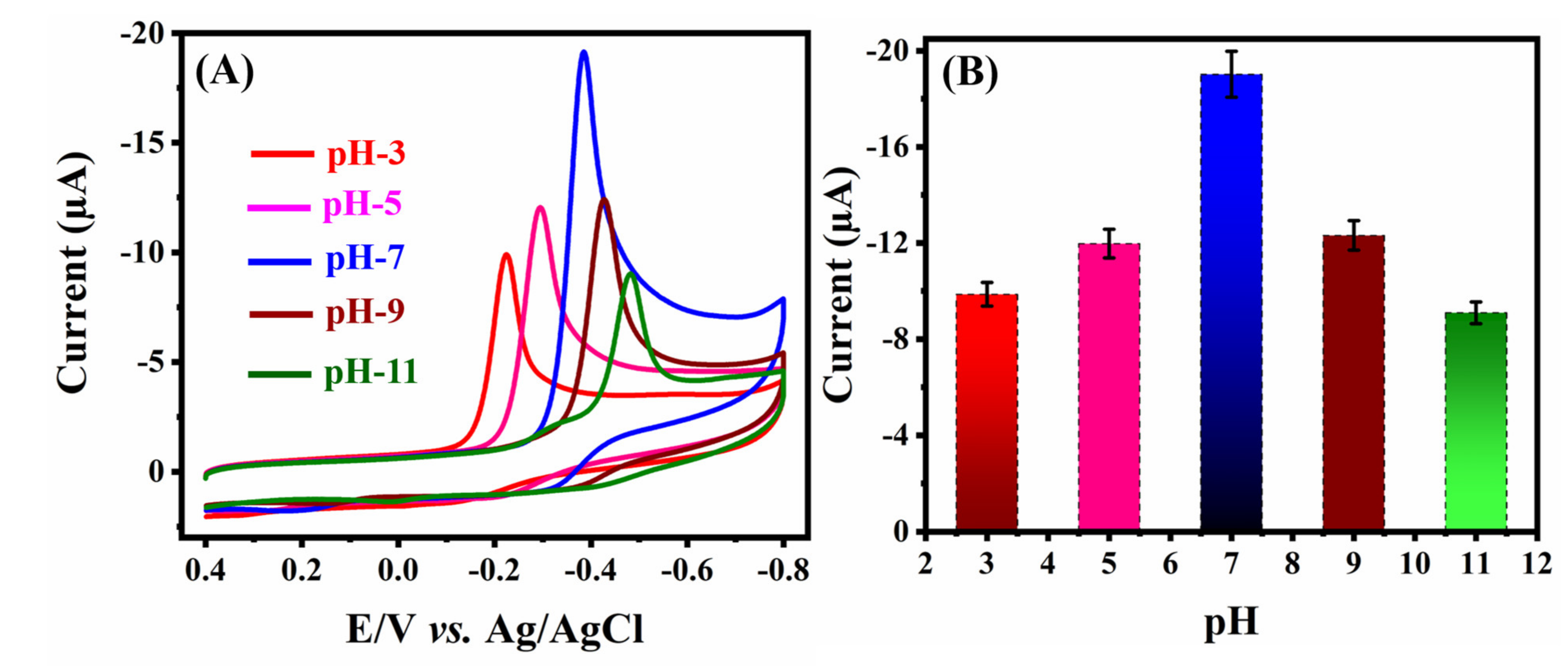
2.1. Electrochemical Characterization and Application
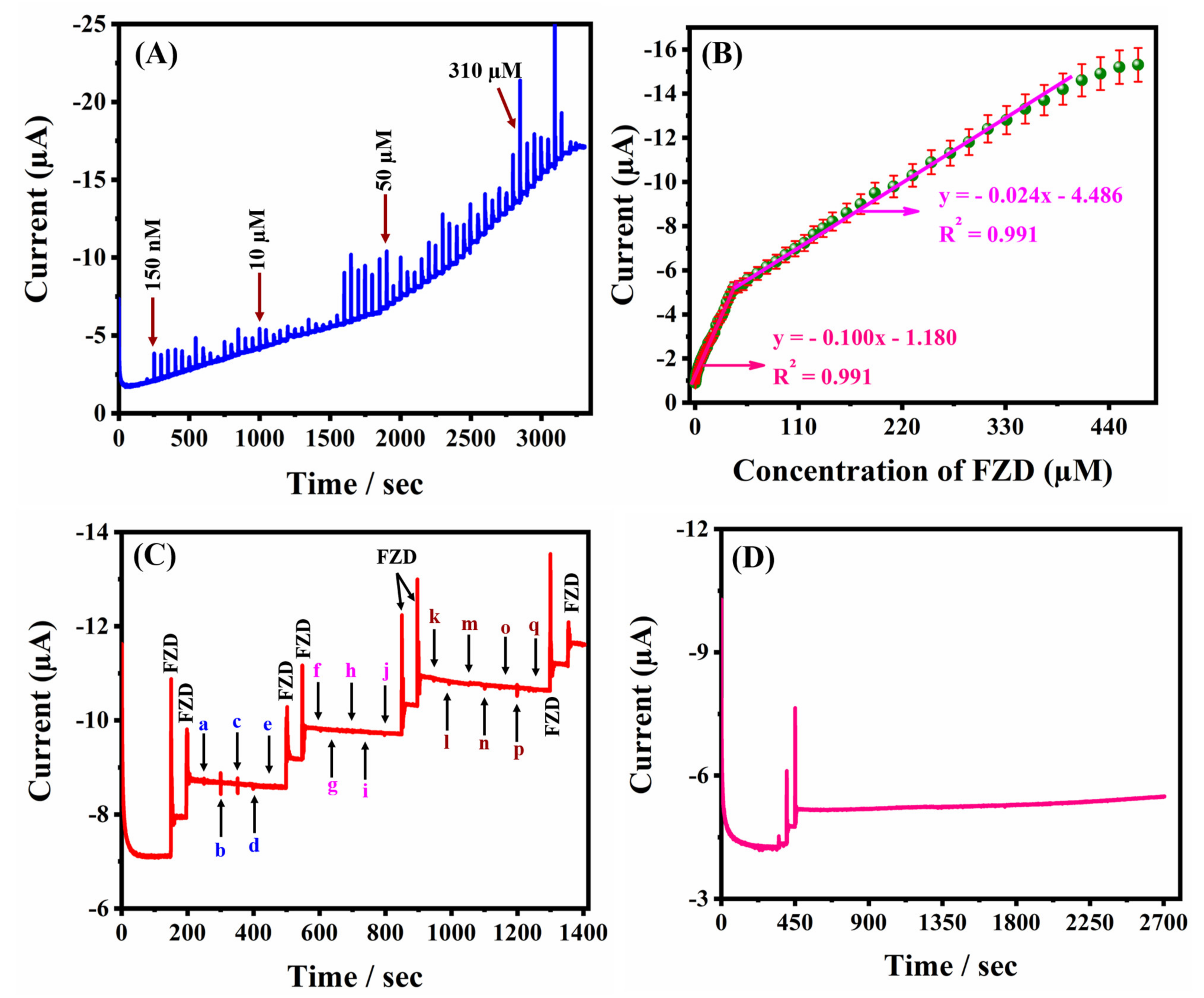
2.2. Amperometric(i-t) Determination of FZD at MMO/PC Composite
2.3. Selectivity and Stability Test
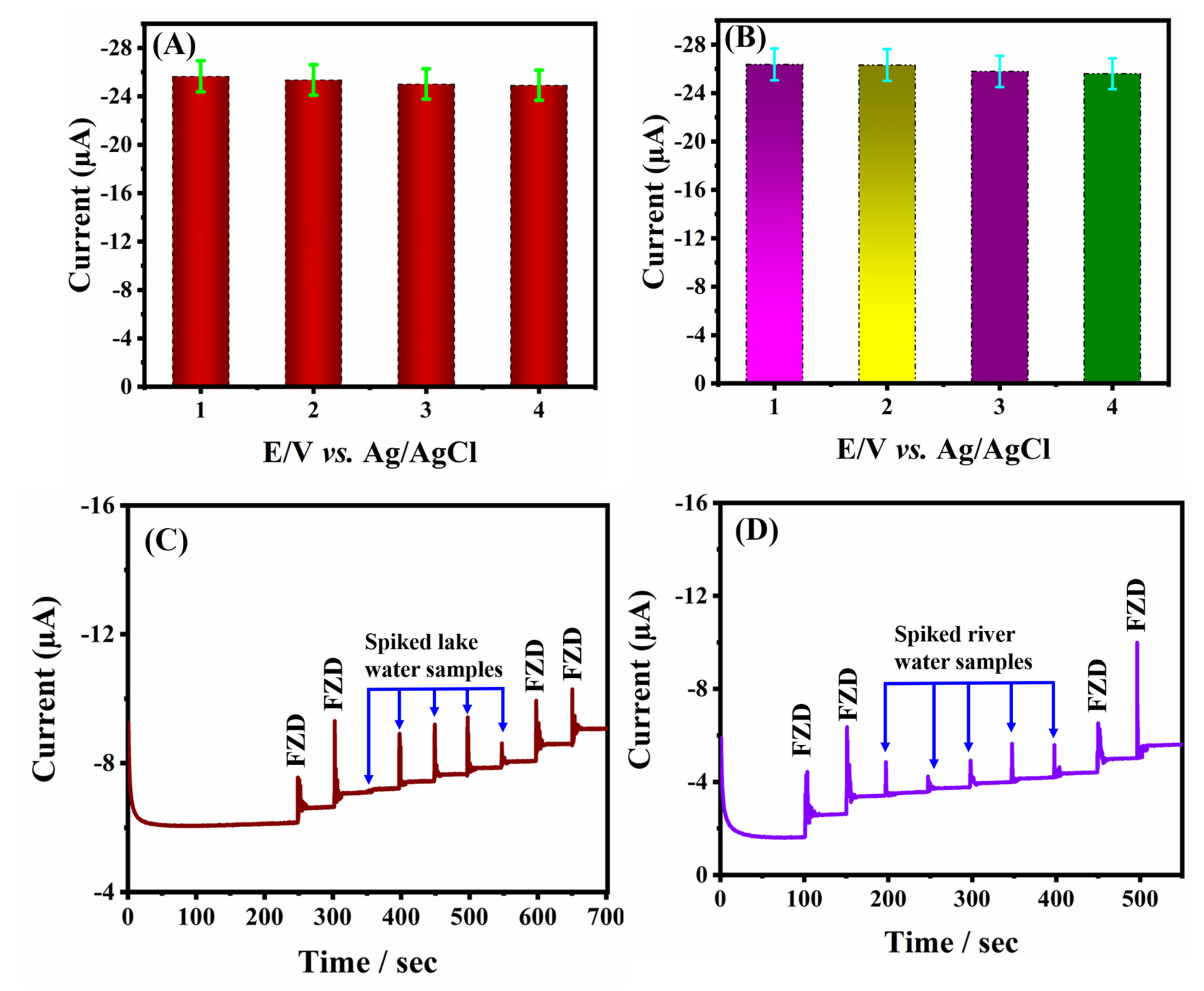
2.4. Repeatability, Reproducibility, and Real-Sample Analysis
3. Experimental
3.1. Chemical Reagent
3.2. Synthesis Procedure of MMO Micro Rods
3.3. Fabrication of MMO@PC Composite
3.4. Preparation of MMO/PC/GCE
3.5. Characterization and Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carignan, G.; Macintosh, A.I.; Sved, S. An Assay for Furazolidone Residues by Liquid Chromatography with Electrochemical Detection Applicable to Depletion Studies in Pigs. J. Agric. Food Chem. 1990, 38, 716–720. [Google Scholar] [CrossRef]
- Xing, C.; Jing, X.; Zhang, X.; Yuan, J. Ultrasensitive indirect competitive ELISA and strip sensor for detection of furazolidone metabolite in animal tissues. Food Agric. Immunol. 2017, 28, 1269–1282. [Google Scholar] [CrossRef] [Green Version]
- MacNeil, J.D. Drug residues in animal tissues. J. AOAC Int. 2001, 84, 191–193. [Google Scholar] [CrossRef] [Green Version]
- Valadez-Salazar, A.; Guiscafre-Gallardo, H.; Sanchez-Garcia, S.; Muñoz, O. Detection of furazolidone in human biological fluids by high performance liquid chromatography. J. Antimicrob. Chemother. 1989, 23, 589–595. [Google Scholar] [CrossRef]
- Botsoglou, N.A. Determination of furazolidone in eggs by high-performance liquid chromatography. J. Agric. Food Chem. 1988, 36, 1224–1227. [Google Scholar] [CrossRef]
- Chu, P.S.; Lopez, M.I. Determination of nitrofuran residues in milk of dairy cows using liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2007, 55, 2129–2135. [Google Scholar] [CrossRef]
- Diblikova, I.; Cooper, K.M.; Kennedy, D.G.; Franek, M. Monoclonal antibody-based ELISA for the quantification of nitrofuran metabolite 3-amino-2-oxazolidinone in tissues using a simplified sample preparation. Anal. Chim. Acta 2005, 540, 285–292. [Google Scholar] [CrossRef]
- Jain, A.K.; Singh, K.P. Evaluation of Antibacterial Studies in Harsingaar. Int. Res. J. Pharm. 2013, 4, 151–153. [Google Scholar] [CrossRef]
- Squella, J.; Bollo, S.; Nunez-Vergara, L. Recent Developments in the Electrochemistry of Some Nitro Compounds of Biological Significance. Curr. Org. Chem. 2005, 9, 565–581. [Google Scholar] [CrossRef]
- Klingler, R.J.; Kochi, J.K. Electron-transfer kinetics from cyclic voltammetry. Quantitative description of electrochemical reversibility. J. Phys. Chem. 1981, 85, 1731–1741. [Google Scholar] [CrossRef]
- Aristov, N.; Habekost, A. Cyclic Voltammetry-A Versatile Electrochemical Method Investigating Electron Transfer Processes. World J. Chem. Educ. 2015, 3, 115–119. [Google Scholar] [CrossRef]
- Karuppiah, C.; Muthupandi, K.; Chen, S.M.; Ali, M.A.; Palanisamy, S.; Rajan, A.; Prakash, P.; Al-Hemaid, F.M.A.; Lou, B.S. Green synthesized silver nanoparticles decorated on reduced graphene oxide for enhanced electrochemical sensing of nitrobenzene in waste water samples. RSC Adv. 2015, 5, 31139–31146. [Google Scholar] [CrossRef]
- Terbouche, A.; Lameche, S.; Ait-Ramdane-Terbouche, C.; Guerniche, D.; Lerari, D.; Bachari, K.; Hauchard, D. A new electrochemical sensor based on carbon paste electrode/Ru(III) complex for determination of nitrite: Electrochemical impedance and cyclic voltammetry measurements. Meas. J. Int. Meas. Confed. 2016, 92, 524–533. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Fakude, C.T.; Mabuba, N.; Peleyeju, G.M.; Arotiba, O.A. The determination of 2-phenylphenol in the presence of 4-chlorophenol using nano-Fe3O4/ionic liquid paste electrode as an electrochemical sensor. J. Colloid Interface Sci. 2019, 554, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Arotiba, O.A. Simultaneous determination of cholesterol, ascorbic acid and uric acid as three essential biological compounds at a carbon paste electrode modified with copper oxide decorated reduced graphene oxide nanocomposite and ionic liquid. J. Colloid Interface Sci. 2020, 560, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, C.; Velmurugan, M.; Chen, S.M.; Devasenathipathy, R.; Karthik, R.; Wang, S.F. Electrochemical Activation of Graphite Nanosheets Decorated with Palladium Nanoparticles for High Performance Amperometric Hydrazine Sensor. Electroanalysis 2016, 28, 808–816. [Google Scholar] [CrossRef]
- Saleh, M.M.; Marzi, K.E.; Naghian, E.; Homayoun, K.A.; Sohouli, E.; Plonska-Brzezinska, M.E.; Ali, S.N.; Rahimi-Nasrabadi, M.; Ahmadi, F. Application of carbon nanoonion-NiMoO4-MnWO4 nanocomposite for modification of glassy carbon electrode: Electrochemical determination of ascorbic acid. Microchem. J. 2020, 159, 105470. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Biomolecule-nanoparticle hybrids for electrochemical biosensors. TrAC Trends Anal. Chem. 2009, 28, 96–109. [Google Scholar] [CrossRef]
- Qiu, J.D.; Shi, L.; Liang, R.P.; Wang, G.C.; Xia, X.H. Controllable deposition of a platinum nanoparticle ensemble on a polyaniline/graphene hybrid as a novel electrode material for electrochemical sensing. Chem. A Eur. J. 2012, 18, 7950–7959. [Google Scholar] [CrossRef]
- Zhao, X.H.; Ma, S.N.; Long, H.; Yuan, H.; Tang, C.Y.; Cheng, P.K.; Tsang, Y.H. Multifunctional Sensor Based on Porous Carbon Derived from Metal-Organic Frameworks for Real Time Health Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 3986–3993. [Google Scholar] [CrossRef]
- Yang, Y.; Chiang, K.; Burke, N. Porous carbon-supported catalysts for energy and environmental applications: A short review. Catal. Today 2011, 178, 197–205. [Google Scholar] [CrossRef]
- Kitano, M.; Arai, K.; Kodama, A.; Kousaka, T.; Nakajima, K.; Hayashi, S.; Hara, M. Preparation of a sulfonated porous carbon catalyst with high specific surface area. Catal. Letters 2009, 131, 242–249. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Song, J.Y.; Uddin, N.; Khan, N.A.; Kim, S.; Choi, C.H.; Jhung, S.H. Oxidative denitrogenation with TiO2@porous carbon catalyst for purification of fuel: Chemical aspects. Appl. Catal. B Environ. 2019, 240, 215–224. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Badhulika, S. Sulfonated porous carbon nanosheets derived from oak nutshell based high-performance supercapacitor for powering electronic devices. Renew. Energy 2020, 161, 173–183. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Manavalan, S.; Thanasekaran, P.; Lin, K.C. Research Progress on Porous Carbon Supported Metal/Metal Oxide Nanomaterials for Supercapacitor Electrode Applications. Ind. Eng. Chem. Res. 2020, 59, 6347–6374. [Google Scholar] [CrossRef]
- Imshennik, V.K.; Suzdalev, I.P.; Stavinskaya, O.N.; Shklovskaya, N.I.; Schünemann, V.; Trautwein, A.X.; Winkler, H. Preparation and characterization of porous carbon loaded with iron particles: A possible magnetic carrier of medical drugs. Microporous Mater. 1997, 10, 225–230. [Google Scholar] [CrossRef]
- Fellinger, T.P.; Hasché, F.; Strasser, P.; Antonietti, M. Mesoporous nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 4072–4075. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Liao, Z.; Hu, A.; Li, X.; Saito, N.; Zhang, Z.; Yang, L.; Hirano, S.I. Natural Self-Confined Structure Effectively Suppressing Volume Expansion toward Advanced Lithium Storage. ACS Appl. Mater. Interfaces 2021, 24634–24642. [Google Scholar] [CrossRef]
- Chen, T.; Fu, Y.; Liao, W.; Zhang, Y.; Qian, M.; Dai, H.; Tong, X.; Yang, Q. Fabrication of Cerium-Doped CoMoP/MoP@C Heterogeneous Nanorods with High Performance for Overall Water Splitting. Energy Fuels 2021, 35, 14169–14176. [Google Scholar] [CrossRef]
- Minakshi, M.; Barmi, M.J.; Jones, R.T. Rescaling metal molybdate nanostructures with biopolymer for energy storage having high capacitance with robust cycle stability. Dalt. Trans. 2017, 46, 3588–3600. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Z.; Chen, H.; Chen, Y.; Chen, P.; Xu, Z. Thermal annealing effects on the luminescence and scintillation properties of CaMoO4 single crystal grown by Bridgman method. J. Alloys Compd. 2018, 734, 179–187. [Google Scholar] [CrossRef]
- Karthika, A.; Nikhil, S.; Suganthi, A.; Rajarajan, M. A facile sonochemical approach based on graphene carbon nitride doped silver molybdate immobilized nafion for selective and sensitive electrochemical detection of chromium (VI) in real sample. Adv. Powder Technol. 2020, 31, 1879–1890. [Google Scholar] [CrossRef]
- Yaghoubian, H.; Tajik, S.; Beitollahi, H.; Sarhadi, H.; Sheikhshoaie, I. Fe2MoO4 magnetic nanocomposite modified screen printed graphite electrode as a voltammetric sensor for simultaneous determination of nalbuphine and diclofenac. J. Mater. Sci. Mater. Electron. 2021, 32, 17311–17323. [Google Scholar] [CrossRef]
- Du, D.; Lan, R.; Xu, W.; Beanland, R.; Wang, H.; Tao, S. Preparation of a hybrid Cu2O/CuMoO4 nanosheet electrode for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 17749–17756. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.C.; Kong, L.B.; Lu, C.; Ma, X.J.; Li, X.M.; Luo, Y.C.; Kang, L. Design and synthesis of CoMoO4-NiMoO4·xH 2O bundles with improved electrochemical properties for supercapacitors. J. Mater. Chem. A 2013, 1, 1380–1387. [Google Scholar] [CrossRef]
- Ramkumar, R.; Minakshi, M. Fabrication of ultrathin CoMoO4 nanosheets modified with chitosan and their improved performance in energy storage device. Dalt. Trans. 2015, 44, 6158–6168. [Google Scholar] [CrossRef]
- Li, H.; Xuan, H. Hierarchical design of Ni(OH)2/MnMoO4 composite on reduced graphene oxide/Ni foam for high-performances battery-supercapacitors hybrid device. Int. J. Hydrogen Energy 2021, 46, 38198–38211. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, A.J.; Arshad, M.; Javed, M.S.; Ahmad, A.; Ahmad, S.S.S.; Khan, M.R.; Akram, S.; Zulfiqar; Ali, S.; et al. Charge storage in binder-free 2D-hexagonal CoMoO4 nanosheets as a redox active material for pseudocapacitors. Ceram. Int. 2021, 47, 8659–8667. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Q.; Chang, J.; Zhang, D.; Peng, Y.; Yang, X. Improvement of hydrogen storage performance of MgH2 by MnMoO4 rod composite catalyst. Solid State Sci. 2021, 121, 106750. [Google Scholar] [CrossRef]
- Venkatesh, K.; Rajakumaran, R.; Chen, S.M.; Karuppiah, C.; Yang, C.C.; Ramaraj, S.K.; Ali, M.A.; Al-Hemaid, F.M.A.; El-Shikh, M.S. A novel hybrid construction of MnMoO4 nanorods anchored graphene nanosheets; an efficient electrocatalyst for the picomolar detection of ecological pollutant ornidazole in water and urine samples. Chemosphere 2021, 273, 129665. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Ezhil Vilian, A.T.; Ghoreishian, S.M.; Umapathi, R.; Hwang, S.-K.; Oh, C.W.; Huh, Y.S.; Han, Y.-K. Hybridized 1D–2D MnMoO4–MXene nanocomposites as high-performing electrochemical sensing platform for the sensitive detection of dihydroxybenzene isomers in wastewater samples. J. Hazard. Mater. 2022, 421, 126775. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.Q.; Yang, F.; Zhao, Y.L.; Xu, X.; Xu, L.; Luo, Y.Z. Hierarchical MnMoO4/CoMoO4 heterostructured nanowires with enhanced supercapacitor performance. Nat. Commun. 2011, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, H.; Huang, Y.; Wang, W.; Wei, S. Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors. Carbon, N.Y. 2011, 49, 838–843. [Google Scholar] [CrossRef]
- Ghosh, D.; Giri, S.; Moniruzzaman, M.; Basu, T.; Mandal, M.; Das, C.K. α MnMoO4/graphene hybrid composite: High energy density supercapacitor electrode material. Dalt. Trans. 2014, 43, 11067–11076. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.; Chen, L.; Dai, B.; Yu, F. Three-Dimensional Honeycomb-Like Porous Carbon with Both Interconnected Hierarchical Porosity and Nitrogen Self-Doping from Cotton Seed Husk for Supercapacitor Electrode. Nanomaterials 2018, 8, 412. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Li, W.; Xu, K.; Zhang, Y.; Ji, T.; Zou, R.; Yang, J.; Qin, Z.; Hu, J. MnMoO4·4H2O nanoplates grown on a Ni foam substrate for excellent electrochemical properties. J. Mater. Chem. A 2014, 2, 20723–20728. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Ramachandran, S.P.; Ravi, G.; Ganesh, V.; Sakunthala, A.; Yuvakkumar, R. Transition mixed-metal molybdates (MnMoO4) as an electrode for energy storage applications. Appl. Phys. A Mater. Sci. Process. 2019, 125, 1–11. [Google Scholar] [CrossRef]
- Rong, X.; Qiu, F.; Qin, J.; Zhao, H.; Yan, J.; Yang, D. A facile hydrothermal synthesis, adsorption kinetics and isotherms to Congo Red azo-dye from aqueous solution of NiO/graphene nanosheets adsorbent. J. Ind. Eng. Chem. 2015, 26, 354–363. [Google Scholar] [CrossRef]
- He, B.S.; Du, G.A. A simple and sensitive electrochemical detection of furazolidone based on an Au nanoparticle functionalized graphene modified electrode. Anal. Methods 2017, 9, 4341–4348. [Google Scholar] [CrossRef]
- Fotouhi, L.; Nemati, M.; Heravi, M.M. Electrochemistry and voltammetric determination of furazolidone with a multi-walled nanotube composite film-glassy carbon electrode. J. Appl. Electrochem. 2011, 41, 137–142. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Chen, S.M.; Pandikumar, A.; Lin, K.C. Fabrication of Platinum-Rhenium Nanoparticle-Decorated Porous Carbons: Voltammetric Sensing of Furazolidone. ACS Sustain. Chem. Eng. 2020, 8, 3591–3605. [Google Scholar] [CrossRef]
- DUAN, L.; ZHANG, L. Electrochemical Behavior of Furazolidone at Mulit-walled Carbon Nanotubes Modified Glassy Carbon Electrode and Its Analytical Application. J. Anal. Sci. 2012, 5, 673–676. [Google Scholar]


| Modified Electrodes | Methods | Limit of Detection (µM) | Reference |
|---|---|---|---|
| Gr/Au/GCE | Amperometric | 0.64 | [49] |
| MWCNT/GCE | Cyclic voltammetry | 2.3 | [50] |
| Pt-Re NP/PAC/GCE | Linear sweep Voltammetry | 0.075 | [51] |
| MWCNT/GCE | Differential pulse voltammetry | 0.08 | [52] |
| MMO/PC/RRDE | Amperometric(i-t) | 0.03 | This Work |
| Sample | Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|
| Lake water | 100 | 99.7 | 99.7 |
| 110 | 109.5 | 99.5 | |
| 120 | 118.5 | 98.7 | |
| 130 | 129.5 | 99.6 | |
| 140 | 137.5 | 98.2 | |
| River water | 22 | 21.8 | 99.0 |
| 24 | 24.5 | 102.0 | |
| 26 | 25.5 | 98.2 | |
| 28 | 27.4 | 97.8 | |
| 30 | 30.5 | 101.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babulal, S.M.; Chen, T.-W.; Chen, S.-M.; Al-Onazi, W.A.; Al-Mohaimeed, A.M. Manganese Molybdenum Oxide Micro Rods Adorned Porous Carbon Hybrid Electrocatalyst for Electrochemical Determination of Furazolidone in Environmental Fluids. Catalysts 2021, 11, 1397. https://doi.org/10.3390/catal11111397
Babulal SM, Chen T-W, Chen S-M, Al-Onazi WA, Al-Mohaimeed AM. Manganese Molybdenum Oxide Micro Rods Adorned Porous Carbon Hybrid Electrocatalyst for Electrochemical Determination of Furazolidone in Environmental Fluids. Catalysts. 2021; 11(11):1397. https://doi.org/10.3390/catal11111397
Chicago/Turabian StyleBabulal, Sivakumar Musuvadhi, Tse-Wei Chen, Shen-Ming Chen, Wedad A. Al-Onazi, and Amal M. Al-Mohaimeed. 2021. "Manganese Molybdenum Oxide Micro Rods Adorned Porous Carbon Hybrid Electrocatalyst for Electrochemical Determination of Furazolidone in Environmental Fluids" Catalysts 11, no. 11: 1397. https://doi.org/10.3390/catal11111397
APA StyleBabulal, S. M., Chen, T.-W., Chen, S.-M., Al-Onazi, W. A., & Al-Mohaimeed, A. M. (2021). Manganese Molybdenum Oxide Micro Rods Adorned Porous Carbon Hybrid Electrocatalyst for Electrochemical Determination of Furazolidone in Environmental Fluids. Catalysts, 11(11), 1397. https://doi.org/10.3390/catal11111397







