On the Support Effect and the Cr Promotion of Co Based Catalysts for the Acetic Acid Steam Reforming
Abstract
1. Introduction
| AcOH Steam reforming | ΔH = 131.4 kJ/mol | (1) | |
| Water gas shift | ΔH = −41.1 kJ/mol | (2) | |
| Thermal decomposition | ΔH = 213.7 kJ/mol | (3) | |
| Decarboxylation | ΔH = −33.5 kJ/mol | (4) | |
| Ketonization | ΔH = 16.7 kJ/mol | (5) | |
| Boudouard reaction | ΔH = −172.4 kJ/mol | (6) | |
| Methane decomposition | ΔH = 74.8 kJ/mol | (7) |
2. Results and Discussion
2.1. Catalysts Characterization
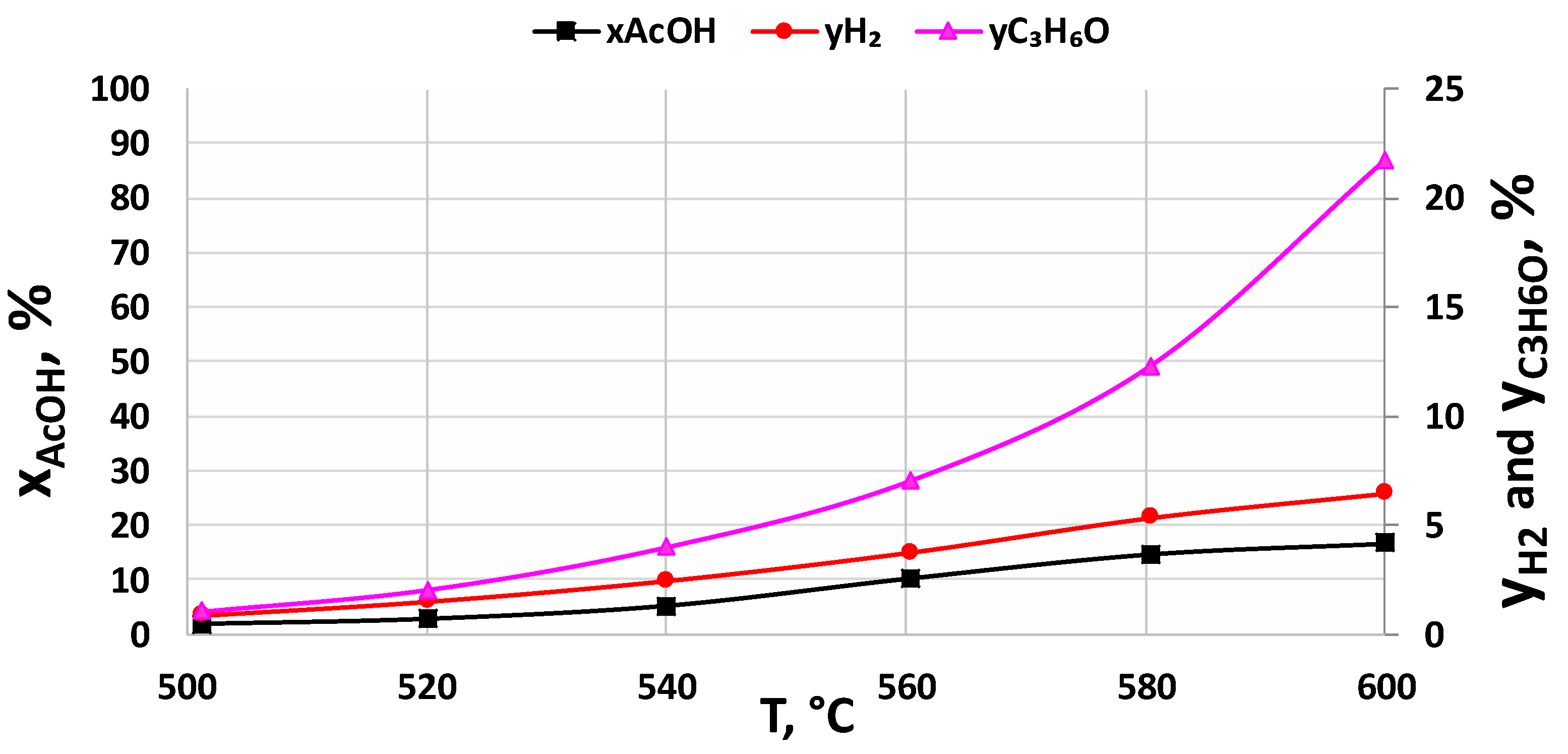
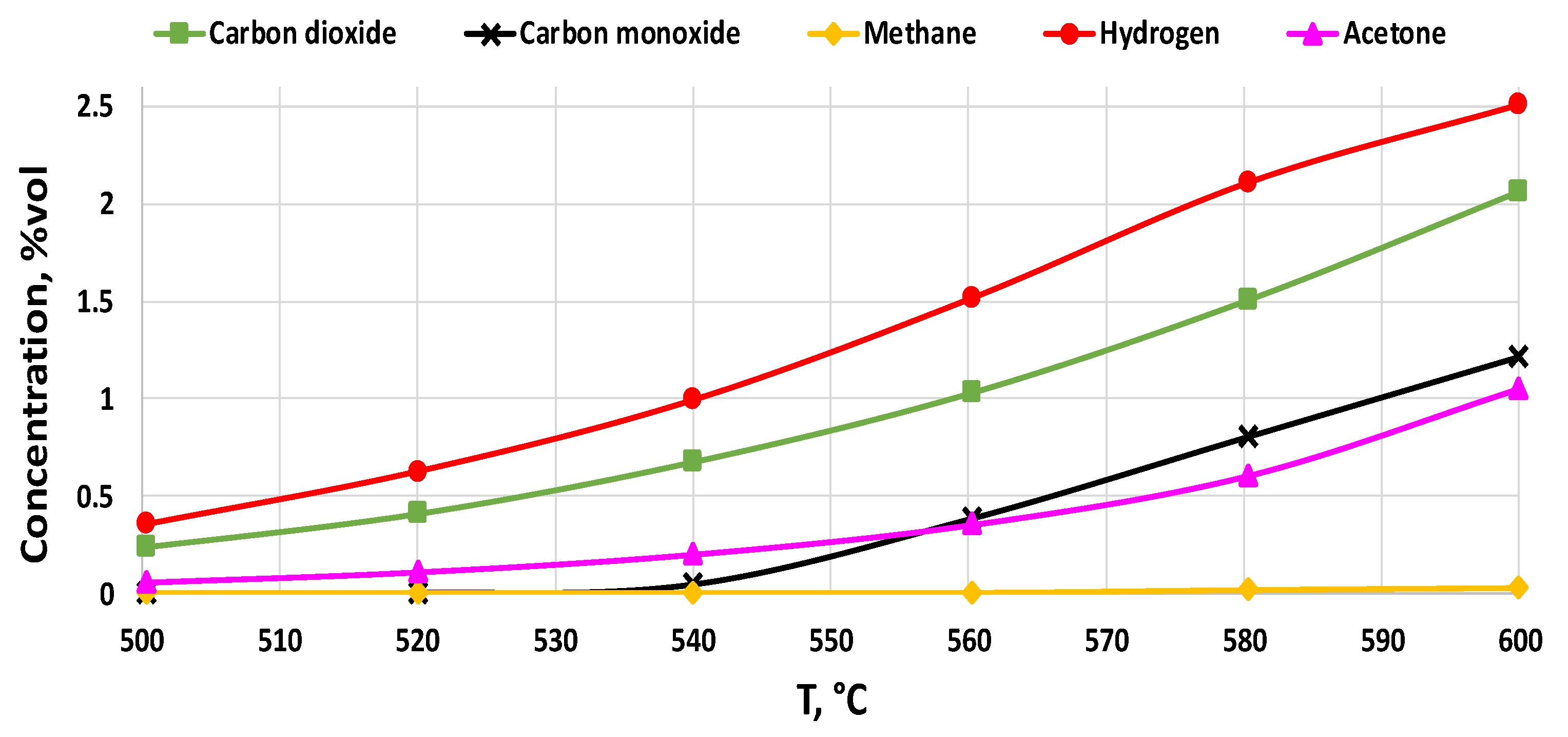
2.2. Activity Tests
2.2.1. Homogeneous Reaction
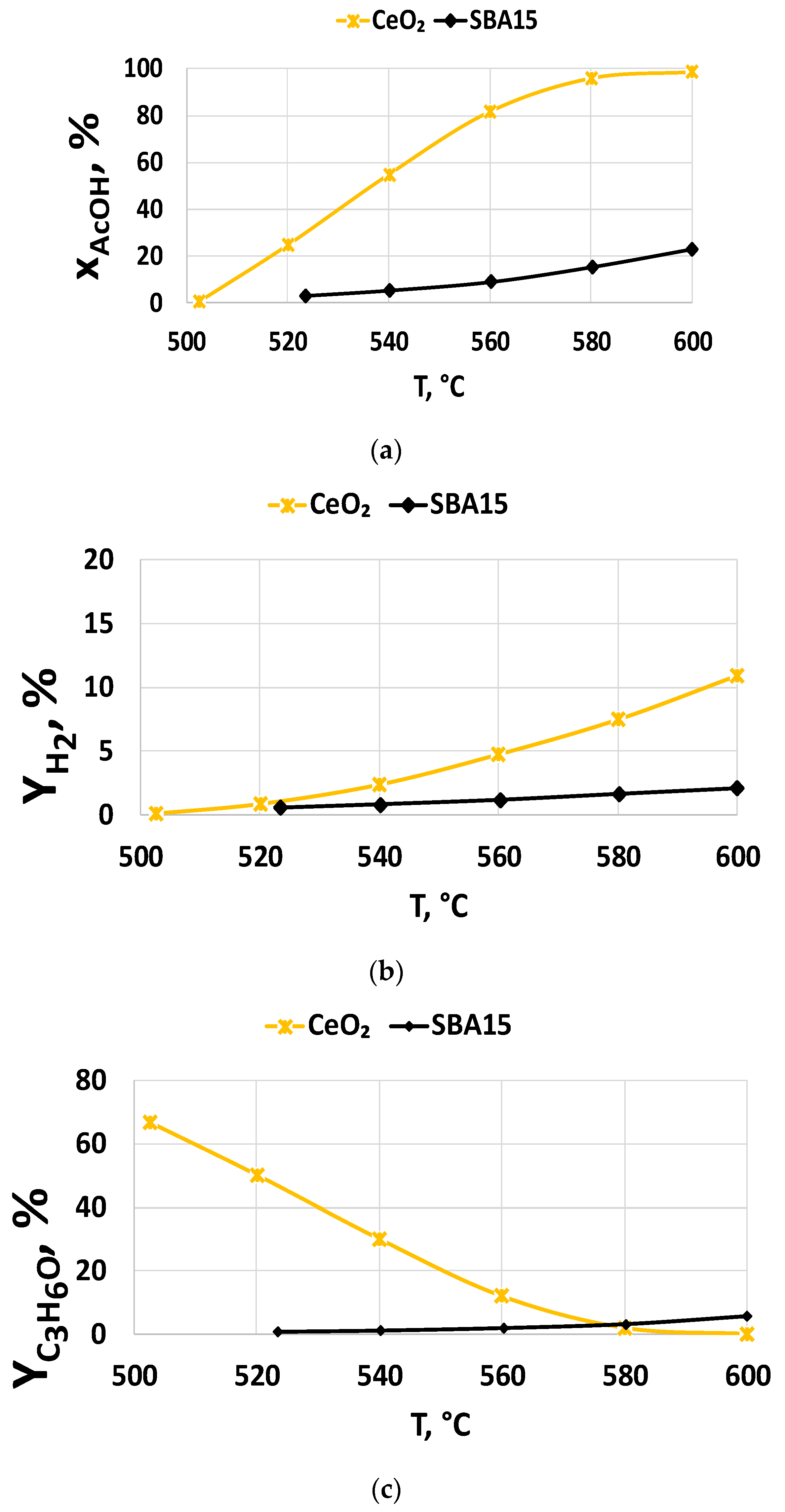
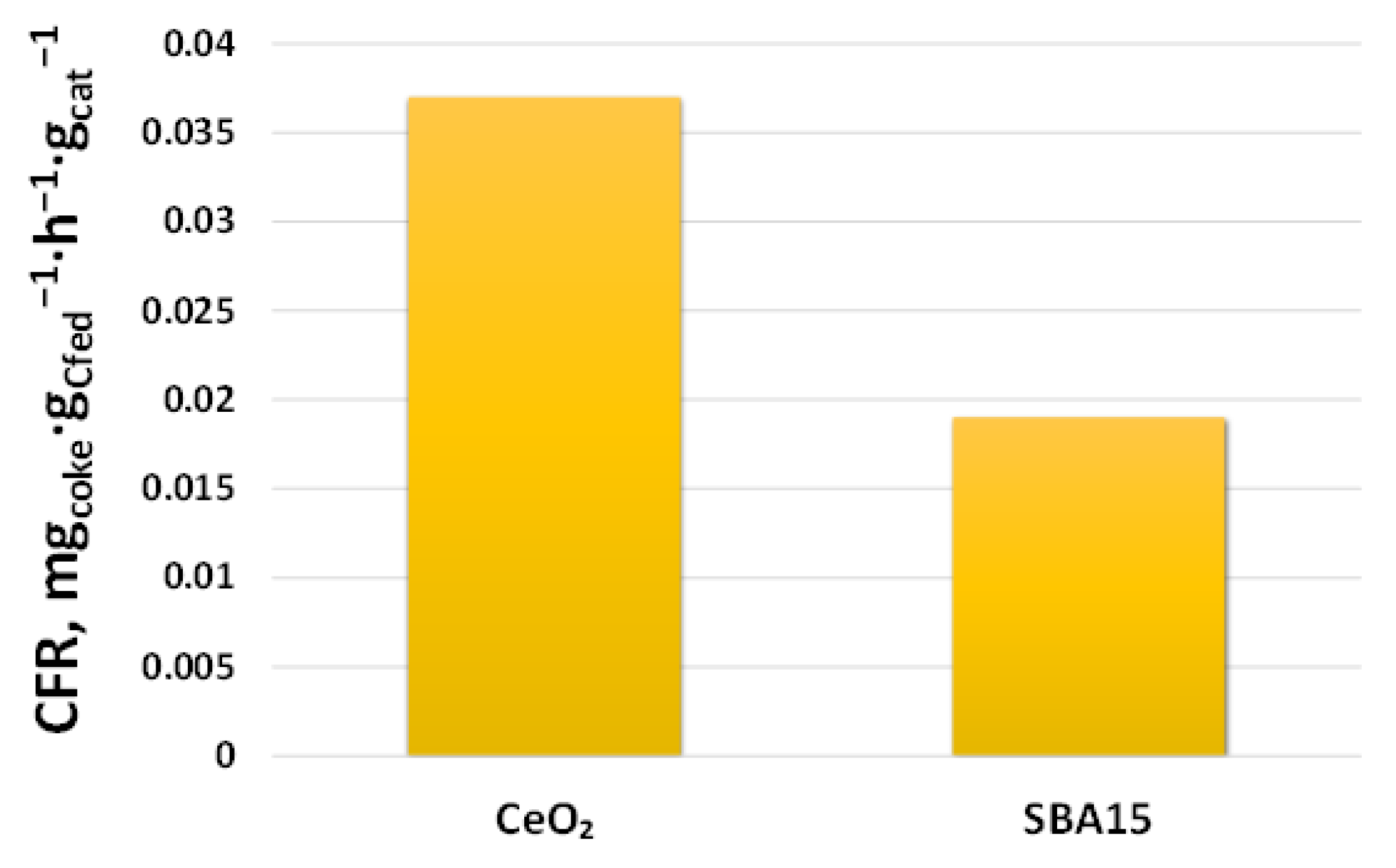
2.2.2. Support Effect
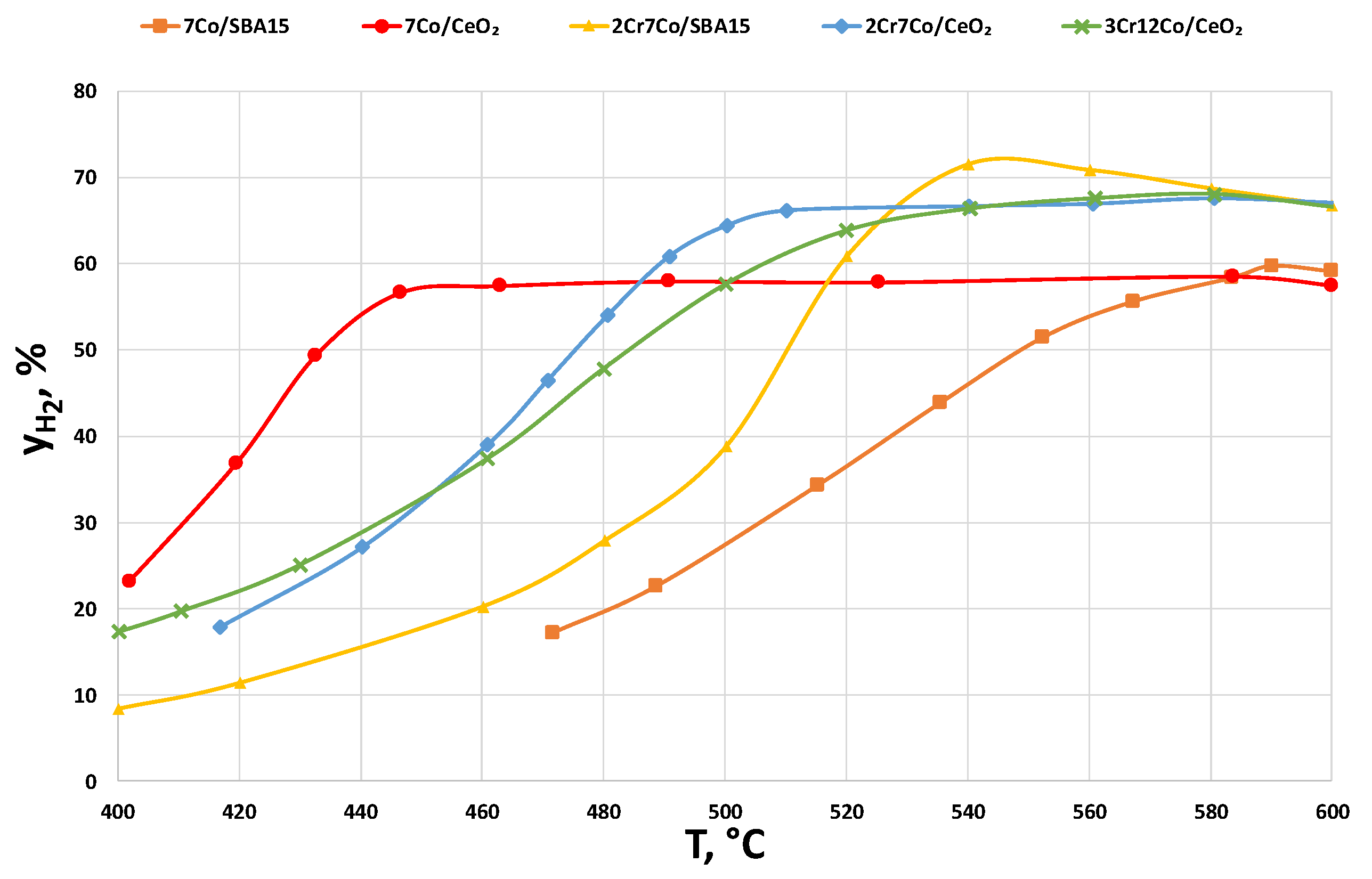
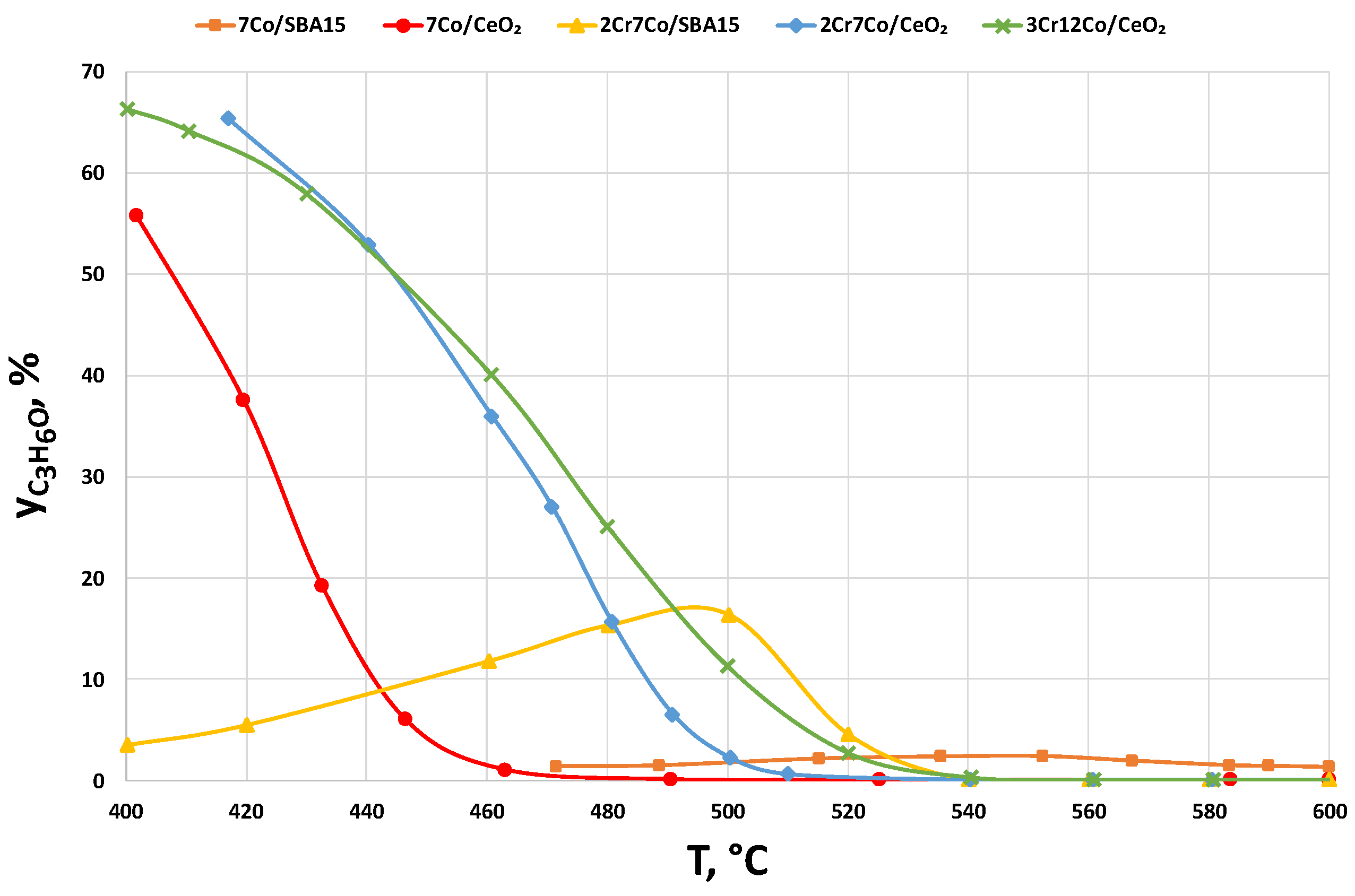
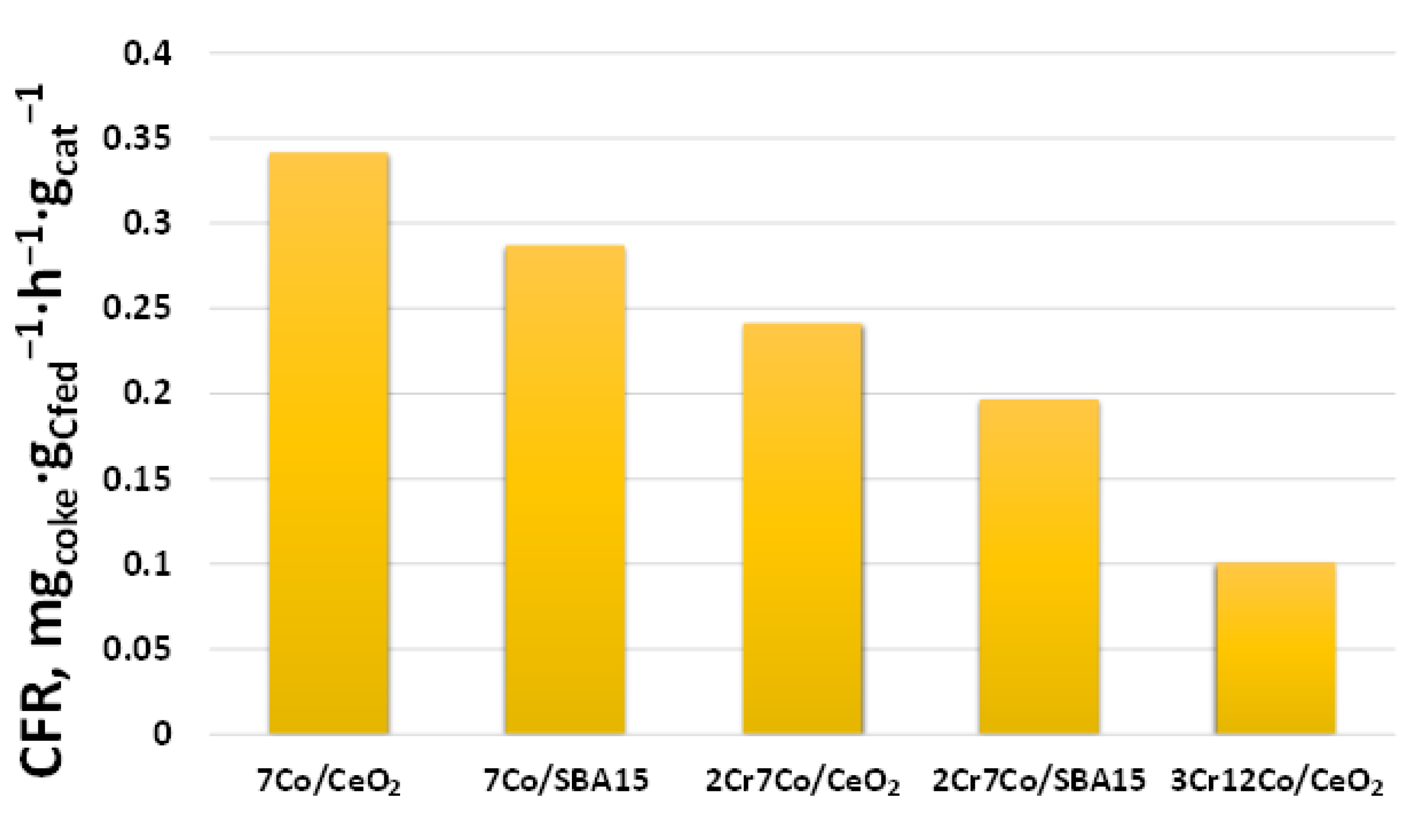
2.2.3. Catalysts Activity Tests
3. Materials and Methods
3.1. Preparation and Characterization of the Catalysts
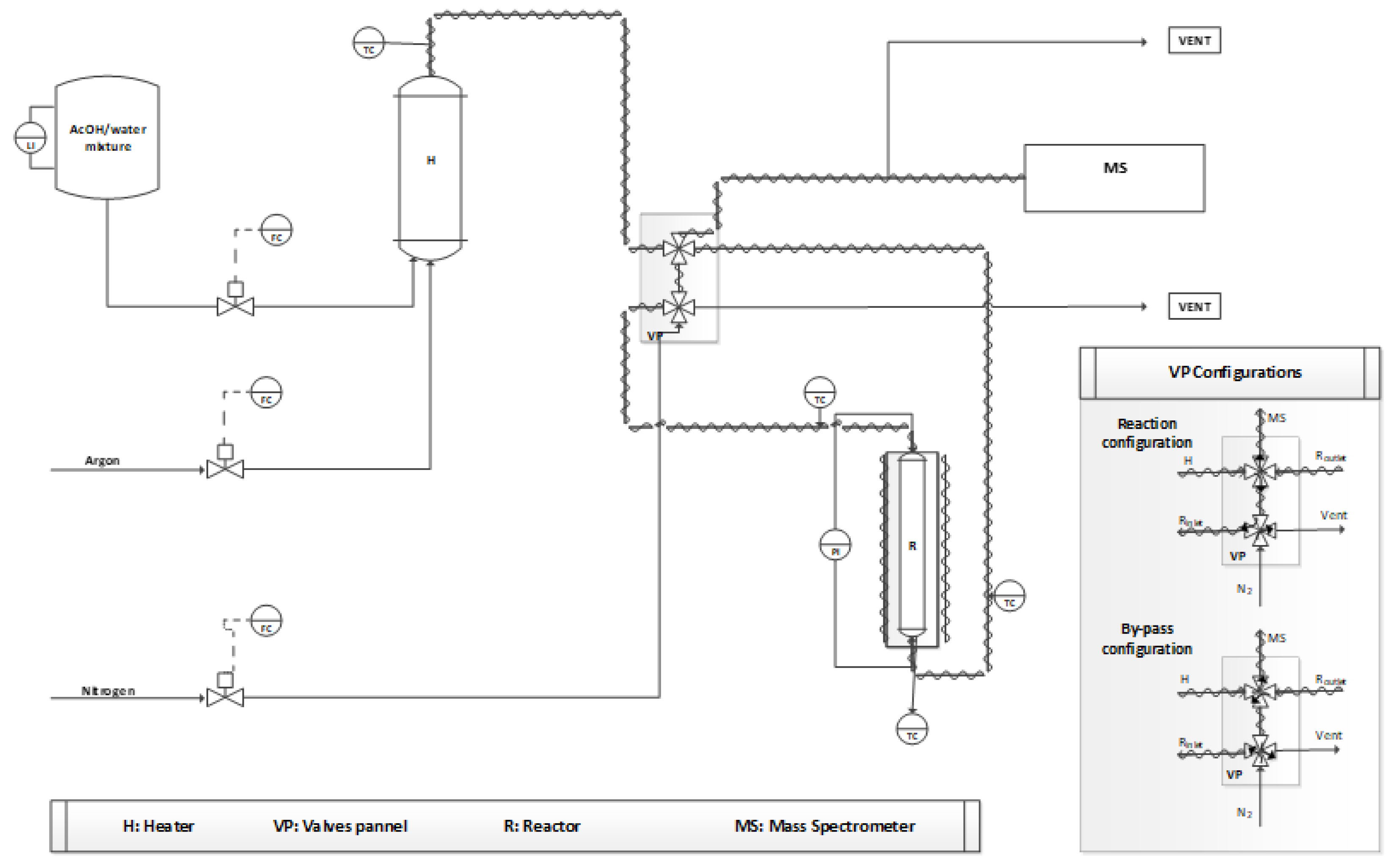
3.2. Experimental Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domine, M.E.; Iojoiu, E.E.; Davidian, T.; Guilhaume, N.; Mirodatos, C. Hydrogen production from biomass-derived oil over monolithic Pt- and Rh-based catalysts using steam reforming and sequential cracking processes. Catal. Today 2008, 565–573. [Google Scholar] [CrossRef]
- Assaf, P.G.M.; Nogueira, F.G.E.; Assaf, E.M. Ni and Co catalysts supported on alumina applied to steam reforming of acetic acid: Representative compound for the aqueous phase of bio-oil derived from biomass. Catal. Today 2013, 213, 2–8. [Google Scholar] [CrossRef]
- Adamu, S.; Xiong, Q.; Bakare, I.A.; Hossain, M.M. Ni/Ce-Al2O3 for optimum hydrogen production from biomass/tar model compounds: Role of support type and ceria modification on desorption kinetics. Int. J. Hydrogen Energy 2019, 44, 15811–15822. [Google Scholar] [CrossRef]
- Bej, B.; Bepari, S.; Pradhan, N.C.; Neogi, S. Production of hydrogen by dry reforming of ethanol over alumina supported nano-NiO/SiO2 catalyst. Catal. Today 2017, 291, 58–66. [Google Scholar] [CrossRef]
- Spallina, V.; Matturro, G.; Ruocco, C.; Meloni, E.; Palma, V.; Fernandez, E.; Melendez, J.; Pacheco Tanaka, A.D.; Viviente Sole, J.L.; van Sint Annaland, M.; et al. Direct route from ethanol to pure hydrogen through autothermal reforming in a membrane reactor: Experimental demonstration, reactor modelling and design. Energy 2018, 143, 666–681. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production. Energy Convers. Manag. 2020, 205, 112–182. [Google Scholar] [CrossRef]
- Pal, P.; Nayak, J. Acetic Acid Production and Purification: Critical Review towards Process Intensification. Sep. Purif. Rev. 2017, 46, 44–61. [Google Scholar] [CrossRef]
- Vashisht, A.; Thakur, K.; Kauldhar, B.S.; Kumar, V.; Yadav, S.K. Waste valorization: Identification of an ethanol tolerant bacterium Acetobacter pasteurianus SKYAA25 for acetic acid production from apple pomace. Sci. Total Environ. 2019, 690, 956–964. [Google Scholar] [CrossRef]
- Huo, Z.; Fang, Y.; Yao, G.; Zeng, X.; Ren, D.; Jin, F. Improved two-step hydrothermal process for acetic acid production from carbohydrate biomass. J. Energy Chem. 2015, 24, 207–212. [Google Scholar] [CrossRef]
- Jin, F.; Zhou, Z.; Kishita, A.; Enomoto, H.; Kishida, H.; Moriya, T. A new hydrothermal process for producing acetic acid from biomass waste. Chem. Eng. Res. Des. 2007, 85, 201–206. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, A.S.K. Comparative study of hydrogen production from steam reforming of acetic acid over synthesized catalysts via MOF and wet impregnation methods. Int. J. Hydrogen Energy 2020, 45, 11512–11526. [Google Scholar] [CrossRef]
- Hu, X.; Yang, J.; Sun, W.; Wang, N.; An, S.; Wang, Q.; Zhang, Y.; Xie, X.; Huang, L. Y-Zr-O solid solution supported Ni-based catalysts for hydrogen production via auto-thermal reforming of acetic acid. Appl. Catal. B Environ. 2020, 278, 119264. [Google Scholar] [CrossRef]
- Cakiryilmaz, N.; Arbag, H.; Oktar, N.; Dogu, G.; Dogu, T. Catalytic performances of Ni and Cu impregnated MCM-41 and Zr-MCM-41 for hydrogen production through steam reforming of acetic acid. Catal. Today 2019, 323, 191–199. [Google Scholar] [CrossRef]
- Chen, G.; Tao, J.; Liu, C.; Yan, B.; Li, W.; Li, X. Hydrogen production via acetic acid steam reforming: A critical review on catalysts. Renew. Sustain. Energy Rev. 2017, 79, 1091–1098. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhong, X.; Xie, X.; Jia, X.; Chen, B.; Wang, N.; Huang, L. Auto-thermal reforming of acetic acid for hydrogen production by ordered mesoporous Ni-xSm-Al-O catalysts: Effect of samarium promotion. Renew. Energy 2020, 145, 2316–2326. [Google Scholar] [CrossRef]
- Choi, I.H.; Hwang, K.R.; Lee, K.Y.; Lee, I.G. Catalytic steam reforming of biomass-derived acetic acid over modified Ni/Γ-Al2O3 for sustainable hydrogen production. Int. J. Hydrogen Energy 2019, 44, 180–190. [Google Scholar] [CrossRef]
- Navarro, R.M.; Guil-Lopez, R.; Ismail, A.A.; Al-Sayari, S.A.; Fierro, J.L.G. Ni- and PtNi-catalysts supported on Al2O3 for acetone steam reforming: Effect of the modification of support with Ce, La and Mg. Catal. Today 2015, 242, 60–70. [Google Scholar] [CrossRef]
- Matas Güell, B.; Babich, I.; Nichols, K.P.; Gardeniers, J.G.E.; Lefferts, L.; Seshan, K. Design of a stable steam reforming catalyst-A promising route to sustainable hydrogen from biomass oxygenates. Appl. Catal. B Environ. 2009, 90, 38–44. [Google Scholar] [CrossRef]
- Pu, J.; Nishikado, K.; Wang, N.; Nguyen, T.T.; Maki, T.; Qian, E.W. Core-shell nickel catalysts for the steam reforming of acetic acid. Appl. Catal. B Environ. 2018, 224, 69–79. [Google Scholar] [CrossRef]
- Resende, K.A.; Ávila-Neto, C.N.; Rabelo-Neto, R.C.; Noronha, F.B.; Hori, C.E. Hydrogen production by reforming of acetic acid using La-Ni type perovskites partially substituted with Sm and Pr. Catal. Today 2015, 242, 71–79. [Google Scholar] [CrossRef]
- Pu, J.; Luo, Y.; Wang, N.; Bao, H.; Wang, X.; Qian, E.W. Ceria-promoted Ni@Al2O3 core-shell catalyst for steam reforming of acetic acid with enhanced activity and coke resistance. Int. J. Hydrogen Energy 2018, 43, 3142–3153. [Google Scholar] [CrossRef]
- Batista da Silva, R.; Brandão, S.T.; Lucotti, A.; Tommasini, M.S.; Castiglioni, C.; Groppi, G.; Beretta, A. Chemical pathways in the partial oxidation and steam reforming of acetic acid over a Rh-Al2O3 catalyst. Catal. Today 2017, 289, 162–172. [Google Scholar] [CrossRef]
- Veiga, S.; Romero, M.; Faccio, R.; Segobia, D.; Duarte, H.; Apesteguía, C.; Bussi, J. Hydrogen-rich gas production by steam and oxidative steam reforming of crude glycerol over Ni-La-Me mixed oxide catalysts (Me=Ce and/or Zr). Catal. Today 2019, 344, 190–198. [Google Scholar] [CrossRef]
- Takanabe, K.; Aika, K.; Seshan, K.; Lefferts, L. Catalyst deactivation during steam reforming of acetic acid over Pt/ZrO2. Chem. Eng. J. 2006, 120, 133–137. [Google Scholar] [CrossRef]
- Pant, K.K.; Mohanty, P.; Agarwal, S.; Dalai, A.K. Steam reforming of acetic acid for hydrogen production over bifunctional Ni-Co catalysts. Catal. Today 2013, 207, 36–43. [Google Scholar] [CrossRef]
- Hu, X.; Dong, D.; Shao, X.; Zhang, L.; Lu, G. Steam reforming of acetic acid over cobalt catalysts: Effects of Zr, Mg and K addition. Int. J. Hydrogen Energy 2017, 42, 4793–4803. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, N.; Yang, L.; Li, M.; Huang, L. Ni-Co bimetallic MgO-based catalysts for hydrogen production via steam reforming of acetic acid from bio-oil. Int. J. Hydrogen Energy 2014, 39, 18688–18694. [Google Scholar] [CrossRef]
- Hu, X.; Lu, G. The inhibition effect of potassium addition on methane formation in steam reforming of acetic acid over alumina-supported cobalt catalysts. Chem. Lett. 2008, 37, 614–615. [Google Scholar] [CrossRef]
- Basagiannis, A.C.; Verykios, X.E. Catalytic steam reforming of acetic acid for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3343–3355. [Google Scholar] [CrossRef]
- Megía, P.J.; Carrero, A.; Calles, J.A.; Vizcaíno, A.J. Hydrogen production from steam reforming of acetic acid as a model compound of the aqueous fraction of microalgae HTL using Co-M/SBA-15 (M: Cu, Ag, Ce, Cr) catalysts. Catalysts 2019, 9, 1013. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.V.N. Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A Gen. 2018, 559, 57–74. [Google Scholar] [CrossRef]
- Chaudhary, V.; Sharma, S. An overview of ordered mesoporous material SBA-15: Synthesis, functionalization and application in oxidation reactions Microporous Mesoporous Macroporous. J. Porous Mater. 2017, 24, 741–749. [Google Scholar] [CrossRef]
- Garcia, L.; French, R.; Czernik, S.; Chornet, E. Catalytic steam reforming of bio-oils for the production of hydrogen: Effects of catalyst composition. Appl. Catal. A Gen. 2000, 201, 225–239. [Google Scholar] [CrossRef]
- Yaseneva, P.; Pavlova, S.; Sadykov, V.; Moroz, E.; Burgina, E.; Dovlitova, L.; Rogov, V.; Badmaev, S.; Belochapkin, S.; Ross, J. Hydrogen production by steam reforming of methanol over Cu-CeZrYOx-based catalysts. Catal. Today 2008, 138, 175–182. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J.; Menon, P.G. Activity and characterization of Cu/Zn, Cu/Cr and Cu/Zr on γ-alumina for methanol reforming for fuel cell vehicles. Appl. Catal. A Gen. 2002, 234, 111–125. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; Megía, P.J. Agglomerated Co-Cr/SBA-15 catalysts for hydrogen production through acetic acid steam reforming. Int. J. Hydrogen Energy 2019, 45, 1–10. [Google Scholar] [CrossRef]
- Pu, J.; Ikegami, F.; Nishikado, K.; Qian, E.W. Effect of ceria addition on Ni-Ru/CeO2-Al2O3 catalysts in steam reforming of acetic acid. Int. J. Hydrogen Energy 2017, 42, 19733–19743. [Google Scholar] [CrossRef]
- Lemonidou, A.A.; Vagia, E.C.; Lercher, J.A. Acetic acid reforming over Rh supported on La2O 3/CeO2-ZrO2: Catalytic performance and reaction pathway analysis. ACS Catal. 2013, 3, 1919–1928. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Meloni, E.; Gallucci, F.; Ricca, A. Enhancing Pt-Ni/CeO2 performances for ethanol reforming by catalyst supporting on high surface silica. Catal. Today 2018, 307, 175–188. [Google Scholar] [CrossRef]
- Cai, W.; Wang, F.; Van Veen, A.C.; Provendier, H.; Mirodatos, C.; Shen, W. Autothermal reforming of ethanol for hydrogen production over an Rh/CeO2 catalyst. Catal. Today 2008, 138, 152–156. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Vita, A.; Italiano, C.; Fabiano, C.; Huang, Y.; Basile, A. The oncoming energy vector: Hydrogen produced in Pd-composite membrane reactor via bioethanol reforming over Ni/CeO2 catalyst. Catal. Today 2016, 259, 368–375. [Google Scholar] [CrossRef]
- Araiza, D.G.; Gómez-Cortés, A.; Díaz, G. Effect of ceria morphology on the carbon deposition during steam reforming of ethanol over Ni/CeO2 catalysts. Catal. Today 2020, 349, 235–243. [Google Scholar] [CrossRef]
- Sutthisripok, W.; Sattayanurak, S.; Sikong, L. Effect of specific surface area on oxygen storage capacity (OSC) and methane steam reforming reactivity of CeO2. J. Porous Mater. 2008, 15, 519–525. [Google Scholar] [CrossRef]
- Martínez, A.; López, C.; Márquez, F.; Díaz, I. Fischer–Tropsch synthesis of hydrocarbons over mesoporous Co/SBA-15 catalysts: The influence of metal loading, cobalt precursor, and promoters. J. Catal. 2003, 220, 486–499. [Google Scholar] [CrossRef]
- Grzybowska, B.; Słoczyński, J.; Grabowski, R.; Wcisło, K.; Kozłowska, A.; Stoch, J.; Zieliński, J. Chromium oxide/alumina catalysts in oxidative dehydrogenation of isobutane. J. Catal. 1998, 178, 687–700. [Google Scholar] [CrossRef]
- Yao, H.C.; Yao, Y.F.Y. Ceria in automotive exhaust catalysts. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- De Lima, S.M.; Adriana, M.; Lídia, O.O.; Graham, U.M.; Jacobs, G.; Davis, B.H.; Mattos, L.; Noronha, F.B. Study of catalyst deactivation and reaction mechanism of steam reforming, partial oxidation, and oxidative steam reforming of ethanol over Co/CeO2 catalyst. J. Catal. 2009, 268, 268–281. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Chen, Y.; Zhang, R.; Yang, S. K-Promoted Co-CeO2 catalyst for the reverse watergas shift reaction. Chem. Lett. 2013, 42, 682–683. [Google Scholar] [CrossRef]
- Costa, A.F.; Cerqueira, H.S.; Falabella, E.; Aguiar, S.; Rollán, J.; Martínez, A. New supports for co-based fischer-tropsch catalyst. Stud. Surf. Sci. Catal. 2007, 167, 141–146. [Google Scholar] [CrossRef]
- El Hassan, N.; Kaydouh, M.N.; Geagea, H.; El Zein, H.; Jabbour, K.; Casale, S.; El Zakhem, H.; Massiani, P. Low temperature dry reforming of methane on rhodium and cobalt based catalysts: Active phase stabilization by confinement in mesoporous SBA-15. Appl. Catal. A Gen. 2016, 520, 114–121. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Arandiyan, H.; Peng, Y.; Chang, H.; Li, J. Low temperature complete combustion of methane over cobalt chromium oxides catalysts. Catal. Today 2013, 201, 12–18. [Google Scholar] [CrossRef]
- Greluk, M.; Rotko, M.; Turczyniak-Surdacka, S. Enhanced catalytic performance of La2O3 promoted Co/CeO2 and Ni/CeO2 catalysts for effective hydrogen production by ethanol steam reforming: La2O3 promoted Co(Ni)/CeO2 catalysts in SRE. Renew. Energy 2020, 155, 378–395. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.; Weng, D.; Lin, F.; Ran, R. MnOx-CeO2-Al2O3 mixed oxides for soot oxidation: Activity and thermal stability. J. Hazard. Mater. 2011, 187, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Carrero, A.; Calles, J.A.; García-Moreno, L.; Vizcaíno, A.J. Production of renewable hydrogen from glycerol steam reforming over bimetallic Ni-(Cu,Co,Cr) catalysts supported on SBA-15 silica. Catalysts 2017, 7, 55. [Google Scholar] [CrossRef]
- Calaza, F.C.; Chen, T.L.; Mullins, D.R.; Xu, Y.; Overbury, S.H. Reactivity and reaction intermediates for acetic acid adsorbed on CeO2(1 1 1). Catal. Today 2015, 253, 65–76. [Google Scholar] [CrossRef]
- Snell, R.W.; Shanks, B.H. Insights into the ceria-catalyzed ketonization reaction for biofuels applications. ACS Catal. 2013, 3, 783–789. [Google Scholar] [CrossRef]
- Wu, Z.; Mann, A.K.P.; Li, M.; Overbury, S.H. Spectroscopic investigation of surface dependent acid base property of ceria nanoshapes. J. Phys. Chem. C 2015, 119, 7340–7350. [Google Scholar] [CrossRef]
- Baylon, R.A.L.; Sun, J.; Martin, K.J.; Venkitasubramanian, P.; Wang, Y. Beyond ketonization: Selective conversion of carboxylic acids to olefins over balanced Lewis acid-base pairs. Chem. Commun. 2016, 52, 4975–4978. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Meloni, E.; Ricca, A. Oxidative steam reforming of ethanol on mesoporous silica supported Pt–Ni/CeO2 catalysts. Int. J. Hydrogen Energy 2017, 42, 1598–1608. [Google Scholar] [CrossRef]

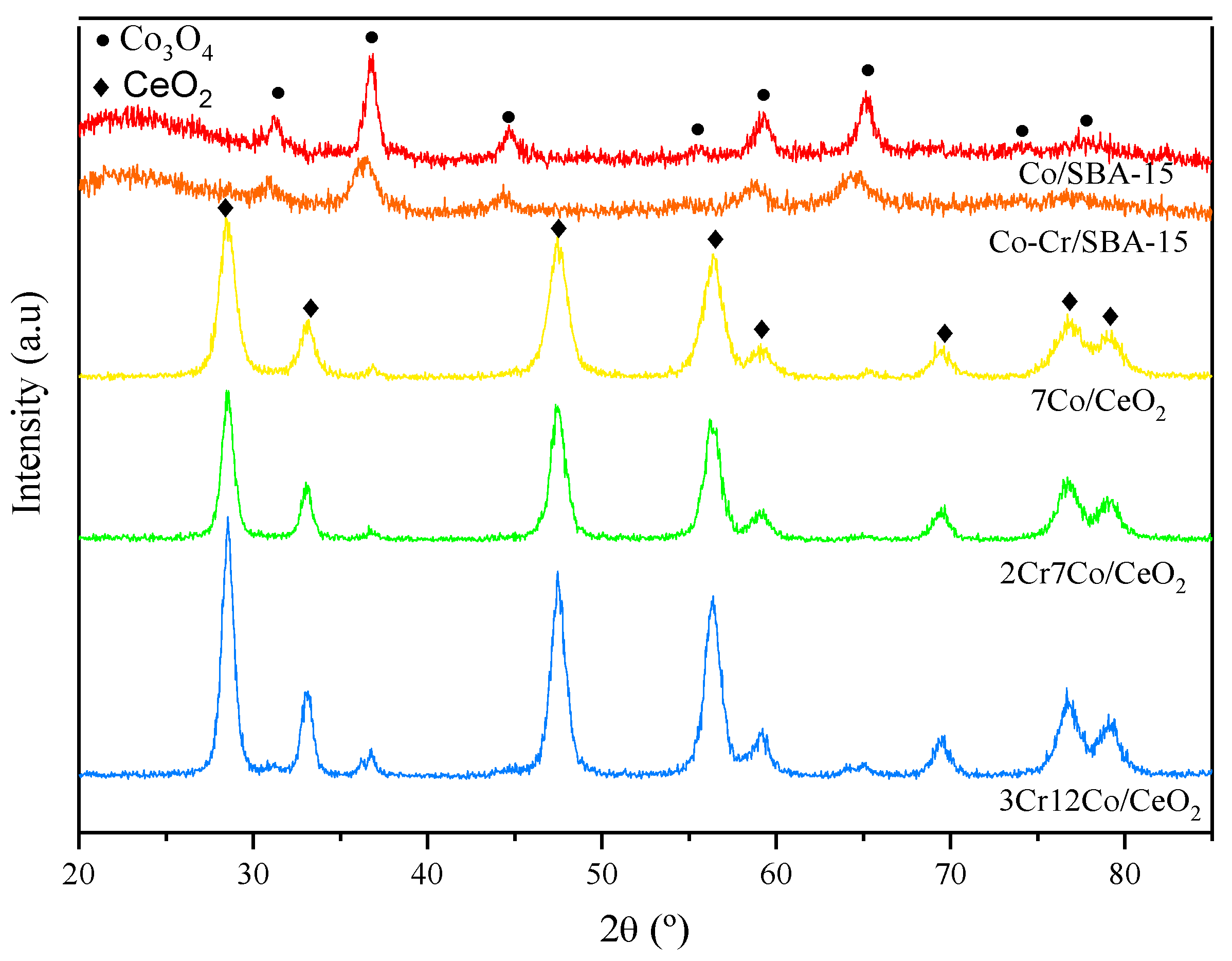
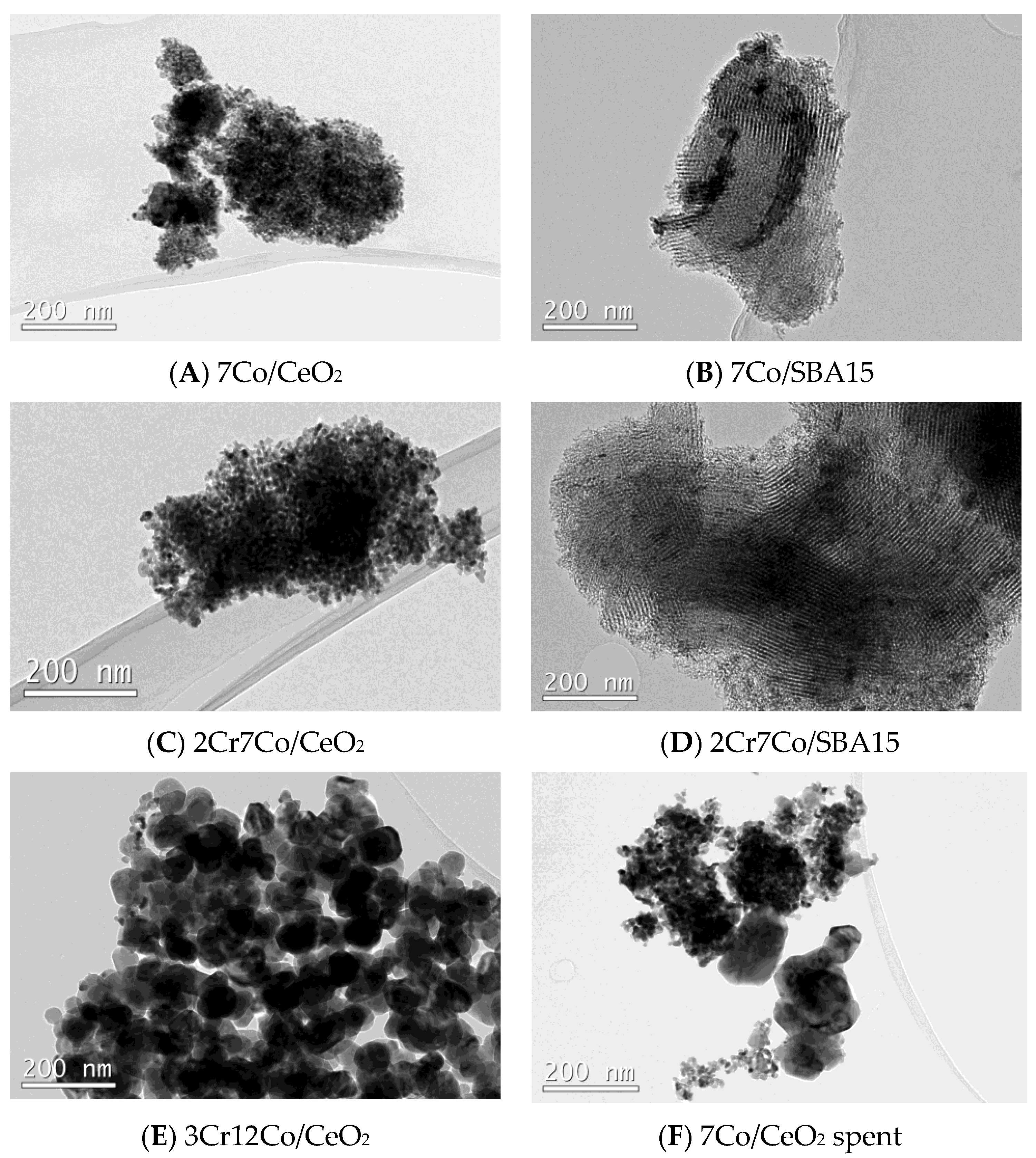

| Sample | SSA, m2 g−1 |
|---|---|
| SBA-15 | 465 |
| 7Co/SBA-15 | 406 |
| 2Cr7Co/SBA-15 | 362 |
| CeO2 | 105 |
| 7Co/CeO2 | 88 |
| 2Cr7Co/CeO2 | 62 |
| 3Cr12Co/CeO2 | 45 |
| Sample | Co Content, wt.% | Cr Content, wt.% | dCo3O4, nm |
|---|---|---|---|
| 7Co/SBA-15 | 6.9 | - | 10 |
| 2Cr7Co/SBA-15 | 6.7 | 1.8 | 6 |
| 7Co/CeO2 | 5.6 | - | 18 |
| 2Cr7Co/CeO2 | 6.4 | 1.9 | 14 |
| 3Cr12Co/CeO2 | 9.5 | 3.0 | 14 |
| Sample | Support | Co Content, wt.% | Cr Content, wt.% |
|---|---|---|---|
| 7Co/SBA-15 | SBA-15 | 7 | - |
| 2Cr7Co/SBA-15 | SBA-15 | 7 | 2 |
| 7Co/CeO2 | CeO2 | 7 | - |
| 2Cr7Co/CeO2 | CeO2 | 7 | 2 |
| 3Cr12Co/CeO2 | CeO2 | 12 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortese, M.; Ruocco, C.; Palma, V.; Megía, P.J.; Carrero, A.; Calles, J.A. On the Support Effect and the Cr Promotion of Co Based Catalysts for the Acetic Acid Steam Reforming. Catalysts 2021, 11, 133. https://doi.org/10.3390/catal11010133
Cortese M, Ruocco C, Palma V, Megía PJ, Carrero A, Calles JA. On the Support Effect and the Cr Promotion of Co Based Catalysts for the Acetic Acid Steam Reforming. Catalysts. 2021; 11(1):133. https://doi.org/10.3390/catal11010133
Chicago/Turabian StyleCortese, Marta, Concetta Ruocco, Vincenzo Palma, Pedro J. Megía, Alicia Carrero, and José A. Calles. 2021. "On the Support Effect and the Cr Promotion of Co Based Catalysts for the Acetic Acid Steam Reforming" Catalysts 11, no. 1: 133. https://doi.org/10.3390/catal11010133
APA StyleCortese, M., Ruocco, C., Palma, V., Megía, P. J., Carrero, A., & Calles, J. A. (2021). On the Support Effect and the Cr Promotion of Co Based Catalysts for the Acetic Acid Steam Reforming. Catalysts, 11(1), 133. https://doi.org/10.3390/catal11010133











