Deactivation of Zeolite Catalysts in the Prins Reaction between Propene and Formaldehyde in the Liquid Phase
Abstract
:1. Introduction
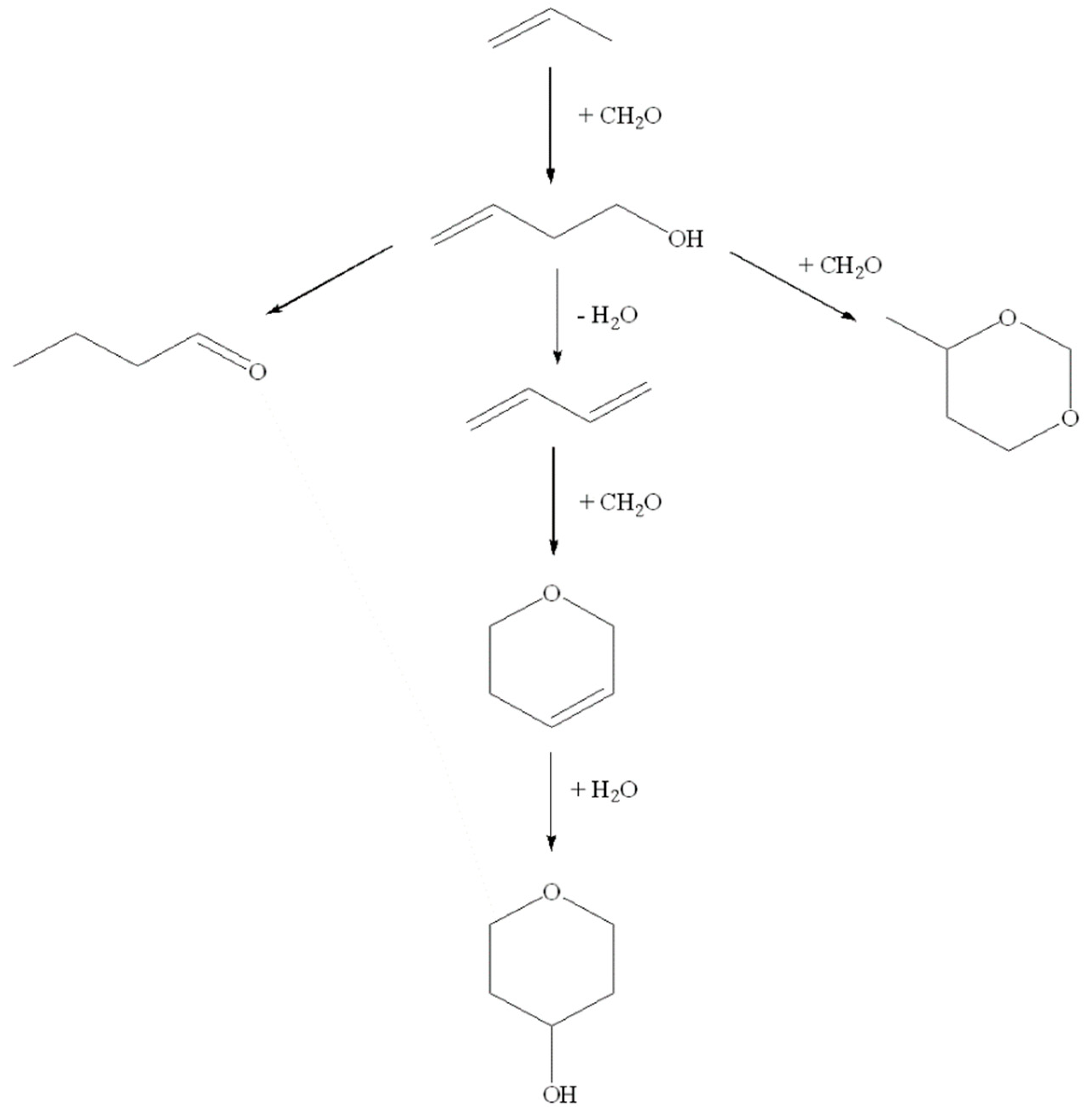
2. Results and Discussion
2.1. Catalysts Characterization
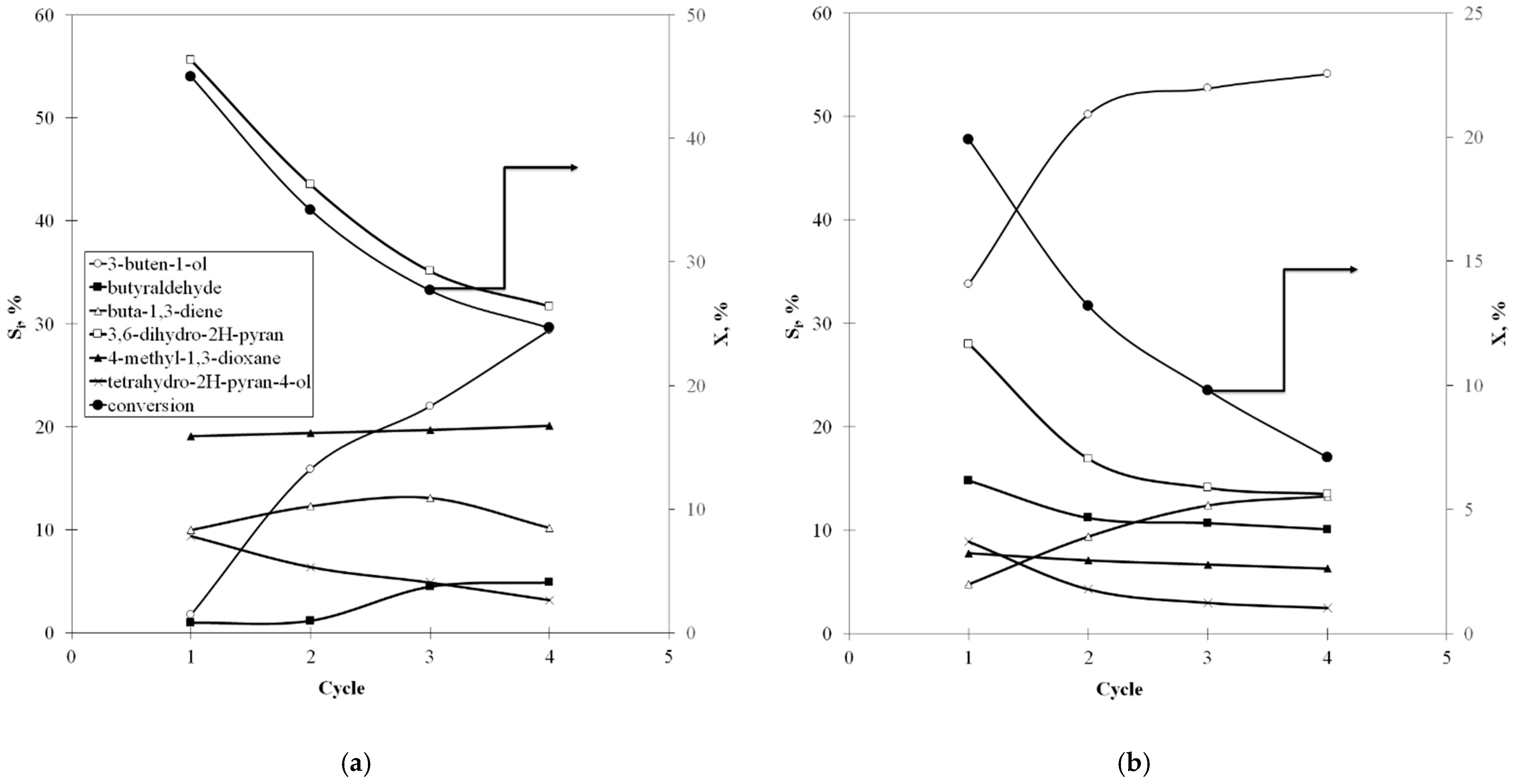
2.2. Catalytic Tests
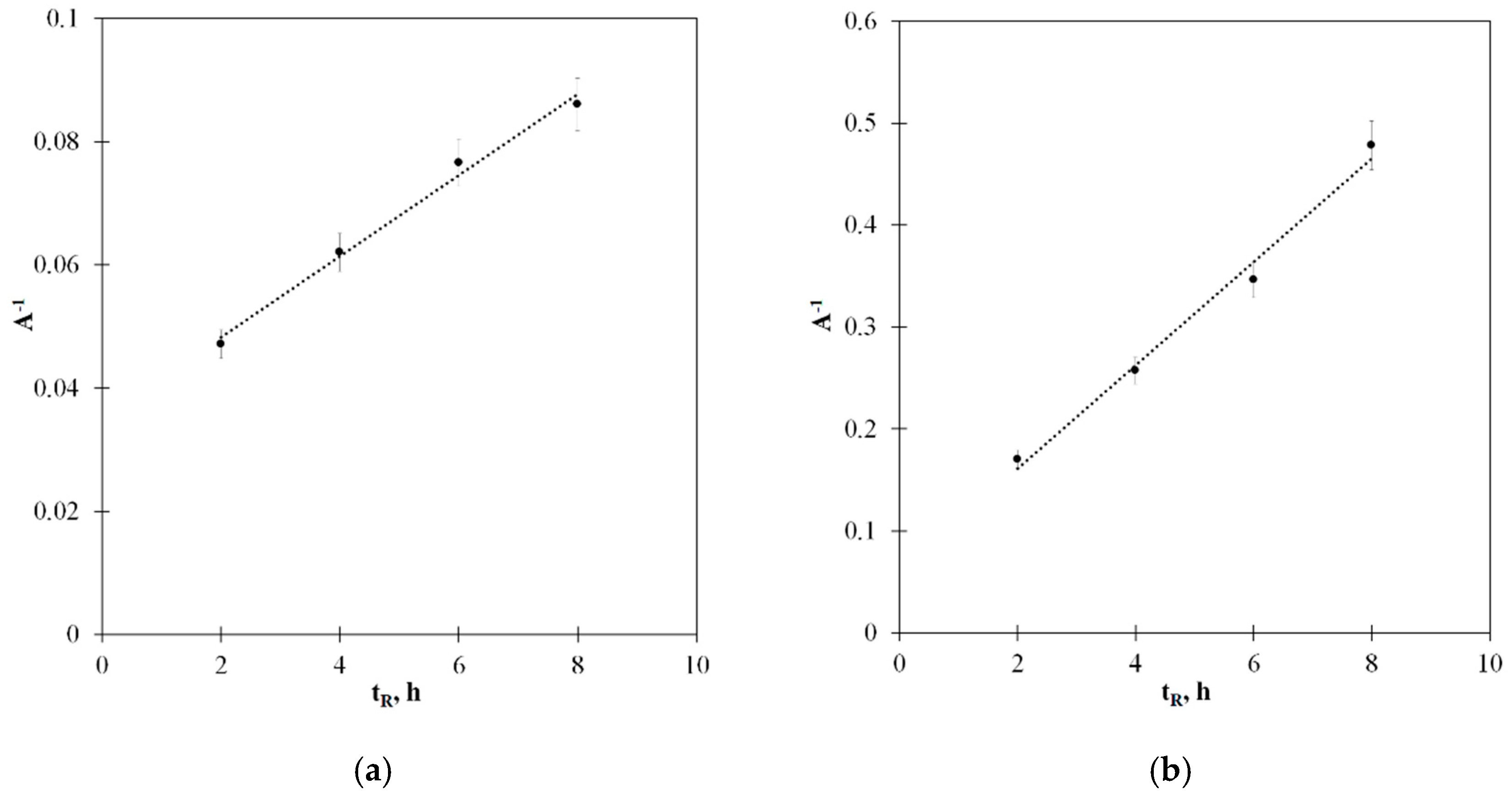
2.3. Catalytic Deactivation
3. Materials and Methods
3.1. Catalysts and Materials
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Markets and Markets. Butadiene Market Worth 14,536.1 Kilotons by 2018. Available online: https://www.marketsandmarkets.com/PressReleases/butadiene.asp (accessed on 11 October 2020).
- Weissermel, K.; Arpe, H.-J. Industrial Organic Chemistry, 3rd ed.; VCH Publishers: New York, NY, USA, 1997; 481p. [Google Scholar]
- Statista. Market Volume of Isoprene in the United States from 2014 to 2025. Available online: https://www.statista.com/statistics/799009/us-isoprene-market-volume/ (accessed on 11 October 2020).
- Torres Galvis, H.M.; De Jong, K.P. Catalysts for production of lower olefins from synthesis gas: A review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Khadzhiev, S.N.; Magomedova, M.V.; Peresypkina, E.G. Mechanism of olefin synthesis from methanol and dimethyl ether over zeolite catalysts: A review. Pet. Chem. 2014, 54, 245–269. [Google Scholar] [CrossRef]
- Khadzhiev, S.N.; Dement’ev, K.I.; Gerzeliev, I.M. Catalytic cracking of alternative raw materials and their mixtures with petroleum fractions over microspherical zeolite-containing catalysts. Pet. Chem. 2014, 54, 1–9. [Google Scholar] [CrossRef]
- Palankoev, T.A.; Dement’ev, K.I.; Kuznetsova, D.V.; Bondarenko, G.N.; Maximov, A.L. Acetone Reaction Pathways as a Model Bio-oxygenate in a hydrocarbon medium on zeolite Y and ZSM-5 catalysts: In Situ FTIR Study. ACS Sustain. Chem. Eng. 2020, 8, 10892–10899. [Google Scholar] [CrossRef]
- Bedenko, S.P.; Dement’ev, K.I.; Tret’yakov, V.F.; Maksimov, A.L. The Prins reaction over heterogeneous catalysts (a Review). Pet. Chem. 2020, 60, 723–730. [Google Scholar] [CrossRef]
- Dumitriu, E.; Gongescu, D.; Hulea, V. Contribution to the study of isobutene condensation with formaldehyde catalyzed by zeolites. Stud. Surf. Sci. Catal. 1993, 78, 669–676. [Google Scholar]
- Dumitriu, E.; Hulea, V.; Hulea, T.; Chelaru, C.; Kaliaguine, S. Selective synthesis of isoprene by Prins condensation using molecular sieves. Stud. Surf. Sci. Catal. 1994, 84, 1997–2004. [Google Scholar]
- Dumitriu, E.; Trong On, D.; Kaliaguine, S. Isoprene by Prins condensation over acidic molecular sieves. J. Catal. 1997, 170, 150–160. [Google Scholar] [CrossRef]
- Dumitriu, E.; Hulea, V.; Fechete, I.; Catrinescu, C.; Auroux, A.; Lacaze, J.-F.; Guimon, C. Prins condensation of isobutylene and formaldehyde over Fe-silicates of MFI structure. Appl. Catal. A Gen. 1999, 181, 15–28. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Li, S.; Caratzoulas, S.; Lobo, R.F. Formaldehyde-isobutene Prins condensation over MFI-type zeolites. Catal. Sci. Technol. R. Soc. Chem. 2018, 8, 5794–5806. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Gould, N.S.; Lobo, R.F. Zeolite-catalyzed formaldehyde-propylene Prins condensation. ChemCatChem 2017, 9, 4417–4425. [Google Scholar] [CrossRef]
- Sangthong, W.; Probst, M.; Limtrakul, J. Computational study of the carbonyl-ene reaction of encapsulated formaldehyde in Na-FAU zeolite. J. Mol. Struct. 2005, 748, 119–127. [Google Scholar] [CrossRef]
- Fu, H.; Xie, S.; Fu, A.; Ye, T. Theoretical study of the carbonyl-ene reaction between formaldehyde and propylene on the MgY zeolite. Comput. Theor. Chem. 2012, 982, 51–57. [Google Scholar] [CrossRef]
- Wannakao, S.; Khongpracha, P.; Limtrakul, J. Density functional theory study of the carbonyl-ene reaction of encapsulated formaldehyde in Cu(I), Ag(I), and Au(I) exchanged FAU zeolites. J. Phys. Chem. A 2011, 115, 12486–12492. [Google Scholar] [CrossRef]
- Hölderich, W.; Hesse, M.; Näumann, F. Zeolites: Catalysts for organic syntheses. Angew. Chem. Int. Ed. Engl. 1988, 27, 226–246. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Fundamental description of deactivation and regeneration of acid zeolites. Stud. Surf. Sci. Catal. 1994, 88, 53–68. [Google Scholar]
- Magnoux, P.; Rabeharitsara, A.; Cerqueira, H.S. Influence of reaction temperature and crystallite size on HBEA zeolite deactivation by coke. Appl. Catal. A Gen. 2006, 304, 142–151. [Google Scholar] [CrossRef]
- Bjørgen, M.; Olsbye, U.; Kolboe, S. Coke precursor formation and zeolite deactivation: Mechanistic insights from hexamethylbenzene conversion. J. Catal. 2003, 215, 30–44. [Google Scholar] [CrossRef]
- Yan, T.; Yang, L.; Dai, W.; Wang, C.; Wu, G.; Guan, N.; Hunger, M.; Li, L. On the deactivation mechanism of zeolite catalyst in ethanol to butadiene conversion. J. Catal. 2018, 367, 7–15. [Google Scholar] [CrossRef]
- Bedenko, S.P.; Kozhevnikov, A.A.; Dement’ev, K.I.; Tret’yakov, V.F.; Maximov, A.L. The Prins condensation between i-butene and formaldehyde over modified BEA and MFI zeolites in liquid phase. Catal. Commun. 2020, 138, 105965. [Google Scholar] [CrossRef]
- Arundale, E.; Mikeska, L.A. The olefin-aldehyde condensation: The Prins reaction. Chem. Rev. 1952, 51, 505–555. [Google Scholar] [CrossRef]
- Flego, C.; Galasso, L.; Kiricsi, I.; Clericiet, M.G. TG-DSC, UV-VIS-IR Studies on catalysts deactivated in alkylation of isobutane with 1-butene. Stud. Surf. Sci. Catal. 1994, 88, 585–590. [Google Scholar]
- Wojciechowski, B.W. The kinetic foundations and the practical application of the time on stream theory of catalyst decay. Catal. Rev. 1974, 9, 79–113. [Google Scholar] [CrossRef]
- Smit, B.; Maesen, T.L.M. Towards a molecular understanding of shape selectivity. Nature 2008, 451, 671–678. [Google Scholar] [CrossRef] [Green Version]
- Schüth, F.; Ward, M.D.; Buriak, J.M. Common pitfalls of catalysis manuscripts submitted to Chemistry of materials. Chem. Mater. 2018, 30, 3599–3600. [Google Scholar] [CrossRef]
- Scott, S.L. A matter of life(time) and death. ACS Catal. 2018, 8, 8597–8599. [Google Scholar] [CrossRef] [Green Version]
- Gayubo, A.G.; Aguayo, A.T.; Olazar, M.; Vivanco, R.; Bilbao, J. Kinetics of the irreversible deactivation of the HZSM-5 catalyst in the MTO process. Chem. Eng. Sci. 2003, 58, 5239–5249. [Google Scholar] [CrossRef]
- Janssens, T.V.W. A new approach to the modeling of deactivation in the conversion of methanol on zeolite catalysts. J. Catal. 2009, 264, 130–137. [Google Scholar] [CrossRef]
| Sample | Si/Al | S, m2/g | VMP, cm3/g | DP, Å | Acid Site Number, μmol/g | S/W Ratio |
|---|---|---|---|---|---|---|
| H-BEA | 12.5 | 540 | 0.22 | 7.2 | 700 | 0.25 |
| H-FAU | 15 | 720 | 0.26 | 10.3 | 390 | 0.55 |
| H-MFI | 15 | 360 | 0.18 | 5.6 | 1120 | 0.60 |
| H-MOR | 10 | 480 | 0.19 | 5.7 | 1450 | 0.38 |
| Sample | X, % | Selectivity, % | ||||||
|---|---|---|---|---|---|---|---|---|
 |  | 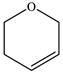 | 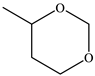 | 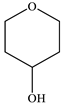 | 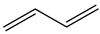 | Other 1 | ||
| H-BEA | 45.0 | 1.8 | 1.0 | 55.6 | 19.1 | 9.4 | 10.0 | 3.1 |
| H-FAU | 41.2 | 3.6 | 1.2 | 43.5 | 32.5 | 6.5 | 9.2 | 3.5 |
| H-MFI | 19.9 | 33.8 | 14.8 | 28.0 | 7.8 | 8.9 | 4.8 | 1.9 |
| H-MOR | 14.0 | 19.3 | 10.5 | 34.9 | 17.3 | 9.0 | 6.4 | 2.6 |
| H-BEA 2 | 19.4 | 4.2 | 1.6 | 59.1 | 21.4 | 6.5 | 5.8 | 1.4 |
| Sample | ΔmI, wt.% | ΔmII, wt.% | QI, J/g | QII, J/g |
|---|---|---|---|---|
| H-BEA | 5.0 | 22.0 | −37.5 | 1235.9 |
| H-FAU | 6.3 | 22.2 | −33.3 | 3127.2 |
| H-MFI | 6.3 | 10.3 | −140.6 | 712.7 |
| H-MOR | 5.9 | 15.1 | −106.1 | 740.3 |
| Sample | k, 10−1 h−1 | A0 |
|---|---|---|
| H-BEA | 1.90 ± 0.05 | 28.5 ± 0.3 |
| H-MFI | 8.40 ± 0.10 | 16.6 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedenko, S.P.; Dement’ev, K.I.; Tret’yakov, V.F. Deactivation of Zeolite Catalysts in the Prins Reaction between Propene and Formaldehyde in the Liquid Phase. Catalysts 2021, 11, 1181. https://doi.org/10.3390/catal11101181
Bedenko SP, Dement’ev KI, Tret’yakov VF. Deactivation of Zeolite Catalysts in the Prins Reaction between Propene and Formaldehyde in the Liquid Phase. Catalysts. 2021; 11(10):1181. https://doi.org/10.3390/catal11101181
Chicago/Turabian StyleBedenko, Stanislav P., Konstantin I. Dement’ev, and Valentin F. Tret’yakov. 2021. "Deactivation of Zeolite Catalysts in the Prins Reaction between Propene and Formaldehyde in the Liquid Phase" Catalysts 11, no. 10: 1181. https://doi.org/10.3390/catal11101181
APA StyleBedenko, S. P., Dement’ev, K. I., & Tret’yakov, V. F. (2021). Deactivation of Zeolite Catalysts in the Prins Reaction between Propene and Formaldehyde in the Liquid Phase. Catalysts, 11(10), 1181. https://doi.org/10.3390/catal11101181






