TiO2–Zeolite Metal Composites for Photocatalytic Degradation of Organic Pollutants in Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Characterization
2.2. Photocatalytic Activity
3. Materials and Methods
3.1. Preparation of Physical Mixture of TiO2 and Zeolite Y
3.2. Synthesis of Pd Nanoparticles and Preparation of Pd-Containing Photocatalysts
3.3. Synthesis of Ag Nanoparticles and Preparation of Ag-Containing Photocatalysts
3.4. Characterization Methods
3.4.1. Nitrogen Sorption
3.4.2. X-ray Diffraction
3.4.3. TEM/STEM
3.4.4. XPS
3.4.5. UV-Vis Measurements
3.4.6. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Burek, P.; Satoh, Y.; Fischer, G.; Kahil, M.T.; Scherzer, A.; Tramberend, S.; Nava, L.F.; Wada, Y.; Eisner, S.; Flörke, M.; et al. Water Futures and Solution. Fast Track Initiative—Final Report ADA Project Number 2725-00/2014. Water Futur. Solut. 2016, 1–113. Available online: http://dare.iiasa.ac.at/111/ (accessed on 10 November 2021).
- Veldkamp, T.I.E.; Wada, Y.; Aerts, J.C.J.H.; Döll, P.; Gosling, S.N.; Liu, J.; Masaki, Y.; Oki, T.; Ostberg, S.; Pokhrel, Y.; et al. Water scarcity hotspots travel downstream due to human interventions in the 20th and 21st century. Nat. Commun. 2017, 8, 15697. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, A.N.; Durham, B. Water recycling and reuse in EUREAU countries: Trends and challenges. Desalination 2008, 218, 3–12. [Google Scholar] [CrossRef]
- Garcia, X.; Pargament, D. Reusing wastewater to cope with water scarcity: Economic, social and environmental considerations for decision-making. Resour. Conserv. Recycl. 2015, 101, 154–166. [Google Scholar] [CrossRef]
- United Nations Development Programme (UNDP). Sustainable Development Goals. Available online: https://www.undp.org/sustainable-development-goals (accessed on 28 September 2021).
- WEDECO, ITT Industries. UV Disinfection and Ozone Oxidation Systems. Available online: https://www.water-technology.net/contractors/disinfection/wedeco2/ (accessed on 28 September 2021).
- MetaWater: Development of Environmentally Friendly Products: Water Technologies. Available online: https://www.metawater.co.jp/eng/csr/environment/product_development/ (accessed on 28 September 2021).
- Chan, S.H.S.; Wu, T.Y.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, R.; Rao, B.; Rakowska, M.; Athanasiou, D.; Millerick, K.; Wei, S.; Lei, X.; Lou, H.H.; Reible, D.D. Removal of Polycyclic Aromatic Hydrocarbons from Water Using Mn(III)-Based Advanced Oxidation Process. J. Environ. Eng. 2021, 147, 04021002. [Google Scholar] [CrossRef]
- Wang, S.; Yi, L.; Halpert, J.E.; Lai, X.; Liu, Y.; Cao, H.; Yu, R.; Wang, D.; Li, Y. A novel and highly efficient photocatalyst based on P 25- graphdiyne nanocomposite. Small 2012, 8, 265–271. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Narciso-da-Rocha, C.; Polo-López, M.I.; Pastrana-Martínez, L.M.; Faria, J.L.; Manaia, C.M.; Fernández-Ibáñez, P.; Nunes, O.C.; Silva, A.M.T. Solar treatment (H2O2, TiO2-P25 and GO-TiO2 photocatalysis, photo-Fenton) of organic micropollutants, human pathogen indicators, antibiotic resistant bacteria and related genes in urban wastewater. Water Res. 2018, 135, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ruiz, B.; Ribao, P.; Diban, N.; Rivero, M.J.; Ortiz, I.; Urtiaga, A. Photocatalytic degradation and mineralization of perfluorooctanoic acid (PFOA) using a composite TiO2 −rGO catalyst. J. Hazard. Mater. 2018, 344, 950–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukahori, S.; Ichiura, H.; Kitaoka, T.; Tanaka, H. Capturing of bisphenol A photodecomposition intermediates by composite TiO2-zeolite sheets. Appl. Catal. B Environ. 2003, 46, 453–462. [Google Scholar] [CrossRef]
- Zhang, G.; Song, A.; Duan, Y.; Zheng, S. Enhanced photocatalytic activity of TiO2/zeolite composite for abatement of pollutants. Microporous Mesoporous Mater. 2018, 255, 61–68. [Google Scholar] [CrossRef]
- Jansson, I.; Suárez, S.; Garcia-Garcia, F.J.; Sánchez, B. Zeolite-TiO2 hybrid composites for pollutant degradation in gas phase. Appl. Catal. B Environ. 2015, 178, 100–107. [Google Scholar] [CrossRef]
- Jiang, N.; Shang, R.; Heijman, S.G.J.; Rietveld, L.C. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review. Water Res. 2018, 144, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Rayalu, S.S.; Labhsetwar, N.K.; Devotta, S. Visible light active zeolite-based photocatalysts for hydrogen evolution from water. Int. J. Hydrog. Energy 2008, 33, 5958–5966. [Google Scholar] [CrossRef]
- López-Muñoz, M.-J.; Van Grieken, R.; Aguado, J.; Marugán, J. Role of the support on the activity of silica-supported TiO2 photocatalysts: Structure of the TiO2/SBA-15 photocatalysts. Catal. Today 2005, 101, 307–314. [Google Scholar] [CrossRef]
- Romay, M.; Diban, N.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. Critical Issues and Guidelines to Improve the Performance of Photocatalytic Polymeric Membranes. Catalysts 2020, 10, 570. [Google Scholar] [CrossRef]
- Corredor, J.; Perez-Peña, E.; Rivero, M.J.; Ortiz, I. Performance of rGO/TiO2 Photocatalytic Membranes for Hydrogen Production. Membranes 2020, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Janssens, R.; Mandal, M.K.; Dubey, K.K.; Luis, P. Slurry photocatalytic membrane reactor technology for removal of pharmaceutical compounds from wastewater: Towards cytostatic drug elimination. Sci. Total Environ. 2017, 599–600, 612–626. [Google Scholar] [CrossRef]
- Marugán, J.; Hufschmidt, D.; López-Muñoz, M.J.; Selzer, V.; Bahnemann, D. Photonic efficiency for methanol photooxidation and hydroxyl radical generation on silica-supported TiO2 photocatalysts. Appl. Catal. B Environ. 2006, 62, 201–207. [Google Scholar] [CrossRef]
- Joseph, C.G.; Taufiq-Yap, Y.H.; Musta, B.; Sarjadi, M.S.; Elilarasi, L. Application of Plasmonic Metal Nanoparticles in TiO2-SiO2 Composite as an Efficient Solar-Activated Photocatalyst: A Review Paper. Front. Chem. 2021, 8, 1283. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, H.A.; Nasr, R.A.; Vannier, R.-N.; Jamil, T.S. Improving of photocatalytic activity of barium ferrate via bismuth and copper co-doping for degradation of paracetamol under visible light irradiation. J. Environ. Sci. 2022, 112, 331–342. [Google Scholar] [CrossRef]
- Wodka, D.; Bielaníska, E.; Socha, R.P.; Elzbieciak-Wodka, M.; Gurgul, J.; Nowak, P.; Warszyníski, P.; Kumakiri, I. Photocatalytic activity of titanium dioxide modified by silver nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Aoyama, J.; Miyakubo, K.; Eguchi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. TiO2 photocatalyst for degradation of organic compounds in water and air supported on highly hydrophobic FAU zeolite: Structural, sorptive, and photocatalytic studies. J. Catal. 2012, 285, 223–234. [Google Scholar] [CrossRef]
- Piedra López, J.G.; González Pichardo, O.H.; Pinedo Escobar, J.A.; de Haro del Río, D.A.; Inchaurregui Méndez, H.; González Rodríguez, L.M. Photocatalytic degradation of metoprolol in aqueous medium using a TiO2/natural zeolite composite. Fuel 2021, 284, 119030. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Ribao, P.; Corredor, J.; Rivero, M.J.; Ortiz, I. Role of reactive oxygen species on the activity of noble metal-doped TiO2 photocatalysts. J. Hazard. Mater. 2019, 372, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, S.; Ribao, P.; Rivero, M.J.; Ortiz, I. Influence of radiation and TiO2 concentration on the hydroxyl radicals generation in a photocatalytic LED reactor. Application to dodecylbenzenesulfonate degradation. Appl. Catal. B Environ. 2015, 178, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Kosydar, R.; Szewczyk, I.; Natkański, P.; Duraczyńska, D.; Gurgul, J.; Kuśtrowski, P.; Drelinkiewicz, A. New insight into the effect of surface oxidized groups of nanostructured carbon supported Pd catalysts on the furfural hydrogenation. Surf. Interfaces 2019, 17, 100379. [Google Scholar] [CrossRef]
- Oćwieja, M.; Adamczyk, Z.; Morga, M.; Michna, A. High density silver nanoparticle monolayers produced by colloid self-assembly on polyelectrolyte supporting layers. J. Colloid Interface Sci. 2011, 364, 39–48. [Google Scholar] [CrossRef] [PubMed]
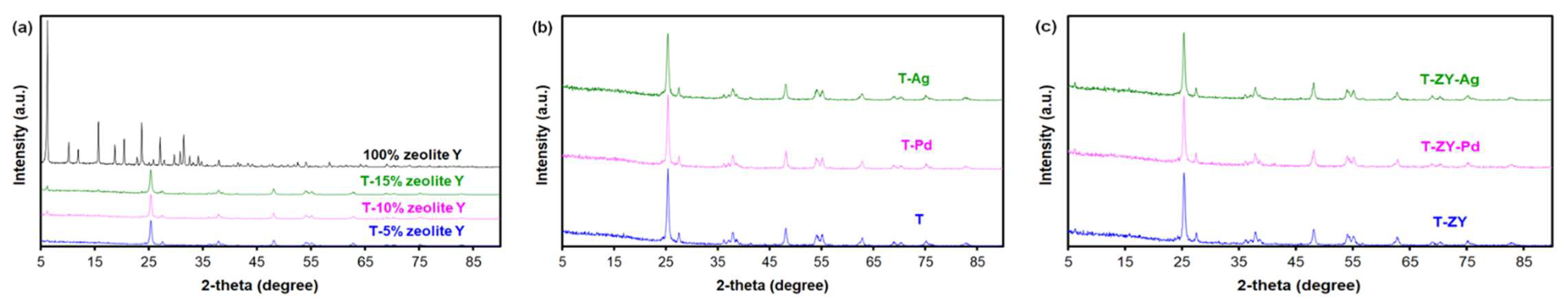
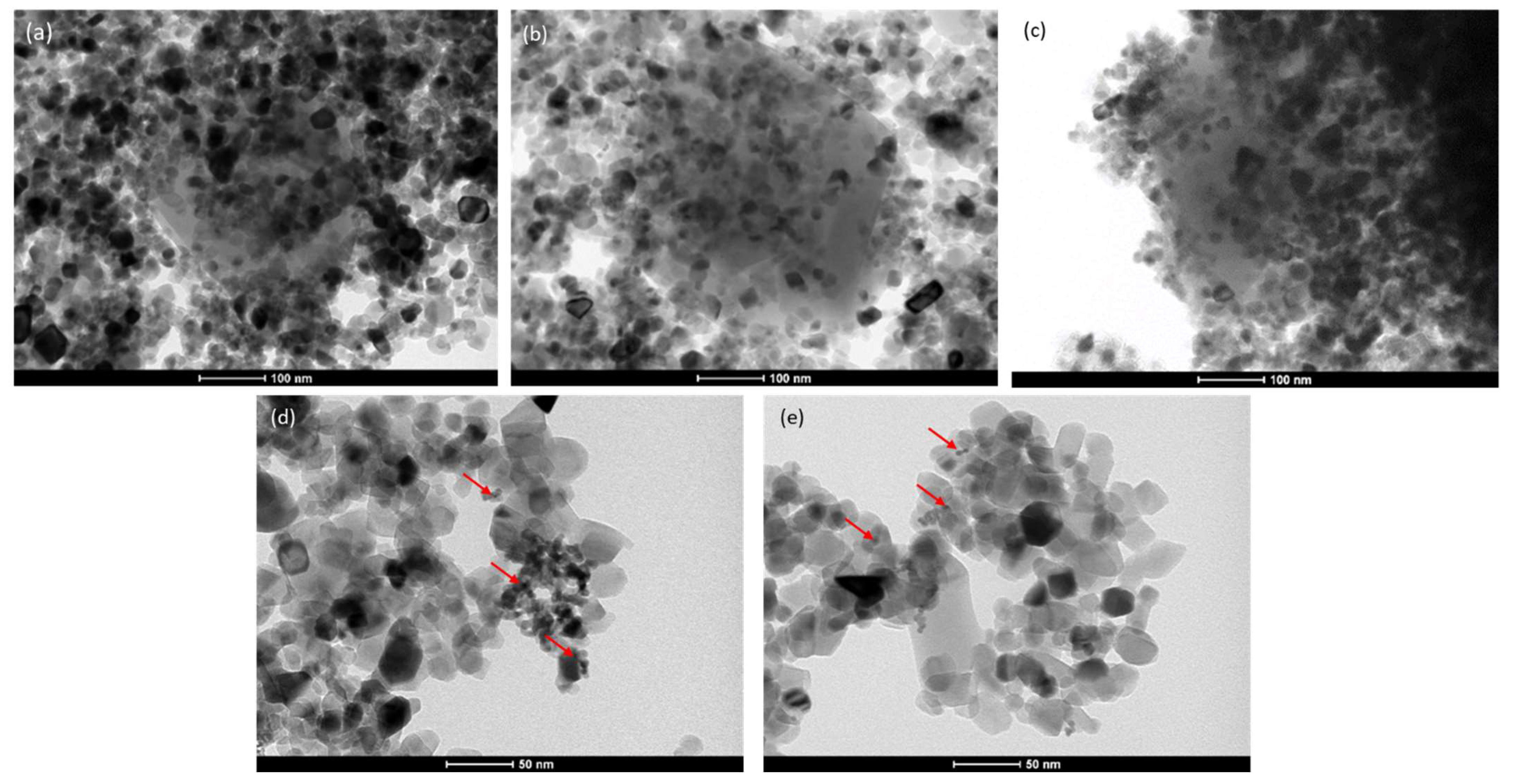
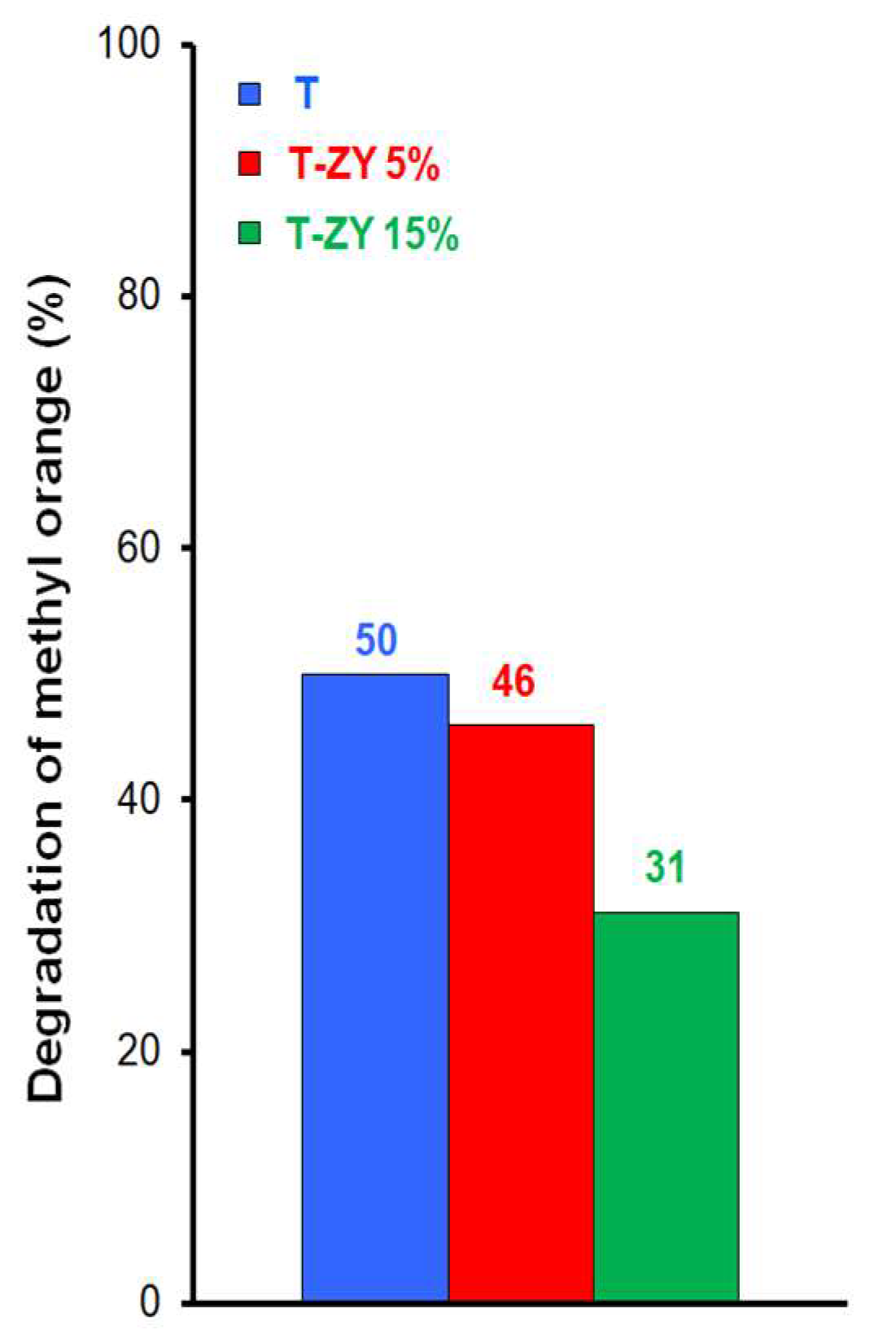

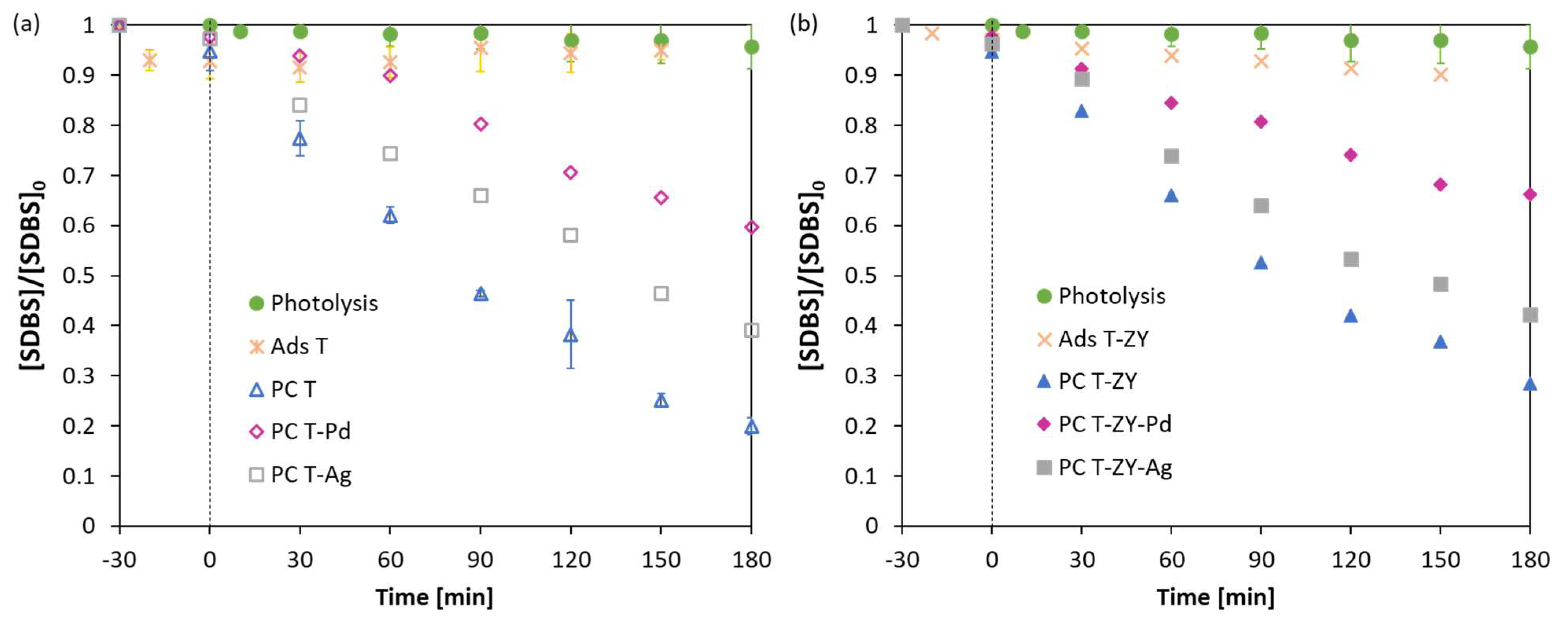
| Sample | Composition | Specific Surface Area, SBET (m2g−1) | Total Pore Volume Vtot (cm3g−1) | Eg (eV) |
|---|---|---|---|---|
| T | TiO2 | 59 | 0.31 | 3.27 |
| T–ZY | TiO2–5% zeolite Y | 84 | 0.26 | 3.20 |
| T–Pd | TiO2-1% Pd | 60 | 0.25 | 3.20 |
| T–ZY–Pd | TiO2–5% zeolite Y-1% Pd | 84 | 0.27 | 3.28 |
| T–Ag | TiO2–0.05% Ag | 59 | 0.26 | 3.22 |
| T–ZY–Ag | TiO2–5% zeolite Y-0.05% Ag | 84 | 0.24 | 3.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diban, N.; Pacuła, A.; Kumakiri, I.; Barquín, C.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. TiO2–Zeolite Metal Composites for Photocatalytic Degradation of Organic Pollutants in Water. Catalysts 2021, 11, 1367. https://doi.org/10.3390/catal11111367
Diban N, Pacuła A, Kumakiri I, Barquín C, Rivero MJ, Urtiaga A, Ortiz I. TiO2–Zeolite Metal Composites for Photocatalytic Degradation of Organic Pollutants in Water. Catalysts. 2021; 11(11):1367. https://doi.org/10.3390/catal11111367
Chicago/Turabian StyleDiban, Nazely, Aleksandra Pacuła, Izumi Kumakiri, Carmen Barquín, Maria J. Rivero, Ane Urtiaga, and Inmaculada Ortiz. 2021. "TiO2–Zeolite Metal Composites for Photocatalytic Degradation of Organic Pollutants in Water" Catalysts 11, no. 11: 1367. https://doi.org/10.3390/catal11111367
APA StyleDiban, N., Pacuła, A., Kumakiri, I., Barquín, C., Rivero, M. J., Urtiaga, A., & Ortiz, I. (2021). TiO2–Zeolite Metal Composites for Photocatalytic Degradation of Organic Pollutants in Water. Catalysts, 11(11), 1367. https://doi.org/10.3390/catal11111367








