Enzymatic Production of Ecodiesel by Using a Commercial Lipase CALB, Immobilized by Physical Adsorption on Mesoporous Organosilica Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Immobilization of Candida Antarctica Lipase B (CALB) on Periodic Mesoporous Organosilica Materials (E-PMO) and on Octyl-MS3030 (E-Octyl-MS3030)
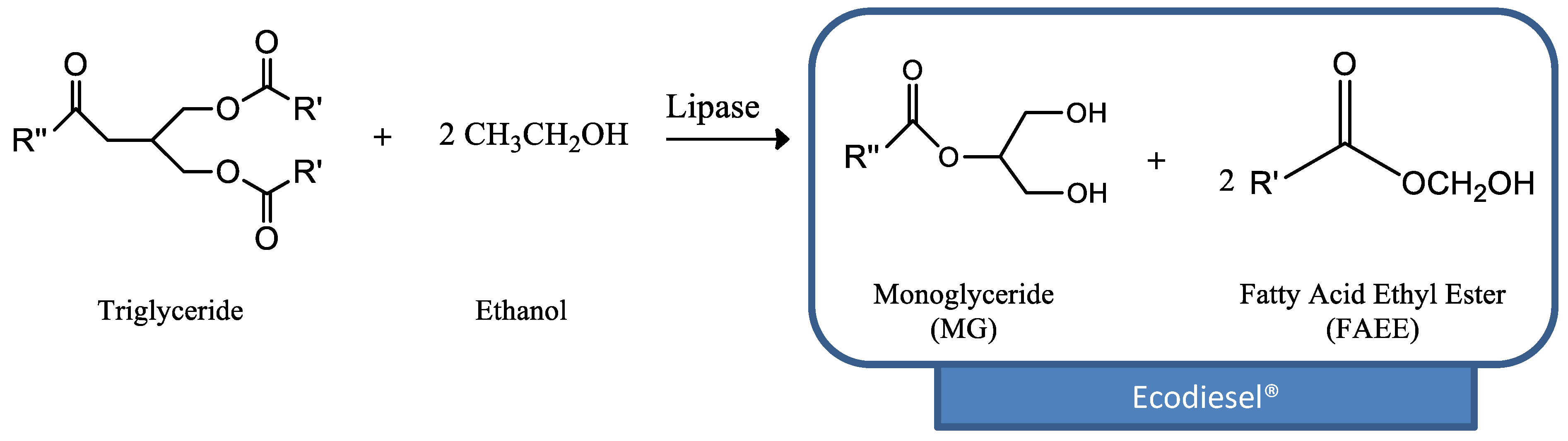
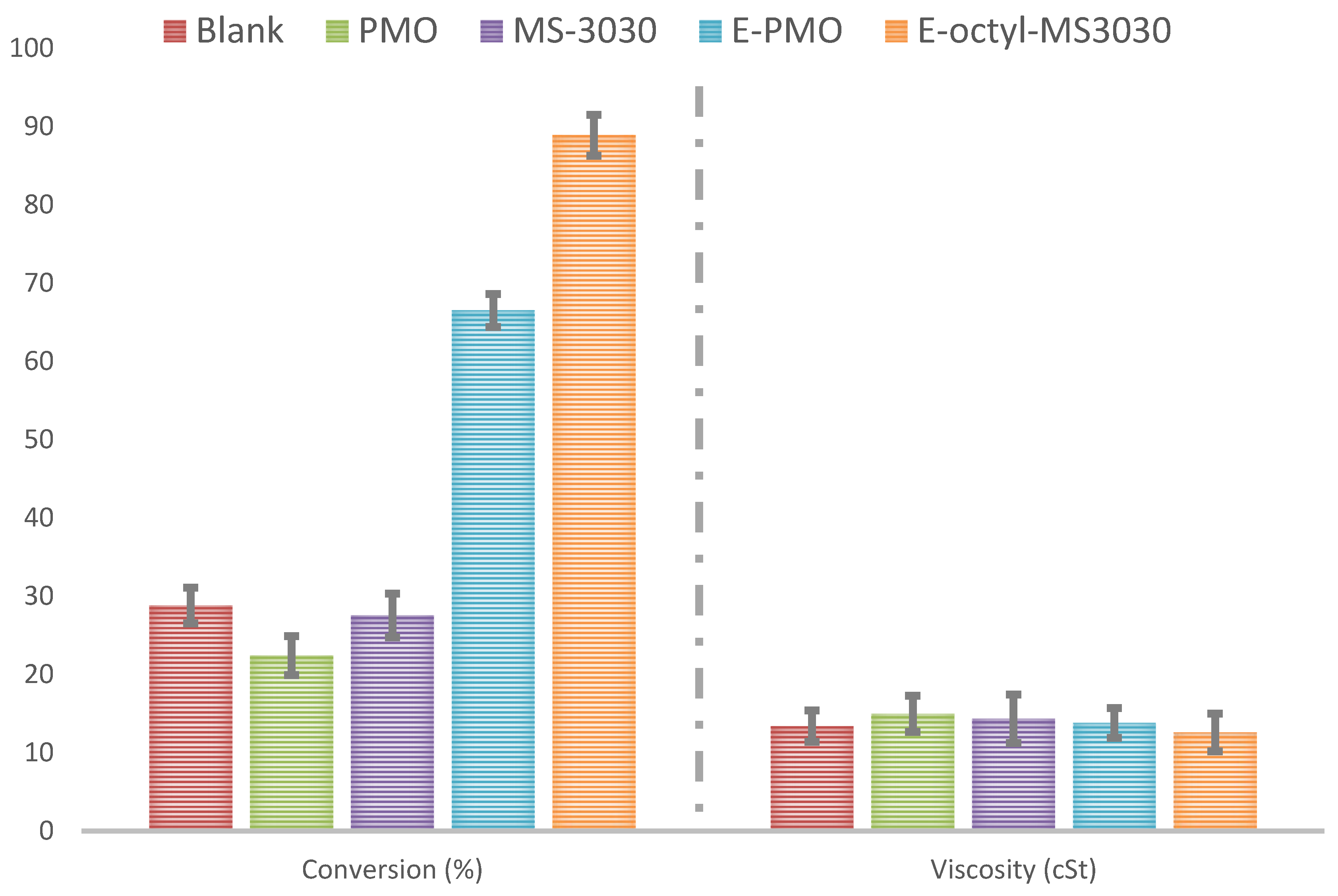
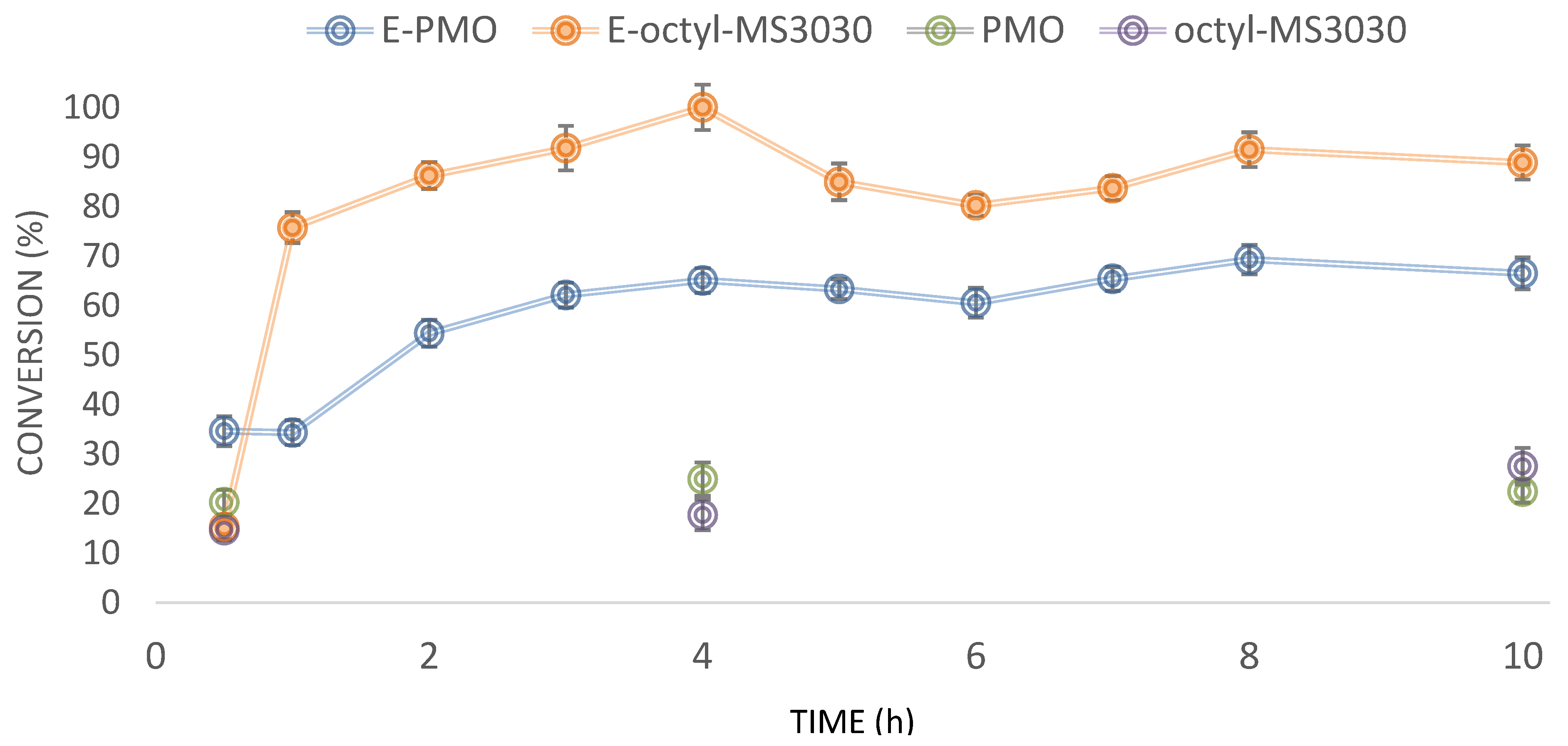
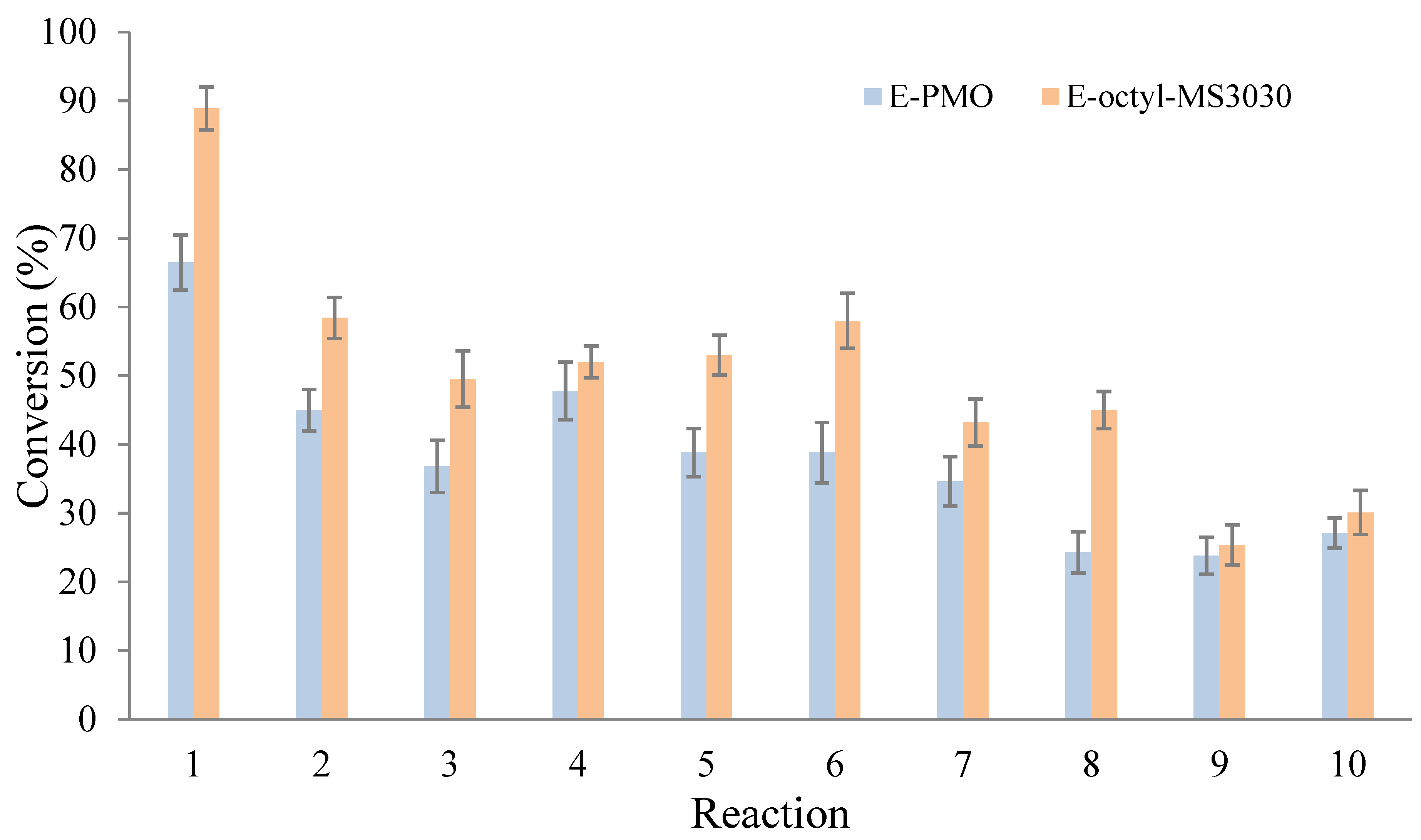
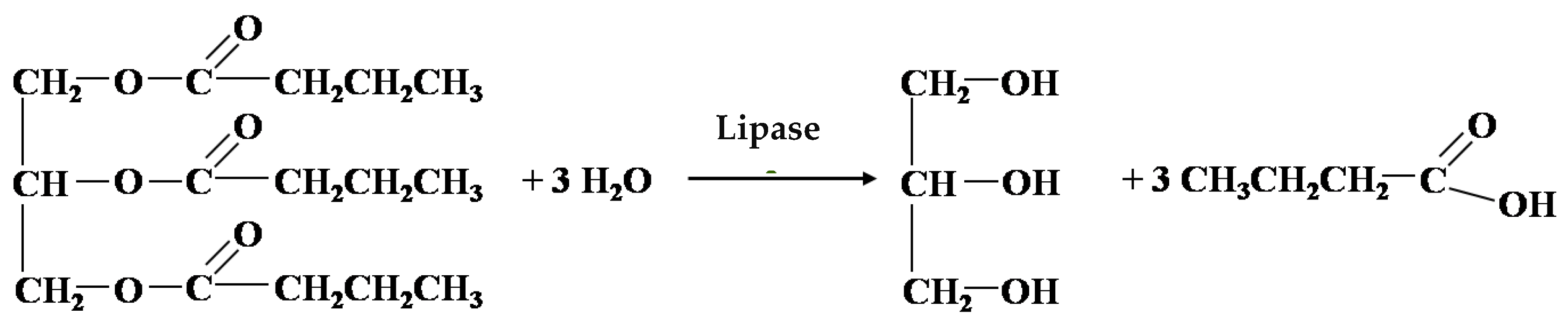
2.2. Transesterification of Sunflower Oil with Ethanol to Produce Ecodiesel
2.3. Comparison with Other Biocatalytic Systems Employed in the Ecodiesel Production
3. Materials and Methods
3.1. Synthesis of the PMO Material
3.2. Synthesis of Octyl-MS3030
3.3. Immobilization of CALB Lipase on PMO and Octyl-MS3030
3.4. Lipase Activity Assay towards the pNPA Hydrolysis
3.5. Lipase Activity Assay towards the Tributyrin Hydrolysis
3.6. Ethanolysis Reaction
3.6.1. Chemicals
3.6.2. Transesterification of Sunflower Oil with Ethanol to Produce Ecodiesel
3.6.3. Analytical Method
3.7. Viscosity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gray, N.; McDonagh, S.; O’Shea, R.; Smyth, B.; Murphy, J.D. Decarbonising ships, planes and trucks: An analysis of suitable low-carbon fuels for the maritime, aviation and haulage sectors. Adv. Appl. Energy 2021, 1, 100008. [Google Scholar] [CrossRef]
- Ortar, N.; Ryghaug, M. Should all cars be electric by 2025? The electric car debate in Europe. Sustainability 2019, 11, 1868. [Google Scholar] [CrossRef] [Green Version]
- Darda, S.; Papalas, T.; Zabaniotou, A. Biofuels journey in Europe: Currently the way to low carbon economy sustainability is still a challenge. J. Clean. Prod. 2019, 208, 575–588. [Google Scholar] [CrossRef]
- Arutyunov, V.S.; Lisichkin, G.V. Energy resources of the 21st century: Problems and forecasts. Can renewable energy sources replace fossil fuels? Russ. Chem. Rev. 2017, 86, 777. [Google Scholar] [CrossRef]
- Zailani, S.; Iranmanesh, M.; Foroughi, B.; Kim, K.; Hyun, S.S. Effects of supply chain practices, integration and closed-loop supply chain activities on cost-containment of biodiesel. Rev. Manag. Sci. 2020, 14, 1299–1319. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A. Biodiesel at the Crossroads: A Critical Review. Catalysts 2019, 9, 1033. [Google Scholar] [CrossRef] [Green Version]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Berbel, J.; Verdugo-Escamilla, C. An overview on glycerol-free processes for the production of renewable liquid biofuels, applicable in diesel engines. Renew. Sustain. Energy Rev. 2015, 42, 1437–1452. [Google Scholar] [CrossRef]
- Caballero, V.; Bautista, F.M.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A.; Hidalgo, J.M.; Luque, R.; Macario, A.; Giordano, G. Sustainable preparation of a novel glycerol-free biofuel by using pig pancreatic lipase: Partial 1, 3-regiospecific alcoholysis of sunflower oil. Process Biochem. 2009, 44, 334–342. [Google Scholar] [CrossRef]
- Luna, D.; Posadillo, A.; Caballero, V.; Verdugo, C.; Bautista, F.M.; Romero, A.A.; Sancho, E.D.; Luna, C.; Calero, J. New biofuel integrating glycerol into its composition through the use of covalent immobilized pig pancreatic lipase. Int. J. Mol. Sci. 2012, 13, 10091–10112. [Google Scholar] [CrossRef] [Green Version]
- Luna, C.; Sancho, E.; Luna, D.; Caballero, V.; Calero, J.; Posadillo, A.; Verdugo, C.; Bautista, F.M.; Romero, A.A. Biofuel that keeps glycerol as monoglyceride by 1, 3-selective ethanolysis with pig pancreatic lipase covalently immobilized on AlPO4 support. Energies 2013, 6, 3879–3900. [Google Scholar] [CrossRef] [Green Version]
- Luna, C.; Verdugo, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Production of a biodiesel-like biofuel without glycerol generation, by using Novozym 435, an immobilized Candida antarctica lipase. Bioresour. Bioprocess. 2014, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Luna, C.; Verdugo, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Biocatalytic behaviour of immobilized Rhizopus oryzae lipase in the 1, 3-selective ethanolysis of sunflower oil to obtain a biofuel similar to biodiesel. Molecules 2014, 19, 11419–11439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calero, J.; Verdugo, C.; Luna, D.; Sancho, E.D.; Luna, C.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Selective ethanolysis of sunflower oil with Lipozyme RM IM, an immobilized Rhizomucor miehei lipase, to obtain a biodiesel-like biofuel, which avoids glycerol production through the monoglyceride formation. New Biotechnol. 2014, 31, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Verdugo, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. A biofuel similar to biodiesel obtained by using a lipase from Rhizopus oryzae, optimized by response surface methodology. Energies 2014, 7, 3383–3399. [Google Scholar] [CrossRef] [Green Version]
- Luna, C.; Luna, D.; Bautista, F.M.; Estevez, R.; Calero, J.; Posadillo, A.; Romero, A.A.; Sancho, E.D. Application of Enzymatic Extracts from a CALB Standard Strain as Biocatalyst within the Context of Conventional Biodiesel Production Optimization. Molecules 2017, 22, 2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calero, J.; Luna, D.; Luna, C.; Bautista, F.M.; Hurtado, B.; Romero, A.A.; Posadillo, A.; Estevez, R. Rhizomucor miehei Lipase Supported on Inorganic Solids, as Biocatalyst for the Synthesis of Biofuels: Improving the Experimental Conditions by Response Surface Methodology. Energies 2019, 12, 831. [Google Scholar] [CrossRef] [Green Version]
- Serra, E.; Díez, E.; Díaz, I.; Blanco, R.M. A comparative study of periodic mesoporous organosilica and different hydrophobic mesoporous silicas for lipase immobilization. Microporous Mesoporous Mater. 2010, 132, 487–493. [Google Scholar] [CrossRef]
- Gascón, V.; Díaz, I.; Blanco, R.; Márquez-Álvarez, C. Hybrid periodic mesoporous organosilica designed to improve the properties of immobilized enzymes. RSC Adv. 2014, 4, 34356–34368. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; Dos Santos, J.C.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, A.; Califano, V. Lipase Immobilization in Mesoporous Silica Nanoparticles for Biofuel Production. Catalysts 2021, 11, 629. [Google Scholar] [CrossRef]
- Kim, K.; Lee, O.; Lee, E. Nano-immobilized biocatalysts for biodiesel production from renewable and sustainable resources. Catalysts 2018, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Guisan, J.M.; Bolivar, J.M.; López-Gallego, F.; Rocha-Martín, J. Immobilization of Enzymes and Cells: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Blanco, R.M.; Terreros, P.; Fernández-Pérez, M.; Otero, C.; Díaz-González, G. Functionalization of mesoporous silica for lipase immobilization: Characterization of the support and the catalysts. J. Mol. Catal. B Enzym. 2004, 30, 83–93. [Google Scholar] [CrossRef]
- Gascón-Pérez, V.; Jiménez, M.B.; Molina, A.; Blanco, R.M.; Sánchez-Sánchez, M. Efficient One-Step Immobilization of CaLB Lipase over MOF Support NH2-MIL-53 (Al). Catalysts 2020, 10, 918. [Google Scholar] [CrossRef]
- Venezia, V.; Sannino, F.; Costantini, A.; Silvestri, B.; Cimino, S.; Califano, V. Mesoporous silica nanoparticles for β-glucosidase immobilization by templating with a green material: Tannic acid. Microporous Mesoporous Mater. 2020, 302, 110203. [Google Scholar] [CrossRef]
- Catalano, F.; Alberto, G.; Ivanchenko, P.; Dovbeshko, G.; Martra, G. Effect of silica surface properties on the formation of multilayer or submonolayer protein hard corona: Albumin adsorption on pyrolytic and colloidal SiO2 nanoparticles. J. Phys. Chem. C 2015, 119, 26493–26505. [Google Scholar] [CrossRef] [Green Version]
- Venerando, R.; Miotto, G.; Magro, M.; Dallan, M.; Baratella, D.; Bonaiuto, E.; Zboril, R.; Vianello, F. Magnetic nanoparticles with covalently bound self-assembled protein corona for advanced biomedical applications. J. Phys. Chem. C 2013, 117, 20320–20331. [Google Scholar] [CrossRef]
- Qiao, S.; Yu, C.; Hu, Q.; Jin, Y.; Zhou, X.; Zhao, X.; Lu, G. Control of ordered structure and morphology of large-pore periodic mesoporous organosilicas by inorganic salt. Microporous Mesoporous Mater. 2006, 91, 59–69. [Google Scholar] [CrossRef]
- Guo, W.; Park, J.-Y.; Oh, M.-O.; Jeong, H.-W.; Cho, W.-J.; Kim, I.; Ha, C.-S. Triblock copolymer synthesis of highly ordered large-pore periodic mesoporous organosilicas with the aid of inorganic salts. Chem. Mater. 2003, 15, 2295–2298. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef]
- Feng, X.; Fryxell, G.E.; Wang, L.-Q.; Kim, A.Y.; Liu, J.; Kemner, K.M. Functionalized monolayers on ordered mesoporous supports. Science 1997, 276, 923–926. [Google Scholar] [CrossRef]
- Verdugo, C.; Luque, R.; Luna, D.; Hidalgo, J.M.; Posadillo, A.; Sancho, E.D.; Rodriguez, S.; Ferreira-Dias, S.; Bautista, F.; Romero, A.A. A comprehensive study of reaction parameters in the enzymatic production of novel biofuels integrating glycerol into their composition. Bioresour. Technol. 2010, 101, 6657–6662. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, B.; Posadillo, A.; Luna, D.; Bautista, F.; Hidalgo, J.; Luna, C.; Calero, J.; Romero, A.; Estevez, R. Synthesis, Performance and Emission Quality Assessment of Ecodiesel from Castor Oil in Diesel/Biofuel/Alcohol Triple Blends in a Diesel Engine. Catalysts 2019, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Estevez, R.; Aguado-Deblas, L.; Posadillo, A.; Hurtado, B.; Bautista, F.M.; Hidalgo, J.M.; Luna, C.; Calero, J.; Romero, A.A.; Luna, D. Performance and Emission Quality Assessment in a Diesel Engine of Straight Castor and Sunflower Vegetable Oils, in Diesel/Gasoline/Oil Triple Blends. Energies 2019, 12, 2181. [Google Scholar] [CrossRef] [Green Version]
- Aguado-Deblas, L.; Hidalgo-Carrillo, J.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A.; Estevez, R. Diethyl ether as an oxygenated additive for fossil diesel/vegetable oil blends: Evaluation of performance and emission quality of triple blends on a diesel engine. Energies 2020, 13, 1542. [Google Scholar] [CrossRef] [Green Version]

| Biocatalyst | Time (h) | Max. Load (mg/g) a | Enzyme Immob (%) b | Biocat. Act. (UpNPA/g) c | Cat. Eff. (UpNPA/mg) d | Biocat. Act. (UTB/g) e | Cat. Eff. (UTB/mg) f |
|---|---|---|---|---|---|---|---|
| E-PMO | 5 | 122 | 31 | 322 ± 14 | 2.6 | 5563 ± 138 | 45.6 |
| E-PMO | 3.5 | 100 | 25 | 332 ± 19 | 3.3 | 5366 ± 222 | 53.6 |
| E-Octyl-MS3030 | 5 | 288 | 72 | 358 ± 10 | 1.2 | 10336 ± 287 | 35.9 |
| E-Octyl-MS3030 | 3.5 | 236 | 60 | 468 ± 10 | 2.0 | 7293 ± 328 | 30.9 |
| Enzyme | Support | Reaction Time (h) | Conversion (%) | Viscosity (cSt) | Reference |
|---|---|---|---|---|---|
| CALB | PMO | 3 | 62.1 | 13.8 | This work |
| CALB | octyl-MS3030 | 3 | 91.8 | 12.6 | This work |
| PPL | Sepiolite | 24 | 65.1 | 16.6 | [8] |
| PPL | AlPO4 | 24 | 49.1 | 16.6 | [9] |
| PPL | AlPO4-Sepiolite | 24 | 58.7 | 12.9 | [10] |
| ROL | Sepiolite | 2 | 84.0 | - | [12] |
| Nov.435 | Acrylic resin | 24 | 57.9 | 12.3 | [11] |
| LIP. RM IM | Sepiolite | 2 | 0 | 18.8 | [16] |
| LIP. RM IM | Commercial silica | 2 | 83 | 11.6 | [16] |
| Support | msupport (mg) | Ethanol (mL) | Enzymatic Extract (mg/mL) | Enzymatic Extract (mL) | Buffer (50 mM, pH 5.0) (mL) | Enzymatic Solution (mL) |
|---|---|---|---|---|---|---|
| PMO/ Octyl-MS3030 | 500 | 2, 5 | 5, 9 | 139 | 66 | 205 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna, C.; Gascón-Pérez, V.; López-Tenllado, F.J.; Bautista, F.M.; Verdugo-Escamilla, C.; Aguado-Deblas, L.; Calero, J.; Romero, A.A.; Luna, D.; Estévez, R. Enzymatic Production of Ecodiesel by Using a Commercial Lipase CALB, Immobilized by Physical Adsorption on Mesoporous Organosilica Materials. Catalysts 2021, 11, 1350. https://doi.org/10.3390/catal11111350
Luna C, Gascón-Pérez V, López-Tenllado FJ, Bautista FM, Verdugo-Escamilla C, Aguado-Deblas L, Calero J, Romero AA, Luna D, Estévez R. Enzymatic Production of Ecodiesel by Using a Commercial Lipase CALB, Immobilized by Physical Adsorption on Mesoporous Organosilica Materials. Catalysts. 2021; 11(11):1350. https://doi.org/10.3390/catal11111350
Chicago/Turabian StyleLuna, Carlos, Victoria Gascón-Pérez, Francisco J. López-Tenllado, Felipa M. Bautista, Cristóbal Verdugo-Escamilla, Laura Aguado-Deblas, Juan Calero, Antonio A. Romero, Diego Luna, and Rafael Estévez. 2021. "Enzymatic Production of Ecodiesel by Using a Commercial Lipase CALB, Immobilized by Physical Adsorption on Mesoporous Organosilica Materials" Catalysts 11, no. 11: 1350. https://doi.org/10.3390/catal11111350









