Abstract
Local structure of Pd1 single sites on the surface of Pd1In1 intermetallic nanoparticles supported on α-Al2O3 was investigated by the combination of CO-DRIFTS spectroscopy and DFT. CO-DRIFTS spectra of PdIn/Al2O3 catalyst exhibit only one asymmetric absorption band of linearly adsorbed CO comprising two peaks at 2065 and 2055 cm−1 attributable to CO molecules coordinated to Pd1 sites located at (110) and (111) facets of PdIn nanoparticles. The absence of bridged or hollow-bonded CO bands indicates that multipoint adsorption on PdIn nanoparticles is significantly hindered or impossible. DFT results show that on (110) facet multipoint CO adsorption is hindered due to large distance between neighboring Pd atoms (3.35 Å). On (111) facet multipoint CO adsorption on surface palladium atoms is impossible, since adjacent Pd atoms are located below the surface plane.
1. Introduction
Single-atom alloys (SAA) is an important class of so-called single-site catalysts widely used in state-of-the-art catalysis [1,2,3,4]. As a rule, SAA catalysts contain supported nanoparticles of substitution alloy, on the surface of which the active metal atoms are isolated from each other by the atoms of inactive (or less active) component, thus forming a system of isolated single-atom sites. SAA catalysts have received increased attention in recent years due to their enhanced selectivity in a number of reactions and regeneration capability.
A significant drawback of these systems is insufficient stability of “single site” surface structure, especially under conditions of adsorbate induced segregation. Thus, the adsorption of molecules with a high adsorption energy (for example: CO, ethylene, acetylene) leads to the enrichment of the surface with the active component, which is frequently accompanied by the formation of multiatomic centers deteriorating surface SAA structure [5,6,7,8].
Unlike substitution alloys, intermetallic compounds (IMCs) can provide a much more stable structure of isolated surface active sites due to several reasons. Thus, the IMCs possess a well-organized crystal structure, which is more stable than solid solution alloys due to the high formation enthalpy. The enthalpic driving force stabilizes IMC surface structure by preventing or minimizing surface segregation even in the presence of adsorbates [9,10]. It is important that IMCs tend to form a system of single-atom active sites on their surface [1,11,12]. Thus, theoretical analysis of formic acid decomposition over SAA-like CuPt catalysts revealed intermetallic Cu3Pt as a promising material for this process [13], since its surface structure exhibits only Pt1 sites isolated by Cu component. This conclusion is in agreement with the results reported elsewhere [14,15,16].
An excellent example of single-site intermetallic catalyst is PdIn compositions, which demonstrate a favorable performance for selective semihydrogenation of substituted alkynes, direct formic acid fuel cell processes, production of enhanced magnetic resonance signals using parahydrogen, etc. [17,18,19,20,21]. The specific catalytic properties of PdIn IMCs are attributed to the formation of single Pd1 sites on their surface, isolated from each other by indium atoms [12,17,20,22,23], which prevents or hinders multipoint adsorption of reacting molecules. Thus, the authors of [17] using computational chemistry modeling with density functional theory (DFT), predicted high ethylene selectivity in acetylene hydrogenation for PdIn(110) surface due to destabilization of multisite adsorption on Pd1 sites, which favors ethylene desorption rather than its overhydrogenation to ethane. However, the local structure of single-atom Pd1 sites on atomic level is not clear yet.
It should be noted that the study of the structure of isolated atomic centers on the surface of bimetallic nanoparticles is a difficult task. The most surface-sensitive techniques (XPS, Auger spectroscopy, etc.) do not provide detailed information about the top atomic layer, but analyze surface and subsurface atomic layers (up to 5 nm in depth). Fortunately, the convenient method for studying surface structure of Pd single-site catalysts is the infrared spectroscopy of adsorbed CO. This is due to the fact that CO is adsorbed only by surface atoms, and it is possible to distinguish isolated Pd1 and multiatomic Pdm (m > 1) centers by the presence of linear-, bridge- or threefold hollow-bonded CO bands. Note that bridged and threefold hollow-bonded CO adsorption is energetically more favorable than linear CO adsorption on Pd surface [24,25] and usually the signals of multiply coordinated CO tend to prevail in infrared spectra. Therefore, convincing evidence of the formation of Pd1 single-site surface structure is the complete disappearance of bridged and triple bonded CO adsorption signals and the presence of linearly bonded CO only, suggesting that CO coordination to several Pd atoms is unfavorable [18,19,20,26,27,28,29,30,31,32].
There are several indications that the formation of PdIn intermetallic compound leads to a decrease in the relative intensity, or disappearance of multiply-bonded CO (bridged and threefold hollow bonded) and to a “red” shift of linearly adsorbed CO by 10–25 cm−1 toward lower frequency in comparison with monometallic palladium surface. Thus, the authors of [27] reported disappearance of bridged CO for freshly reduced PdIn catalyst in contrast to monometallic Pd. Similar observations were presented elsewhere [33,34]. Results of our previous CO-DRIFTS study of Pd1In1/Al2O3 are in good agreement with these data and demonstrate the disappearance of multiply-bonded CO species on the surface of PdIn nanoparticles [18,30,35]. However, it is still unclear which characteristic features of PdIn surface structure impede the multipoint adsorption of CO.
It should be noted that the formation of isolated Pd1 sites on SAA surface usually occurs in the catalyst with a low fraction of palladium and is explained by a dilution of Pd atoms and their surroundings by atoms of the second component, which is present in significant abundance. However, for Pd1In1 intermetallics isolation of Pd atoms by their surrounding with In atoms seems unlikely, since the fraction of In atoms is not sufficient, and the disappearance of bridged CO should be explained by a specific local atomic structure around surface Pd atoms.
Therefore, this study was focused on investigation of the local atomic structure of Pd1 isolated single sites on the surface of PdIn intermetallic nanoparticles by combining experimental (CO-DRIFTS) and theoretical (DFT calculations) approaches. Since CO-DRIFTS provides information only on the upper surface layer, the combination with DFT modeling can be an informative method for revealing the local atomic surroundings of Pd1 surface sites.
2. Results and Discussion
2.1. The Choice of Catalytic System
The reference Pd/Al2O3 and bimetallic PdIn/Al2O3 catalysts were prepared via incipient wetness impregnation of α-Al2O3 by aqueous solution of Pd and In nitrates followed by hydrogen reduction in order to avoid the formation of monometallic Pd or In species. Experimental details can be found in Supplementary Materials. The α-Al2O3 was used as a carrier for the catalyst preparation. This choice was dictated by several factors. First, the highly-crystalline structure of α-Al2O3 allows reliable study of Pd1In1 nanoparticles by XRD analysis, since α-Al2O3 diffraction pattern exhibits narrow reflexes that do not overlap characteristic signals of metallic Pd, In, and PdIn. Second, since relatively large bimetallic particles are formed over α-Al2O3 due to low BET surface area, the fraction of edged and corner atoms is low and contribution of CO adsorption on these atoms can be neglected. Third, high chemical inertness of this carrier allows one to minimize metal-support interaction effects.
2.2. Transmission Electron Microscopy
The microstructure of the samples was studied by transmission electron microscopy (TEM), using an HT7700 instrument (Hitachi, Japan). More details can be found in Supplementary Materials. Figure S1 presents TEM images of Pd/Al2O3 and PdIn/Al2O3 catalysts. The monometallic catalyst (Figure S1a) contains almost spherical well-distributed Pd particles with average diameter of ca. 30 nm. Bimetallic catalyst contains the regular spherical nanosized PdIn particles ranged from 10 to 30 nm.
2.3. X-ray Diffraction
Powder XRD patterns were obtained on a Bruker D8 ADVANCE X-ray diffractometer (Cu Kα, Ni-filter, LYNX-EYE detector, reflection geometry). For details, please see Supplementary Materials. Figure 1 depicts XRD patterns of parent α-Al2O3 carrier, monometallic Pd/Al2O3, and intermetallic Pd1In1/Al2O3 catalysts. The reference XRD patterns are also displayed. α-Al2O3 diffraction peaks were detected at 2θ of ca. 35.3°, 37.9°, and 43.4° (ICSD N°10425) indicating a highly-crystalline structure of the support [29,36,37]. For the Pd/Al2O3 catalyst, a characteristic peak was observed at 40.2 and a noticeable reflex at 46.7 attributed to the (111) and (200) facets of Pd, respectively (ICSD N°180870) [18,31,38].
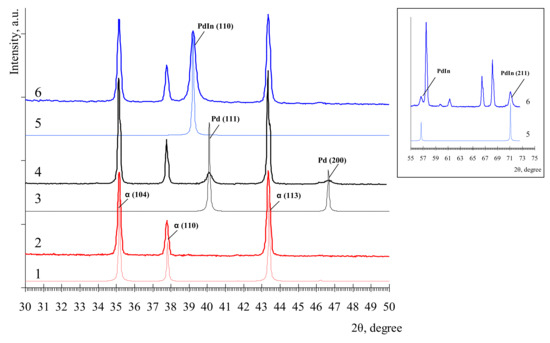
Figure 1.
Powder X-ray diffraction patterns of α-Al2O3 (2), Pd/α-Al2O3 (4), and Pd-In/α-Al2O3 (6). The reference XRD patterns from PDF-4 2018 are also displayed (1, 3 and 5, respectively). Inset represents an additional set of XRD signals attributed to PdIn IMCs at 2q > 55°.
PdIn/Al2O3 (Figure 1) reflexes of PdIn intermetallic phase with cubic structure of the CsCl type were observed at ca. 39.2°, 56.6°, and 71.0°, corresponding to (110) and (211) facets of Pd1In1 (ICSD N°59473) [29,32,39]. It is important that no peaks characteristic of monometallicPd0 were observed. It should also be mentioned that no metallic In0 particles were detected by XRD due to the absence of characteristic XRD reflections at 32.9°, 36.2°, 39.1°, 56.5°, 63.1°, 67.0°, and 69.0° assigned to the (011), (002), (110), (020), (013), (121), and (022) In crystal planes, respectively. Note that this data agrees well with EXAFS results reported previously [40].
2.4. CO-DRIFTS
Diffuse reflectance infrared Fourier transform spectroscopy was performed with a Tensor 27 Bruker spectrometer equipped with a high temperature cell (Harrick) and liquid-nitrogen-cooled MCT detector. Spectra were recorded at 50 °C under continuous 0.5% CO/He flow (30 cm3/min). For details, please refer to Supplementary Materials. Typical CO-DRIFT spectra of the reference Pd/Al2O3 and the intermetallic PdIn/Al2O3 are displayed in Figure 2. The DRIFTS spectrum of CO adsorbed on the reference monometallic Pd-catalyst exhibits two broad bands at 2150–2050 and 2200–1800 cm−1 (Figure 2a). The band at ~2100–2000 cm−1 corresponds to CO molecules linearly adsorbed on Pd atoms, whereas the broad peak at ~2000–1800 cm−1 is attributable to bridged and hollow-bonded CO on Pd surface [41]. Lower intensity of linearly adsorbed CO bands can be explained by the large size of Pd particles (~20–30 nm), and the prevalence of terrace atoms on their surface, which favors multiply adsorbed CO.
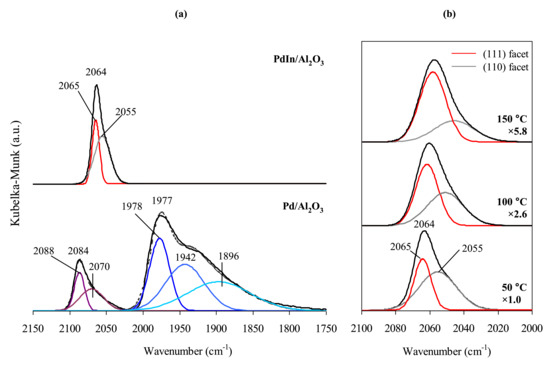
Figure 2.
CO-DRIFTS data for Pd/Al2O3 and PdIn/Al2O3 catalysts (a) Deconvolution of CO−DRIFTS spectra of Pd/Al2O3 and PdIn/Al2O3 catalysts; (b) CO−DRIFTS spectra with fitted bands for PdIn/Al2O3 catalyst at different temperatures.
For PdIn/Al2O3, the only intense asymmetric absorption band was detected centered at 2064 cm−1 attributable to CO linearly adsorbed on Pd surface sites of PdIn nanoparticles. It should be mentioned that CO-DRIFTS spectrum of PdIn catalyst does not exhibit characteristic bands at 2080–2084 cm−1 indicative for CO adsorption on monometallic Pd particles. This observation suggests formation of only PdIn species and agrees with XRD and EXAFS results (see above).
In contrast to Pd/Al2O3, spectrum of CO adsorbed on PdIn/Al2O3 does not exhibit bands of bridged or hollow-bonded CO within 2000–1800 cm−1 region. These observations are in a good agreement with the data reported by Furukawa [27], Hirano [33], and results of our previous studies [18,35], and indicate that multiple coordination of CO on the surface of PdIn intermetallic nanoparticles is unfavorable. As mentioned above, this observation can be explained by the disappearance of Pdn ensembles (n ≥ 2) on PdIn surface [27,33] capable of accommodating bridged CO species [35] similarly to the PdGa IMCs [22]. This idea is in line with the results of detailed study of bulk structure and physical properties of InPd IMC [21].
The band corresponding with linear CO on PdIn (2064 cm−1) is shifted toward a lower frequency relative to the band of CO adsorbed on monometallic Pd nanoparticles (~2084 cm−1), which is in a good agreement with the data reported by Wu [34] and Furukawa [27].
In order to gain insight into specific surface arrangements of surface structure of PdIn nanoparticles determining CO adsorption, it is informative to analyze possible reasons of the asymmetry of the band at 2064 cm−1. Tentatively, its assignment can be made on the basis of the simplified PdIn intermetallic model proposed in [17]. In accordance to this model, PdIn crystallite surface is made up of two facets, (110) and (111) (see Figure 3a,b). The contribution of the (110) facet to the total surface area is ~83%, while only 17% of (111) facet.
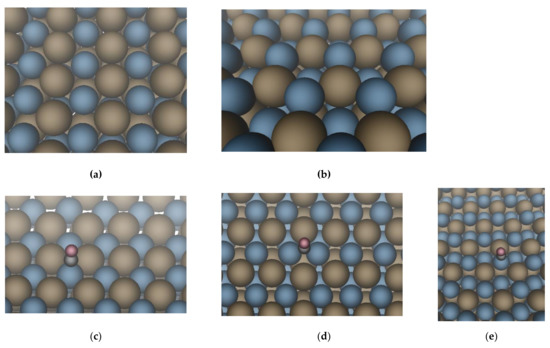
Figure 3.
Surface geometry of PdIn(110) (a) and PdIn(111) (b); (c) CO linearly adsorbed on PdIn(110); (d) bridge-bonded CO on PdIn(110); (e) CO linearly adsorbed on PdIn(111). Pd atoms are blue and In atoms are brown.
The model allows us to suggest that peak asymmetry of linear adsorbed CO in the FTIR spectra of PdIn/Al2O3 catalyst is attributable to CO adsorption on (110) and (111) facets in accordance with the results of DFT calculations (see data below). Deconvolution of the linearly adsorbed CO peak revealed two bands with maxima at 2065 and 2055 cm−1, corresponding to CO adsorption on (111) and (110) facets, respectively (Figure 2b). It should be noted that the ratio of integrated intensities of the absorption bands for (111) and (110) facets calculated from spectral data is approximately 48 and 52%. The discrepancy with the data reported in [17] may be related to differences in structure of bimetallic PdIn nanoparticles, or either different extinction coefficients for CO molecules adsorbed on various facets, different adsorption strengths (see below), or both.
To investigate the strength of CO adsorption on different PdIn facets the temperature-dependent experiment was performed (Figure 2b). It is shown that the increase in temperature leads to the change in peak shape and its shift towards lower wavenumbers. The peak maximum shifts from 2064 to 2057 cm−1 with temperature increase from 50 to 150 °C.
The results of spectra deconvolution show the pronounced decrease in the intensity of the peak corresponding to CO adsorption on (110) facet, while the intensity of the peak related to (111) facet changes to a much lesser extent (Table 1). The steep increase in ICO(111)/ICO(110) intensity ratio indicated that CO molecules bound to (111) facet of PdIn much stronger than to (110). This observation is in good agreement with the results of DFT calculation (Table 2) which show markedly higher Eads on PdIn (111) compared to Pd(110): −0.77 and −0.39 eV, respectively.

Table 1.
Dependences of CO bands positions and their relative intensities on adsorption temperature.

Table 2.
DFT calculated CO adsorption energies and wavenumbers for various adsorption sites on the Pd and PdIn surfaces.
For spectra collected at 50 °C, the FWHM is 11.6 cm−1 and 25.5 cm−1 for peaks with maximum at ~2065 cm−1 and 2055 cm−1, respectively. This difference is significant, but not as large as in a previously reported study, where FWHM was ~10, 25, and 40 cm−1 for CO adsorbed on metallic Pt [41]. The broadening of CO adsorption peak is presumably attributable to inhomogeneity of adsorption sites and can be related to different sizes of metallic particles [42].
In order to exclude the possible influence of gaseous or physisorbed CO, we performed the experiment by purging adsorbed CO with helium flow at 50 °C. The results of this experiment were found to be in a good agreement with the results obtained by variation of adsorption temperature and also revealed stronger adsorption of CO on (111) facet (see Figure S2).
2.5. DFT Calculations
For interpretation of the observed spectral data the DFT calculations were performed. Details can be found in Supplementary Materials.
Table 2 shows calculated CO adsorption energies and wavenumbers for various adsorption sites on the surfaces of Pd and PdIn.
The calculated values for linear, bridge, and hollow-bonded CO for Pd(111) and Pd(100) facets were in agreement with DRIFTS results. Calculated CO adsorption energies are consistent with the fact that CO preferentially adsorbs on bridge and hollow sites.
With DFT calculations the adsorption behavior of CO at the two different facets of PdIn intermetallic compound were investigated and the results are presented in Figure 3a–e and Table 2. It is of interest to analyze the DRIFTS data on CO adsorption on the surface of intermetallic nanoparticles by DFT calculations in two aspects: (1) the absence of signals from multiply coordinated CO species, and (2) the position of absorption bands and the adsorption energy of linear forms of adsorbed CO.
DFT calculations indicate that CO does not bind to In atoms, but only to the sites consisting of Pd. This limits the possible adsorption modes of CO on PdIn surface significantly. DFT results also show that on the (110) surface CO is capable of binding both bridged and linearly to Pd (Figure 3 c,d respectively). However, the energy of CO adsorption in bridged position on the (110) facet is negligible (−0.09 eV), therefore this band is not detectable under the conditions of our experiments. Such a low energy of adsorption compared to the energy of adsorption of the bridged form of CO on monometallic palladium (−1.51 to 1.71 eV, see Table 2) stems from the different geometry of the bridge site on intermetallic PdIn (110) and monometallic Pd. For monometallic Pd the distance between neighboring Pd atoms is 2.8 Å, whereas on PdIn(110) the corresponding distance is 3.34 Å (Figure 3a and Figure S3). Therefore, to facilitate the CO adsorption at the bridge site a quite heavy reconstruction of the surface takes place, where the interatomic Pd-Pd distance at the bridge site is shortened to 2.96 Å.
On the (111) surface, CO can only bind linearly to Pd as shown in Figure 3e. Adsorption of bridge-bonded CO on PdIn(111) facet is impossible due to specific atomic geometry of Pd site on the surface of (111) facet. As can be seen from Figure 3e and Figure S3, the Pd atoms adjacent to the Pd atom are located significantly below the surface plane. Since the nearest Pd surface atom is at a distance of 4.73 Å, this makes bridging CO adsorption impossible. Thus, the calculated data is consisted with the absence of additional bands in the range of 2000–1800 cm−1 in the CO-DRIFTS spectra of PdIn/Al2O3 sample and clearly show that only linear CO adsorption on isolated Pd1 sites is possible on the surface of intermetallic PdIn nanoparticles.
DFT study of linear CO adsorption indicates that CO adsorbed on (111) and (110) facets vibrates with a frequency value of 2065 and 2058 cm−1, which is in qualitative agreement with experimentally observed values: 2064 and 2055 cm−1 (Table 2). It is remarkable that DFT calculations also show stronger binding of linear CO on the (111) facet with an adsorption energy of −0.77 eV compared to an adsorption energy of −0.39 eV on the (110) surface. Different strength of CO adsorption is in agreement with the experimental data on different stability of linear CO species revealed by variation of CO adsorption temperature (Figure 2b). Thus, the preferential decrease in the intensity of the peak at 2055 cm−1 with increasing adsorption temperature is in agreement with weaker CO binding on (110) facet.
Different stability of CO adsorbed on (111) and (110) facets can be explained as follows. The surface Pd-atoms in both surface terminations have a coordination of 8 nearest neighbors; however, in the surface layer of the (111) surface each Pd-atom is surrounded by 4 Pd and 4 In, whereas each surface Pd in the (110)-surface is surrounded by 2 Pd and 6 In. In order to address the effect of the local environment on the reactivity of surface Pd, we calculated the d-band center of the surface Pd-atom to which CO bind. According to the d-band model proposed in [43], the strength of the adsorption decreases as the d-states are shifted down in energy. We find a downshift in the d-states energy going from PdIn(111) (−1.6 eV) to PdIn(110) (−2.4 eV), which explains the weaker binding of CO to Pd on the surface of PdIn(110).
3. Conclusions
In summary, supported intermetallic PdIn nanoparticles were synthesized and their surface structure was studied by combination of experimental and theoretical methods: CO-DRIFT spectroscopy and DFT calculations. Results of CO-DRIFTS revealed only linear CO adsorption on PdIn nanoparticles as indicated by two CO stretching vibration bands at 2065 and 2055 cm−1, corresponding to CO adsorption on (111) and (110) facets. The assignment of the bands was confirmed by DFT calculation. Both experimental and theoretical methods have shown that multipoint adsorption of CO on the surface of PdIn nanoparticles is unfavorable. On the PdIn (110) facet the energy of CO adsorption in bridged position was found to be negligible (−0.09 eV) because of too large a distance between neighboring Pd (3.35 Å). Adsorption of bridge-bonded CO on PdIn(111) facet is impossible since palladium atoms adjacent to the surface palladium atom are located significantly below the surface plane, while the nearest Pd surface atom is at a distance of 4.73 Å, which excludes bridging adsorption.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11111376/s1. See the Supplementary Materials for details of catalyst preparation, experimental section, TEM images, and details of TEM, XRD, CO-DRIFTS experiments and additional computational details and CO-DRIFTS spectra. Figure S1: Representative TEM images for Pd/Al2O3 (left) and PdIn/Al2O3 (right) catalysts. Figure S2: CO-DRIFTS data for PdIn/Al2O3 catalyst: (a) CO desorption from PdIn/Al2O3 in He flow at 50 °C; (b) peak deconvolution after 0, 14 and 24 min of the experiment. Before the desorption experiment PdIn sample was reduced in situ at 500 °C in 5%H2/Ar flow. After cooling to 50 °C He flow, flow of 0.5 vol.% CO/He was introduced to the cell and the spectra were collected. Then the adsorbed CO was purged by helium flow (30 L/min) at 50 °C for 30min with subsequent recording of spectra. Figure S3: Scheme of the neighboring Pd positions on the Pd (110) and Pd (111) surface planes. Pd atoms are blue and In atoms are brown. Table S1: Parameters used for DFT calculations.
Author Contributions
All authors contributed equally to this work. A.Y.S. initiated the idea and supervised this research; G.N.B. prepared all materials for TEM, XRD, and CO-DRIFTS analysis; N.S.S. and P.V.M. performed CO-DRIFTS analysis and interpreted the data; A.V.B. performed XRD analysis; P.V.M. interpreted the XRD data; I.S.M. interpreted TEM data; H.F. performed DFT calculations; I.S.M. and A.Y.S. wrote the manuscript with the help of N.S.S., P.V.M., A.V.B., G.N.B., and H.F. All authors contributed to the discussion of the results reported in the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant N° 19-13-00285.
Acknowledgments
Authors are grateful to the Department of Structural Studies of the N.D. Zelinsky Institute of Organic Chemistry RAS for studying the samples by electron microscopy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hannagan, R.T.; Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Single-Atom Alloy Catalysis. Chem. Rev. 2020, 120, 12044–12088. [Google Scholar] [CrossRef] [PubMed]
- Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Single-Atom alloys as a Reductionist approach to the Rational Design of Heterogeneous Catalysis. Acc. Chem. Res. 2019, 52, 237–247. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective Hydrogenation over Supported Metal Catalysts: From Nanoparticles to Single Atoms. Chem. Rev. 2020, 120, 683–733. [Google Scholar] [CrossRef]
- Wang, A.; Jun, L.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- McCue, A.J.; Anderson, J.A. CO induced surface segregation as a means of improving surface composition and enhancing performance of CuPd bimetallic catalysts. J. Catal. 2015, 329, 538–546. [Google Scholar] [CrossRef]
- Bukhtiyarov, A.V.; Panafidin, M.A.; Chetyrin, I.A.; Prosvirin, I.P.; Mashkovsky, I.S.; Smirnova, N.S.; Markov, P.V.; Zubavichus, Y.V.; Stakheev, A.Y.; Bukhtiyarov, V.I. Intermetallic Pd-In/HOPG model catalysts: Reversible tuning the surface structure by O2-induced segregation. Appl. Surf. Sci. 2020, 525, 146493. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Smirnova, N.S.; Markov, P.V.; Baeva, G.N.; Bragina, G.O.; Rassolov, A.V.; Mashkovsky, I.S. Adsorption-Induced Segregation as a Method for the Target-Oriented Modification of the Surface of a Bimetallic Pd-Ag Catalyst. Kinet. Catal. 2018, 59, 610–617. [Google Scholar] [CrossRef]
- Smirnova, N.S.; Markov, P.V.; Baeva, G.N.; Rassolov, A.V.; Mashkovsky, I.S.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Panafidin, M.A.; Zubavichus, Y.V.; Bukhtiyarov, V.I.; et al. CO-induced segregation as an efficient tool to control the surface composition and catalytic performance of PdAg/Al2O3 catalyst. Mendeleev Commun. 2019, 29, 547–549. [Google Scholar] [CrossRef]
- Meyer, R.J.; Zhang, Q.; Kryczka, A.; Gomez, C.; Todorovic, R. Perturbation of Reactivity with Geometry: How Far Can We Go? ACS Catal. 2018, 8, 566–570. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Komatsu, T. Intermetallic Compounds: Promising Inorganic Materials for Well-Structured and Electronically Modified Reaction Environments for Efficient Catalysis. ACS Catal. 2017, 7, 735–765. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Peter, S.C. Synthetically tuned electronic and geometrical properties of intermetallic compounds as effective heterogeneous catalysts. Prog. Solid State Chem. 2018, 52, 1–30. [Google Scholar] [CrossRef]
- Armbrüster, M.; Behrens, M.; Cinquini, F.; Föttinger, K.; Grin, Y.; Haghofer, A.; Klötzer, B.; Knop-Gericke, A.; Lorenz, H.; Ota, A.; et al. How to Control the Selectivity of Palladium-based Catalysts in Hydrogenation Reactions: The Role of Subsurface Chemistry. ChemCatChem 2012, 4, 1048–1063. [Google Scholar] [CrossRef]
- Yoo, J.S.; Abild-Pedersen, F.; Nørskov, J.K.; Studt, F. Theoretical Analysis of Transition-Metal Catalysts for Formic Acid Decomposition. ACS Catal. 2014, 4, 1226–1233. [Google Scholar] [CrossRef]
- Shen, Y.G.; O’Connor, D.J.; Wandelt, K.; MacDonald, R.J. Studies of Surface Composition and Structure of Cu3Pt(111) by Low Energy Alkali Ion Scattering. Surf. Sci. 1995, 328, 21–31. [Google Scholar] [CrossRef]
- Schneider, U.; Castro, G.R.; Wandelt, K. Adsorption on Ordered Cu3Pt(111): Site Selectivity. Surf. Sci. 1993, 287−288, 146–150. [Google Scholar] [CrossRef]
- Shen, Y.; O’Connor, J.; MacDonald, R.J. The Interaction of CO with the Cu3Pt(111) Surface. Surf. Sci. 1992, 269−270, 321–325. [Google Scholar]
- Feng, Q.; Zhao, S.; Wang, Y.; Dong, J.; Chen, W.; He, D.; Wang, D.; Yang, J.; Zhu, Y.; Zhu, H.; et al. Isolated Single-Atom Pd Sites in Intermetallic Nanostructures: High Catalytic Selectivity for Semihydrogenation of Alkynes. J. Am. Chem. Soc. 2017, 139, 7294–7301. [Google Scholar] [CrossRef] [PubMed]
- Mashkovsky, I.S.; Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Rassolov, A.V.; Yakushev, I.A.; Vargaftik, M.N.; Stakheev, A.Y. Highly-Ordered PdIn Intermetallic Nanostuctures Obtained from Heterobimetallic Acetate Complex: Formation and Catalytic Properties in Diphenylacetylene hydrogenation. Nanomaterials 2018, 8, 769. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Peng, M.; Ling, L.; Wang, B. PdIn Intermetallic Material with Isolated Single-Atom Pd Sites—A Promising Catalyst for Direct Formic Acid Fuel Cell. Chem. Eng. Sci. 2019, 199, 64–78. [Google Scholar] [CrossRef]
- Burueva, D.B.; Kovtunov, K.V.; Bukhtiyarov, A.V.; Barskiy, D.A.; Prosvirin, I.P.; Mashkovsky, I.S.; Baeva, G.N.; Bukhtiyarov, V.I.; Stakheev, A.Y.; Koptyug, I.V. Selective Single-Site Pd−In Hydrogenation Catalyst for Production of Enhanced Magnetic Resonance Signals Using Parahydrogen. Chem. Eur. J. 2018, 24, 2547–2553. [Google Scholar] [CrossRef]
- Wencka, M.; Hahne, M.; Kocjan, A.; Vrtnik, S.; Koželj, P.; Korže, D.; Jagličić, Z.; Sorić, M.; Popčević, P.; Ivkov, J.; et al. Physical properties of the InPd intermetallic catalyst. Intermetallics 2014, 55, 56–65. [Google Scholar] [CrossRef]
- Kovnir, K.; Armbrüster, M.; Teschner, D.; Venkov, T.V.; Szentmiklósi, L.; Jentoft, F.C.; Knop-Gericke, A.; Grin, Y.; Schlögl, R. In situ surface characterization of the intermetallic compound PdGa—A highly selective hydrogenation catalyst. Surf. Sci. 2009, 603, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Villaseca, S.A.; Friedrich, M.; Teschner, D.; Knop-Gericke, A.; Armbrüster, M. Addressing electronic effects in the semi-hydrogenation of ethyne by InPd2 and intermetallic Ga–Pd compounds. J. Catal. 2016, 338, 265–272. [Google Scholar] [CrossRef]
- Ryczkowski, J. IR spectroscopy in catalysis. Catal. Today 2001, 68, 263–381. [Google Scholar] [CrossRef]
- Yudanov, I.V.; Sahnoun, R.; Neyman, K.M.; Rösch, N.; Hoffmann, J.; Schauermann, S.; Johánek, V.; Unterhalt, H.; Rupprechter, G.; Libuda, J.; et al. CO Adsorption on Pd Nanoparticles: Density Functional and Vibrational Spectroscopy Studies. J. Phys. Chem. B. 2003, 107, 255–264. [Google Scholar] [CrossRef]
- Garcia-Trenco, A.; Regoutz, A.; White, E.R.; Payne, D.J.; Shaffer, M.S.P.; Williams, C.K. PdIn intermetallic nanoparticles for the Hydrogenation of CO2 to Methanol. Appl. Catal. B. Env. 2018, 220, 9–18. [Google Scholar] [CrossRef]
- Furukawa, S.; Endo, M.; Komatsu, T. Bifunctional Catalytic System Effective for Oxidative Dehydrogenation of 1-Butene and n-Butane Using Pd-Based Intermetallic Compounds. ACS Catal. 2014, 4, 3533–3542. [Google Scholar] [CrossRef]
- Cao, Y.; Sui, Z.; Zhu, Y.; Zhou, X.; Chen, D. Selective Hydrogenation of Acetylene over Pd-In/Al2O3 Catalyst: Promotional Effect of Indium and Composition-Dependent Performance. ACS Catal. 2017, 7, 7835–7846. [Google Scholar] [CrossRef]
- Chauruka, S.R.; Hassanpour, A.; Brydson, R.; Roberts, K.J.; Ghadiri, M.; Stitt, H. Effect of mill type on the size reduction and phase transformation of gamma alumina. Chem. Eng. Sci. 2015, 134, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Markov, P.V.; Smirnova, N.S.; Baeva, G.N.; Bukhtiyarov, A.V.; Mashkovsky, I.S.; Stakheev, A.Y. Intermetallic PdxIny/Al2O3 catalysts with isolated single-atom Pd sites for one-pot hydrogenation of diphenylacetylene into trans-stilbene. Mendeleev Commun. 2020, 30, 468–471. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Smirnova, N.S.; Markov, P.V.; Baeva, G.N.; Bragina, G.O.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Stakheev, A.Y. Tuning the surface structure and catalytic performance of PdIn/Al2O3 in selective liquid-phase hydrogenation by mild oxidative-reductive treatments. Mendeleev Commun. 2018, 28, 603–605. [Google Scholar] [CrossRef]
- Markov, P.V.; Bukhtiyarov, A.V.; Mashkovsky, I.S.; Smirnova, N.S.; Prosvirin, I.P.; Vinokurov, Z.S.; Panafidin, M.A.; Baeva, G.N.; Zubavichus, Y.V.; Bukhtiyarov, V.I.; et al. PdIn/Al2O3 Intermetallic Catalyst: Structure and Catalytic Characteristics in Selective Hydrogenation of Acetylene. Kinet. Catal. 2019, 60, 842–850. [Google Scholar] [CrossRef]
- Hirano, T.; Kazahaya, Y.; Nakamura, A.; Miyao, T.; Naito, S. Remarkable effect of addition of In and Pb on the reduction of N2O by CO over SiO2 supported Pd catalysts. Catal. Lett. 2007, 117, 73–78. [Google Scholar] [CrossRef]
- Wu, Z.; Wegener, E.C.; Tseng, H.-T.; Gallagher, J.R.; Harris, J.W.; Diaz, R.E.; Ren, Y.; Ribeiro, F.H.; Miller, J.T. Pd–In intermetallic alloy nanoparticles: Highly selective ethane dehydrogenation catalysts. Catal. Sci. Technol. 2016, 6, 6965–6976. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Smirnova, N.S.; Krivoruchenko, D.S.; Baeva, G.N.; Mashkovsky, I.S.; Yakushev, I.A.; Vargaftik, M.N. Single-atom Pd sites on the surface of Pd–In nanoparticles supported on γ-Al2O3: A CO-DRIFTS study. Mendeleev Commun. 2017, 27, 515–517. [Google Scholar] [CrossRef]
- Matori, K.A.; Wah, L.C.; Hashim, M.; Ismail, I.; Hafiz, M.; Zaid, M. Phase Transformations of α-Alumina Made from Waste Aluminum via a Precipitation Technique. Int. J. Mol. Sci. 2012, 13, 16812–16821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wu, K.; Cao, J.; Wang, Y. Controlled synthesis of α-Al2O3 via the hydrothermal-pyrolysis method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012004. [Google Scholar] [CrossRef]
- Lorenz, H.; Turner, S.; Lebedev, O.I.; Tendeloo, G.V.; Klötzer, B.; Rameshan, C.; Pfaller, K.; Penner, S. Pd–In2O3 interaction due to reduction in hydrogen: Consequences for methanol steam reforming. Appl. Catal. A Gen. 2010, 374, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Yea, J.; Gea, Q.; Liu, C. Effect of PdIn bimetallic particle formation on CO2 reduction over the Pd–In/SiO2 catalyst. Chem. Eng. Sci. 2015, 135, 193–201. [Google Scholar] [CrossRef]
- Smirnova, N.S.; Khramov, E.V.; Baeva, G.N.; Markov, P.V.; Bukhtiyarov, A.V.; Zubavichus, Y.V.; Stakheev, A.Y. An Investigation into the Bulk and Surface Phase Transformations of Bimetallic Pd-In/Al2O3 Catalyst during Reductive and Oxidative Treatments In Situ. Catalysts 2021, 11, 859. [Google Scholar] [CrossRef]
- Lentz, C.; Panahian Jand, S.; Melke, J.; Roth, C.; Kaghazchi, P. DRIFTS study of CO adsorption on Pt nanoparticles supported by DFT calculations. J. Mol. Catal. A Chemical. 2017, 426, 1–9. [Google Scholar] [CrossRef]
- Meunier, F.C. Relevance of IR Spectroscopy of Adsorbed CO for the Characterization of Heterogeneous Catalysts Containing Isolated Atoms. J. Phys. Chem. C. 2021, 125, 21810–21823. [Google Scholar] [CrossRef]
- Lear, T.; Marshall, R.; Lopez-Sanchez, A.J.; Jackson, S.D. The application of infrared spectroscopy to probe the surface morphology of alumina-supported palladium catalysts. J. Chem. Phys. 2005, 123, 174706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).