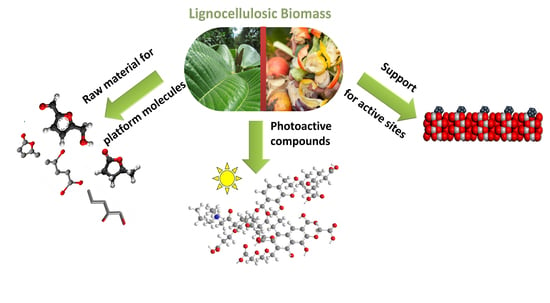
Lignocellulose Biomass as a Multifunctional Tool for Sustainable Catalysis and Chemicals: An Overview
Abstract
1. Introduction
2. Chemistry of Lignocellulose Biomass
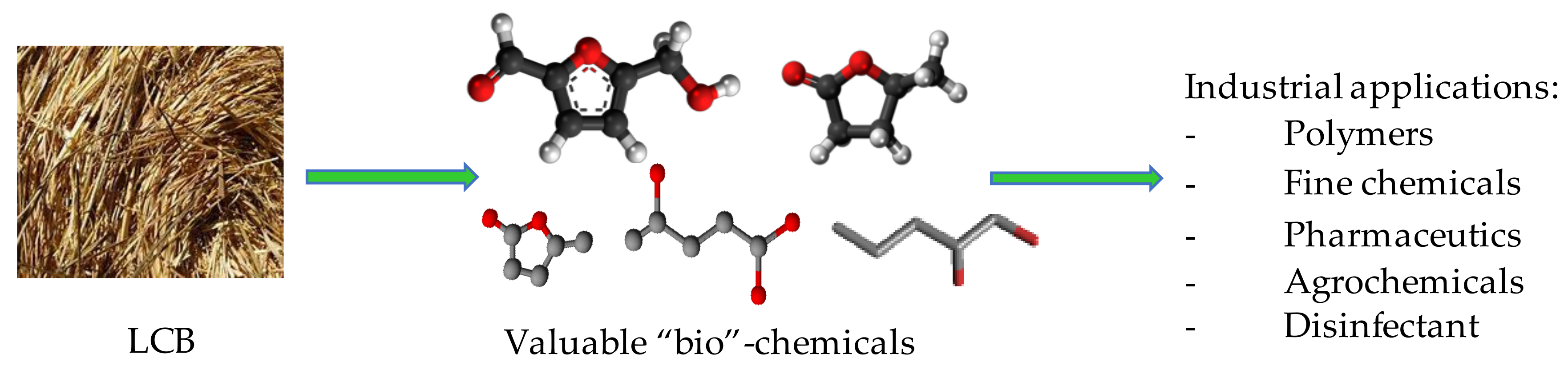
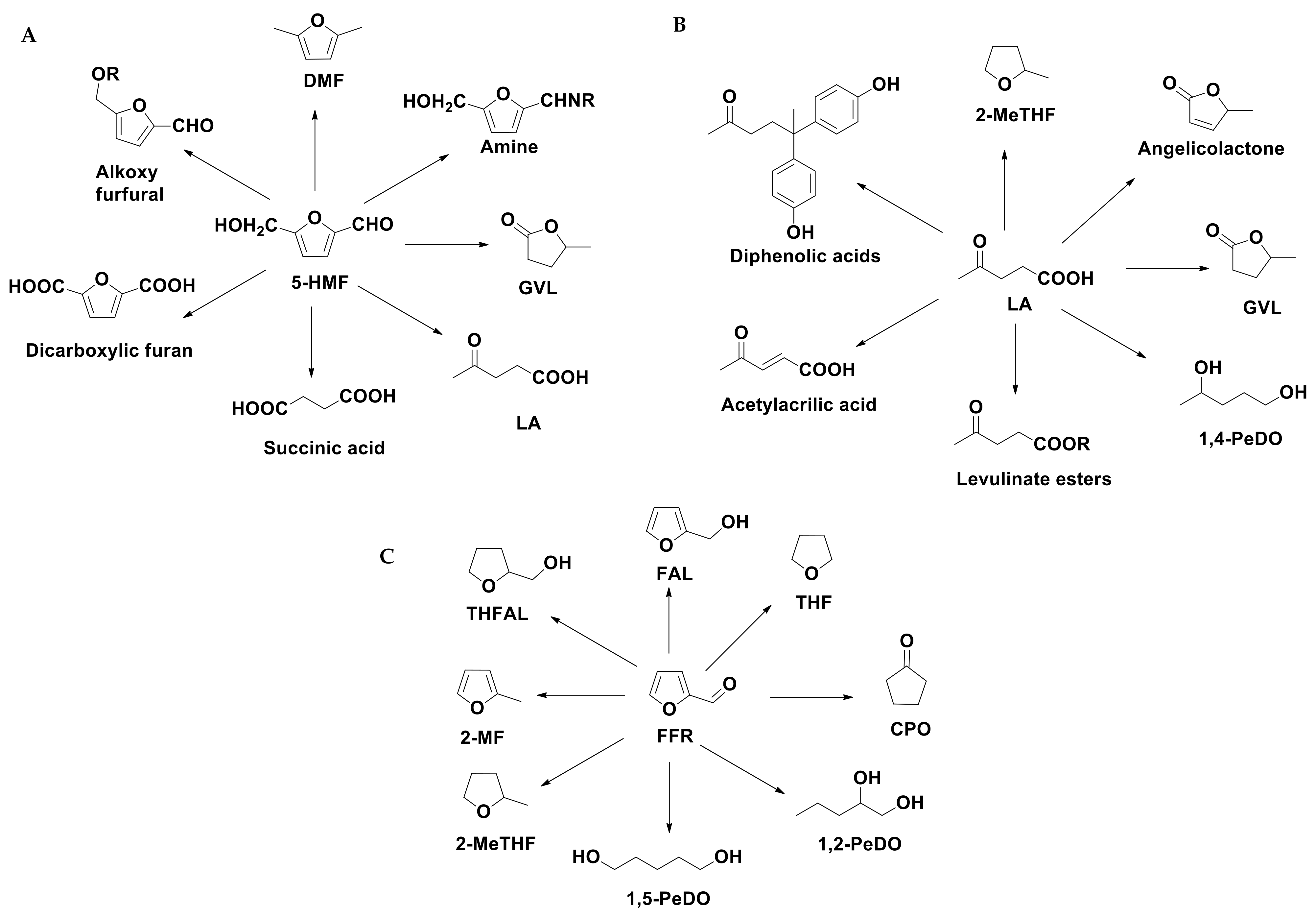
3. Conversion of LCB into Valuable Chemicals
4. Photoactive Humic-Like Substances for Water Remediation
5. LCB as a Support for a Sustainable Catalysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fridays for Future Webpage. Available online: https://fridaysforfuture.org/ (accessed on 5 January 2021).
- Spratt, D.; Dunlop, I. Existential climate-related security risk: A scenario approach. Discuss. Pap. 2019, 1–10. [Google Scholar]
- Xu, Y.; Ramanathan, V. Well below 2 °C: Mitigation strategies for avoiding dangerous to catastrophic climate changes. Proc. Natl. Acad. Sci. USA 2017, 114, 10315–10323. [Google Scholar] [CrossRef] [PubMed]
- Glanemann, N.; Willner, S.N.; Levermann, A. Paris climate agreement passes the cost-benefit test. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McGlade, C.; Ekins, P. The geographical distribution of fossil fuels unused when limiting global warming to 2 °C. Nature 2015, 517, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Levi, P.G.; Cullen, J.M. Mapping global flows of chemicals: From fossil fuel feedstocks to chemical products. Environ. Sci. Technol. 2018, 52, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.C.; De Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef]
- Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 5 January 2021).
- Ritchie, H.; Roser, M. CO2 and Greenhouse Gas Emissions. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 5 January 2021).
- Govorushko, S. Environmental problems of extraction, transportation, and use of fossil fuels. In Fossil Fuels; Kumar, R., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 1–84. ISBN 978-1-62808-412-2. [Google Scholar]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Marks, S.J.; Clair-Caliot, G.; Taing, L.; Bamwenda, J.T.; Kanyesigye, C.; Rwendeire, N.E.; Kemerink-Seyoum, J.S.; Kansiime, F.; Batega, D.W.; Ferrero, G. Water supply and sanitation services in small towns in rural–urban transition zones: The case of Bushenyi-Ishaka Municipality, Uganda. NPJ Clean Water 2020, 3, 1–9. [Google Scholar] [CrossRef]
- UNICEF: Drinking Water Report. Available online: https://data.unicef.org/topic/water-and-sanitation/drinking-water/ (accessed on 5 January 2021).
- Tummino, M.L.; Testa, M.L.; Malandrino, M.; Gamberini, R.; Prevot, A.B.; Magnacca, G.; Laurenti, E. Green waste-derived substances immobilized on SBA-15 silica: Surface properties, adsorbing and photosensitizing activities towards organic and inorganic substrates. Nanomaterials 2019, 9, 162. [Google Scholar] [CrossRef]
- Englande, A.J.; Krenkel, P.; Shamas, J. Wastewater treatment &water reclamation. In Reference Module in Earth Systems and Environmental Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 1–32. ISBN 9780124095489. [Google Scholar]
- Contaminants of Emerging Concern Including Pharmaceuticals and Personal Care Products. Available online: https://www.epa.gov/wqc/contaminants-emerging-concern-including-pharmaceuticals-and-personal-care-products (accessed on 5 January 2021).
- Tummino, M.L.; Nisticò, R.; Riedo, C.; Fabbri, D.; Cerruti, M.; Magnacca, G. Waste cleaning waste: Combining alginate with biowaste-derived substances in hydrogels and films for water cleanup. Chem. A Eur. J. 2020, 26. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ching, Y.; Chuah, C. Applications of lignocellulosic fibers and lignin in bioplastics: A review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Tillman, D.A.; Duong, D.N.B.; Harding, N.S. Blending coal with biomass. In Solid Fuel Blending; Elsevier: Oxford, UK, 2012; pp. 125–200. [Google Scholar]
- Mikkola, J.; Sklavounos, E.; King, A.W.T.; Virtanen, P. The biorefinery and green chemistry. In Ionic Liquids in the Biorefinery Concept: Challenges and Perspectives; The Royal Society of Chemistry: Cambridge, UK, 2016; pp. 1–37. ISBN 9781782622598. [Google Scholar]
- Kamm, B.; Kamm, M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2004, 64, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna water purification by using adsorbents: A review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Adsul, M.G.; Singhvi, M.S.; Gaikaiwari, S.A.; Gokhale, D.V. Development of biocatalysts for production of commodity chemicals from lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4304–4312. [Google Scholar] [CrossRef]
- Govil, T.; Wang, J.; Samanta, D.; David, A.; Tripathi, A.; Rauniyar, S.; Salem, D.R.; Sani, R.K. Lignocellulosic feedstock: A review of a sustainable platform for cleaner production of nature’s plastics. J. Clean. Prod. 2020, 270, 122521. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef] [PubMed]
- Balla, V.K.; Kate, K.H.; Satyavolu, J.; Singh, P.; Tadimeti, J.G.D. Additive manufacturing of natural fiber reinforced polymer composites: Processing and prospects. Compos. Part B Eng. 2019, 174, 106956. [Google Scholar] [CrossRef]
- Wang, D.; Cai, Z.; Zhang, Z.; Xu, X.; Yu, H. Laboratory Investigation of lignocellulosic biomass as performance improver for bituminous materials. Polymers 2019, 11, 1253. [Google Scholar] [CrossRef] [PubMed]
- Kamil, M.; Ramadan, K.; Ghenai, C.; Olabi, A.G.; Nazzal, I.T. Emissions from combustion of second-generation biodiesel produced from seeds of date palm fruit (Phoenix dactylifera L.). Appl. Sci. 2019, 9, 3720. [Google Scholar] [CrossRef]
- Moncada, J.; Tamayo, J.A.; Cardona, C.A. Integrating first, second, and third generation biorefineries: Incorporating microalgae into the sugarcane biorefinery. Chem. Eng. Sci. 2014, 118, 126–140. [Google Scholar] [CrossRef]
- Tummino, M.L.; Tolardo, V.; Malandrino, M.; Sadraei, R.; Magnacca, G.; Laurenti, E. A way to close the loop: Physicochemical and adsorbing properties of soybean hulls recovered after soybean peroxidase extraction. Front. Chem. 2020, 8, 763. [Google Scholar] [CrossRef]
- Saveyn, H.; Eder, P. End-of-Waste Criteria for Biodegradable Waste Subjected to Biological Treatment (Compost & Digestate): Technical Proposals; European Commission, Joint Research Centre: Sevilla, Spain, 2014; ISBN 9789279350627. [Google Scholar]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Awasthi, M.K.; Dussap, C.G.; Pandey, A. Assessing the impact of industrial waste on environment and mitigation strategies: A comprehensive review. J. Hazard. Mater. 2020, 398, 123019. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukhopadhyay, S.; Hashim, M.A.; Gupta, B. Sen contemporary environmental issues of landfill leachate: Assessment and remedies. Crit. Rev. Environ. Sci. Technol. 2015, 45, 472–590. [Google Scholar] [CrossRef]
- Pavlas, M.; Dvořáček, J.; Pitschke, T.; Peche, R. Biowaste Treatment and Waste-To-Energy—Environmental Benefits. Energies 2020, 13, 1994. [Google Scholar] [CrossRef]
- Pognani, M.; Barrena, R.; Font, X.; Sánchez, A. A complete mass balance of a complex combined anaerobic/aerobic municipal source-separated waste treatment plant. Waste Manag. 2012, 32, 799–805. [Google Scholar] [CrossRef]
- Chan, Y.C.; Sinha, R.K.; Wang, W. Emission of greenhouse gases from home aerobic composting, anaerobic digestion and vermicomposting of household wastes in Brisbane (Australia). Waste Manag. Res. 2011, 29, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Tabasso, S.; Berto, S.; Rosato, R.; Marinos, J.A.T.; Ginepro, M.; Zelano, V.; Daniele, P.G.; Montoneri, E. Chemical modeling of acid-base properties of soluble biopolymers derived from municipal waste treatment materials. Int. J. Mol. Sci. 2015, 16, 3405–3418. [Google Scholar] [CrossRef] [PubMed]
- Bouallagui, H.; Touhami, Y.; Ben Cheikh, R.; Hamdi, M. Bioreactor performance in anaerobic digestion of fruit and vegetable wastes. Process Biochem. 2005, 40, 989–995. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Karpichev, Y.; Pandey, A.; Chandra Kuhad, R.; Bhat, R.; Punia, R.; Aghbashlo, M.; Tabatabaei, M.; Gupta, V.K. Advancement in valorization technologies to improve utilization of bio-based waste in bioeconomy context. Renew. Sustain. Energy Rev. 2020, 131, 109965. [Google Scholar] [CrossRef]
- Beuel, P.; Rieker, C.; Bursche, J. Comparative life-cycle-assessment of pretreatment processes for the production of biofuels from lignocellulosic residues. Int. Energy Sustain. Conf. IESC 2019. [Google Scholar] [CrossRef]
- Rebello, S.; Anoopkumar, A.N.; Aneesh, E.M.; Sindhu, R.; Binod, P.; Pandey, A. Sustainability and life cycle assessments of lignocellulosic and algal pretreatments. Bioresour. Technol. 2020, 301, 122678. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, A.; Korres, N.E.; Rathore, D.; Sevda, S.; Pant, D. Sustainable utilization of crop residues for energy generation: A life cycle assessment (LCA) perspective. Bioresour. Technol. 2020, 303, 122964. [Google Scholar] [CrossRef]
- Yilmaz, N.; Kodama, Y.; Numata, K. Revealing the architecture of the cell wall in living plant cells by bioimaging and enzymatic degradation. Biomacromolecules 2020, 21, 95–103. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Maleki, S.S.; Mohammadi, K.; Ji, K.S. Characterization of cellulose synthesis in plant cells. Sci. World J. 2016, 2016, 8641373. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and opportunities in the characterization of cellulose—An important regulator of cell wall growth and mechanics. Front. Plant Sci. 2019, 9, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kuna, E.; Behling, R.; Valange, S.; Chatel, G.; Colmenares, J.C. Sonocatalysis: A potential sustainable pathway for the valorization of lignocellulosic biomass and derivatives. Top. Curr. Chem. 2017, 41, 375. [Google Scholar] [CrossRef]
- Haider, K.M.; Guggenberger, G. Organic matter | Genesis and formation. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 93–101. ISBN 978-0-12-348530-4. [Google Scholar]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Panwar, D.; Kaira, G.S. Bioprocesses for enzyme production using agro-industrial wastes: Technical challenges and commercialization potential. In Agro-Industrial Wastes as Feedstock for Enzyme Production: Apply and Exploit the Emerging and Valuable Use Options of Waste Biomass; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 61–93. ISBN 9780128026120. [Google Scholar]
- Guilherme, A.A.; Dantas, P.V.F.; Santos, E.S.; Fernandes, F.A.N.; Macedo, G.R. Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugar cane bagasse. Braz. J. Chem. Eng. 2015, 32, 23–33. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Peng, H.J.; Tang, C.; Lieder, M.; Zhang, Q.; Titirici, M.M. Porous carbon derived from rice husks as sustainable bioresources: Insights into the role of micro-/mesoporous hierarchy in hosting active species for lithium-sulphur batteries. Green Chem. 2016, 18, 5169–5179. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit peel waste: Characterization and its potential uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefinery 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Rusanen, A.; Lappalainen, K.; Kärkkäinen, J.; Tuuttila, T.; Mikola, M.; Lassi, U. Selective hemicellulose hydrolysis of Scots pine sawdust. Biomass Convers. Biorefinery 2019, 9, 283–291. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A review on the partial and complete dissolution and fractionation of wood and lignocelluloses using imidazolium ionic liquids. Polymers 2020, 12, 195. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.C.; Hu, H.Q.; Xie, F.J.; Wei, X.Y.; Fan, X. Structural characterization of lignin and its degradation products with spectroscopic methods. J. Spectrosc. 2017, 2017. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, E.; Bond, J.Q.; Dumesic, J.A.; Román-Leshkov, Y. Role of Acid Catalysis in the Conversion of Lignocellulosic Biomass to Fuels and Chemicals. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 261–288. ISBN 9780444563309. [Google Scholar]
- Werpy, T.; Petersen, G. Top value added chemicals from biomass volume I. US Natl. Renew. Energy Lab. 2004, 1–76. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Tomishige, K. Production of 1,5-pentanediol from biomass via furfural and tetrahydrofurfuryl alcohol. Catal. Today 2012, 195, 136–143. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Wright, W.R.H.; Palkovits, R. Development of heterogeneous catalysts for the conversion of levulinic acid to γ-valerolactone. ChemSusChem 2012, 5, 1657–1667. [Google Scholar] [CrossRef]
- Testa, M.L.; Corbel-Demailly, L.; La Parola, V.; Venezia, A.M.; Pinel, C. Effect of Au on Pd supported over HMS and Ti doped HMS as catalysts for the hydrogenation of levulinic acid to γ-valerolactone. Catal. Today 2015, 257, 291–296. [Google Scholar] [CrossRef]
- Delhomme, C.; Schaper, L.A.; Zhang-Preße, M.; Raudaschl-Sieber, G.; Weuster-Botz, D.; Kühn, F.E. Catalytic hydrogenation of levulinic acid in aqueous phase. J. Organomet. Chem. 2013, 724, 297–299. [Google Scholar] [CrossRef]
- Nzediegwu, E.; Dumont, M.J. Chemo-catalytic transformation of cellulose and cellulosic-derived waste materials into platform chemicals. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011, 13, 520–540. [Google Scholar] [CrossRef]
- Chen, S.S.; Maneerung, T.; Tsang, D.C.W.; Ok, Y.S.; Wang, C.H. Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem. Eng. J. 2017, 328, 246–273. [Google Scholar] [CrossRef]
- Dawes, G.J.S.; Scott, E.L.; Le Nôtre, J.; Sanders, J.P.M.; Bitter, J.H. Deoxygenation of biobased molecules by decarboxylation and decarbonylation—A review on the role of heterogeneous, homogeneous and bio-catalysis. Green Chem. 2015, 17, 3231–3250. [Google Scholar] [CrossRef]
- Rinaldi, R.; Palkovits, R.; Schüth, F. Depolymerization of cellulose using solid catalysts in ionic liquids. Angew. Chem. Int. Ed. 2008, 47, 8047–8050. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Liu, J.; Fu, W.; Tang, T.; Wu, D. Improved activity for cellulose conversion to levulinic acid through hierarchization of ETS-10 zeolite. ACS Sustain. Chem. Eng. 2017, 5, 5800–5809. [Google Scholar] [CrossRef]
- Imteyaz Alam, M.; De, S.; Dutta, S.; Saha, B. Solid-acid and ionic-liquid catalyzed one-pot transformation of biorenewable substrates into a platform chemical and a promising biofuel. RSC Adv. 2012, 2, 6890–6896. [Google Scholar] [CrossRef]
- Li, S.; Qian, E.W.; Shibata, T.; Hosomi, M. Catalytic hydrothermal saccharification of rice straw using mesoporous silica-based solid acid catalysts. J. Jpn. Pet. Inst. 2012, 55, 250–260. [Google Scholar] [CrossRef]
- Jeong, J.; Antonyraj, C.A.; Shin, S.; Kim, S.; Kim, B.; Lee, K.Y.; Cho, J.K. Commercially attractive process for production of 5-hydroxymethyl-2-furfural from high fructose corn syrup. J. Ind. Eng. Chem. 2013, 19, 1106–1111. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Van Der Schaaf, J.; Schouten, J.C.; Nijhuis, T.A. Fructose dehydration to 5-hydroxymethylfurfural over solid acid catalysts in a biphasic system. ChemSusChem 2012, 5, 1812–1819. [Google Scholar] [CrossRef]
- Weingarten, R.; Conner, W.C.; Huber, G.W. Production of levulinic acid from cellulose by hydrothermal decomposition combined with aqueous phase dehydration with a solid acid catalyst. Energy Environ. Sci. 2012, 5, 7559–7574. [Google Scholar] [CrossRef]
- Chen, S.S.; Wang, L.; Yu, I.K.M.; Tsang, D.C.W.; Hunt, A.J.; Jérôme, F.; Zhang, S.; Ok, Y.S.; Poon, C.S. Valorization of lignocellulosic fibres of paper waste into levulinic acid using solid and aqueous BrØnsted acid. Bioresour. Technol. 2018, 247, 387–394. [Google Scholar] [CrossRef]
- Alonso, D.M.; Gallo, J.M.R.; Mellmer, M.A.; Wettstein, S.G.; Dumesic, J.A. Direct conversion of cellulose to levulinic acid and gamma-valerolactone using solid acid catalysts. Catal. Sci. Technol. 2013, 3, 927–931. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, Z.; Xue, L.; Wang, X.; Jiang, Z. Highly efficient preparation of HMF from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction. Appl. Catal. B Environ. 2016, 196, 50–56. [Google Scholar] [CrossRef]
- Sun, Z.; Cheng, M.; Li, H.; Shi, T.; Yuan, M.; Wang, X.; Jiang, Z. One-pot depolymerization of cellulose into glucose and levulinic acid by heteropolyacid ionic liquid catalysis. RSC Adv. 2012, 2, 9058–9065. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Catalytic hydrolysis of cellulose and oil palm biomass in ionic liquid to reducing sugar for levulinic acid production. Fuel Process. Technol. 2014, 128, 490–498. [Google Scholar] [CrossRef]
- Lai, D.M.; Deng, L.; Guo, Q.X.; Fu, Y. Hydrolysis of biomass by magnetic solid acid. Energy Environ. Sci. 2011, 4, 3552–3557. [Google Scholar] [CrossRef]
- Yang, F.; Li, Y.; Zhang, Q.; Sun, X.; Fan, H.; Xu, N.; Li, G. Selective conversion of cotton cellulose to glucose and 5-hydroxymethyl furfural with SO42-/MxOysolid superacid catalyst. Carbohydr. Polym. 2015, 131, 9–14. [Google Scholar] [CrossRef]
- Huang, Y.; Chao, P.Y.; Cheng, T.Y.; Ho, Y.; Lin, C.T.; Hsu, H.Y.; Wong, J.J.; Tsai, T.C. Design of sulfonated mesoporous silica catalyst for fructose dehydration guided by difructose anhydride intermediate incorporated reaction network. Chem. Eng. J. 2016, 283, 778–788. [Google Scholar] [CrossRef]
- Shao, Y.; Du, W.; Gao, Z.; Sun, K.; Zhang, Z.; Li, Q.; Zhang, L.; Zhang, S.; Liu, Q.; Hu, X. Sulfated TiO2 nanosheets catalyzing conversion of biomass derivatives: Influences of the sulfation on distribution of Brønsted and Lewis acidic sites. J. Chem. Technol. Biotechnol. 2020, 95, 1337–1347. [Google Scholar] [CrossRef]
- Testa, M.L.; Miroddi, G.; Russo, M.; La Parola, V.; Marcì, G. Dehydration of fructose to 5-HMF over acidic TiO2 catalysts. Materials 2020, 13, 1178. [Google Scholar] [CrossRef]
- Drago, C.; Liotta, L.F.; La Parola, V.; Testa, M.L.; Nicolosi, G. One-pot microwave assisted catalytic transformation of vegetable oil into glycerol-free biodiesel. Fuel 2013, 113, 707–711. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; Estevez, R.; Russo, M.; La Parola, V.; Bautista, F.M.; Testa, M.L. Microwave-assisted glycerol etherification over sulfonic acid catalysts. Materials 2020, 13, 1584. [Google Scholar] [CrossRef] [PubMed]
- Date, N.S.; La Parola, V.; Rode, C.V.; Testa, M.L. Ti-doped Pd-Au catalysts for one-pot hydrogenation and ring opening of furfural. Catalysts 2018, 8, 252. [Google Scholar] [CrossRef]
- Koso, S.; Furikado, I.; Shimao, A.; Miyazawa, T.; Kunimori, K.; Tomishige, K. Chemoselective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol. Chem. Commun. 2009, 2035–2037. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabal-Telleria, I.; Requies, J.; Güemez, M.B.; Arias, P.L. Dehydration of d-xylose to furfural using selective and hydrothermally stable arenesulfonic SBA-15 catalysts. Appl. Catal. B Environ. 2014, 145, 34–42. [Google Scholar] [CrossRef]
- Shirotori, M.; Nishimura, S.; Ebitani, K. One-pot synthesis of furfural from xylose using Al2 O3 –Ni-Al layered double hydroxide acid-base bi-functional catalyst and sulfonated resin. Chem. Lett. 2016, 45, 194–196. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Yeap, J.H.; Wong, H.C.; Dumesic, J.A. Production of furfural from lignocellulosic biomass using beta zeolite and biomass-derived solvent. Top. Catal. 2013, 56, 1775–1781. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, G.; Yu, K.; Yu, H.; Wang, X. Furfural production from biomass–derived carbohydrates and lignocellulosic residues via heterogeneous acid catalysts. Ind. Crops Prod. 2017, 98, 68–75. [Google Scholar] [CrossRef]
- O’Neil, R.; Ahmad, M.N.; Vanoye, L.; Aiouache, F. Kinetics of aqueous phase dehydration of xylose into furfural catalyzed by ZSM-5 zeolite. Ind. Eng. Chem. Res. 2009, 48, 4300–4306. [Google Scholar] [CrossRef]
- Paulino, P.N.; Perez, R.F.; Figueiredo, N.G.; Fraga, M.A. Tandem dehydration-transfer hydrogenation reactions of xylose to furfuryl alcohol over zeolite catalysts. Green Chem. 2017, 19, 3759–3763. [Google Scholar] [CrossRef]
- Song, S.; Di, L.; Wu, G.; Dai, W.; Guan, N.; Li, L. Meso-Zr-Al-beta zeolite as a robust catalyst for cascade reactions in biomass valorization. Appl. Catal. B Environ. 2017, 205, 393–403. [Google Scholar] [CrossRef]
- Dias, A.S.; Pillinger, M.; Valente, A.A. Dehydration of xylose into furfural over micro-mesoporous sulfonic acid catalysts. J. Catal. 2005, 229, 414–423. [Google Scholar] [CrossRef]
- Kaiprommarat, S.; Kongparakul, S.; Reubroycharoen, P.; Guan, G.; Samart, C. Highly efficient sulfonic MCM-41 catalyst for furfural production: Furan-based biofuel agent. Fuel 2016, 174, 189–196. [Google Scholar] [CrossRef]
- Deng, T.; Xu, G.; Fu, Y. One-pot cascade conversion of xylose to furfuryl alcohol over a bifunctional Cu/SBA-15-SO3H catalyst. Chin. J. Catal. 2020, 41, 404–414. [Google Scholar] [CrossRef]
- Canhaci, S.J.; Perez, R.F.; Borges, L.E.P.; Fraga, M.A. Direct conversion of xylose to furfuryl alcohol on single organic–inorganic hybrid mesoporous silica-supported catalysts. Appl. Catal. B Environ. 2017, 207, 279–285. [Google Scholar] [CrossRef]
- Perez, R.F.; Canhaci, S.J.; Borges, L.E.P.; Fraga, M.A. One-step conversion of xylose to furfuryl alcohol on sulfated zirconia-supported Pt catalyst—Balance between acid and metal sites. Catal. Today 2017, 289, 273–279. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Morales, G.; Paniagua, M.; Hernández, B.; Osatiashtiani, A.; Lee, A.F.; Wilson, K. ZrO2-SBA-15 catalysts for the one-pot cascade synthesis of GVL from furfural. Catal. Sci. Technol. 2018, 8, 4485–4493. [Google Scholar] [CrossRef]
- Zhu, S.; Xue, Y.; Guo, J.; Cen, Y.; Wang, J.; Fan, W. Integrated conversion of hemicellulose and furfural into γ-valerolactone over Au/ZrO2 Catalyst Combined with ZSM-5. ACS Catal. 2016, 6, 2035–2042. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Millán, G.G.; Sixta, H. Towards the green synthesis of furfuryl alcohol in a one-pot system from xylose: A review. Catalysts 2020, 10, 1101. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Requies, J.; Güemez, M.B.; Arias, P.L. Pore size tuning of functionalized SBA-15 catalysts for the selective production of furfural from xylose. Appl. Catal. B Environ. 2012, 115–116, 169–178. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Adani, F.; Spagnol, M.; Nierop, K.G.J. Biochemical origin and refractory properties of humic acid extracted from maize plants: The contribution of lignin. Biogeochemistry 2007, 82, 55–65. [Google Scholar] [CrossRef]
- Lee, J.G.; Yoon, H.Y.; Cha, J.Y.; Kim, W.Y.; Kim, P.J.; Jeon, J.R. Artificial humification of lignin architecture: Top-down and bottom-up approaches. Biotechnol. Adv. 2019, 37, 107416. [Google Scholar] [CrossRef] [PubMed]
- Zingaretti, D.; Lombardi, F.; Baciocchi, R. Soluble organic substances extracted from compost as amendments for Fenton-like oxidation of contaminated sites. Sci. Total Environ. 2018, 619–620, 1366–1374. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bulkowska, K.; Kierklo, K. Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil. Chemosphere 2015, 136, 42–49. [Google Scholar] [CrossRef]
- Guo, X.X.; Liu, H.T.; Wu, S.B. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Eyheraguibel, B.; Silvestre, J.; Morard, P. Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize. Bioresour. Technol. 2008, 99, 4206–4212. [Google Scholar] [CrossRef]
- Deganello, F.; Tummino, M.L.; Calabrese, C.; Testa, M.L.; Avetta, P.; Fabbri, D.; Prevot, A.B.; Montoneri, E.; Magnacca, G. A new, sustainable LaFeO3 material prepared from biowaste-sourced soluble substances. New J. Chem. 2015, 39, 877–885. [Google Scholar] [CrossRef]
- Winarso, S.; Pandutama, M.H.; Purwanto, L.D. Effectivity of humic substance extracted from palm oil compost as liquid fertilizer and heavy metal bioremediation. Agric. Agric. Sci. Procedia 2016, 9, 146–157. [Google Scholar] [CrossRef][Green Version]
- Kałuza-Haładyn, A.; Jamroz, E.; Bekier, J. Humic substances of differently matured composts produced from municipal solid wastes and biomass of energetic plants. Soil Sci. Annu. 2019, 70, 292–297. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology, the Gold Book, 2nd ed.; Blackwell Science: Oxford, UK, 1997. [Google Scholar]
- Michelin, C.; Hoffmann, N. Photosensitization and photocatalysis—Perspectives in organic synthesis. ACS Catal. 2018, 8, 12046–12055. [Google Scholar] [CrossRef]
- Zhao, S.; Xue, S.; Zhang, J.; Zhang, Z.; Sun, J. Dissolved organic matter-mediated photodegradation of anthracene and pyrene in water. Sci. Rep. 2020, 10, 3413. [Google Scholar] [CrossRef] [PubMed]
- Tummino, M.L.; Magnacca, G.; Cimino, D.; Laurenti, E.; Nisticò, R. The innovation comes from the sea: Chitosan and alginate hybrid gels and films as sustainable materials for wastewater remediation. Int. J. Mol. Sci. 2020, 21, 550. [Google Scholar] [CrossRef] [PubMed]
- Arce, V.B.; Mucci, C.R.; Fernández Solarte, A.M.; Torres Sánchez, R.M.; Mártire, D.O. Application of novel fulvic acid-coated magnetite nanoparticles for CO2-Mediated Photoreduction of Cr(VI). Water Air Soil Pollut. 2018, 229, 39. [Google Scholar] [CrossRef]
- Zhan, M.; Yang, X.; Xian, Q.; Kong, L. Photosensitized degradation of bisphenol A involving reactive oxygen species in the presence of humic substances. Chemosphere 2006, 63, 378–386. [Google Scholar] [CrossRef]
- Aguer, J.P.; Richard, C. Influence of the excitation wavelength on the photoiductive properties of humic substances. Chemosphere 1999, 38, 2293–2301. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Fabbri, D.; Pramauro, E.; Baiocchi, C.; Medana, C.; Montoneri, E.; Boffa, V. Sensitizing effect of bio-based chemicals from urban wastes on the photodegradation of azo-dyes. J. Photochem. Photobiol. A Chem. 2010, 209, 224–231. [Google Scholar] [CrossRef]
- Bernabeu, A.; Vercher, R.F.; Santos-Juanes, L.; Simón, P.J.; Lardín, C.; Martínez, M.A.; Vicente, J.A.; González, R.; Llosá, C.; Arques, A.; et al. Solar photocatalysis as a tertiary treatment to remove emerging pollutants from wastewater treatment plant effluents. Catal. Today 2011, 161, 235–240. [Google Scholar] [CrossRef]
- Carlos, L.; Mártire, D.O.; Gonzalez, M.C.; Gomis, J.; Bernabeu, A.; Amat, A.M.; Arques, A. Photochemical fate of a mixture of emerging pollutants in the presence of humic substances. Water Res. 2012, 46, 4732–4740. [Google Scholar] [CrossRef]
- Gomis, J.; Bianco Prevot, A.; Montoneri, E.; González, M.C.; Amat, A.M.; Mártire, D.O.; Arques, A.; Carlos, L. Waste sourced bio-based substances for solar-driven wastewater remediation: Photodegradation of emerging pollutants. Chem. Eng. J. 2014, 235, 236–243. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for advanced oxidation processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Náfrádi, M.; Hlogyik, T.; Cora Pravda, B.; Schrantz, K.; Hernádi, K.; Alapi, T. Comparison of advanced oxidation processes in the decomposition of diuron and monuron-efficiency, intermediates, electrical energy per order and the effect of various matrices. Environ. Sci. Water Res. Technol. 2018, 4, 1345–1360. [Google Scholar] [CrossRef]
- Foszpańczyk, M.; Bilińska, L.; Gmurek, M.; Ledakowicz, S. Heterogeneous oxidation of phenolic compounds with photosensitizing catalysts incorporated into chitosan. Catalysts 2019, 9, 891. [Google Scholar] [CrossRef]
- Testa, M.L.; Tummino, M.L.; Agostini, S.; Avetta, P.; Deganello, F.; Montoneri, E.; Magnacca, G.; Prevot, A.B. Synthesis, characterization and environmental application of silica grafted photoactive substances isolated from urban biowaste. RSC Adv. 2015, 5, 47920–47927. [Google Scholar] [CrossRef]
- Jia, J.; Liu, D.; Tian, J.; Wang, W.; Ni, J.; Wang, X. Visible-light-excited humic acid for peroxymonosulfate activation to degrade bisphenol A. Chem. Eng. J. 2020, 400, 125853. [Google Scholar] [CrossRef]
- Caram, B.; García-Ballesteros, S.; Santos-Juanes, L.; Arques, A.; García-Einschlag, F.S. Humic like substances for the treatment of scarcely soluble pollutants by mild photo-Fenton process. Chemosphere 2018, 198, 139–146. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Baino, F.; Fabbri, D.; Franzoso, F.; Magnacca, G.; Nisticò, R.; Arques, A. Urban biowaste-derived sensitizing materials for caffeine photodegradation. Environ. Sci. Pollut. Res. 2017, 24, 12599–12607. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Qu, R.; Ge, J.; Wang, Z.; Gu, C. Enhanced removal of chlorophene and 17β-estradiol by Mn(III) in a mixture solution with humic acid: Investigation of reaction kinetics and formation of co-oligomerization products. Environ. Sci. Technol. 2018, 52, 13222–13230. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, C.; Sun, Z.; Zhao, X.; Zhou, Q.; Hoffmann, M.R. Synergistic impact of humic acid on the photo-reductive decomposition of perfluorooctanoic acid. Chem. Eng. J. 2019, 360, 1101–1110. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO binary oxide systems: Comprehensive characterization and tests of photocatalytic activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Su, J.; Liang, J.; Wu, B.; Zuo, J.; Zuo, Y. Role of humic substances in the photodegradation of naproxen under simulated sunlight. Chemosphere 2017, 187, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Brahmia, O.; Boulkamh, A. The influence of the structure of humic substances extracted from different soils and peats on their capacity to photosensitize 1-naphthol. J. Mater. Environ. Sci. 2016, 7, 310–318. [Google Scholar]
- Rigobello, E.S.; Campos, S.X.; Azevedo, E.R.D.; Dantas, A.D.B.; Vieira, E.M. Comparative characterization of humic substances extracted from freshwater and peat of different apparent molecular sizes. Ambient. E Agua Interdiscip. J. Appl. Sci. 2017, 12, 774. [Google Scholar] [CrossRef][Green Version]
- Niu, H.; Yang, H.; Tong, L.; Zhong, S.; Liu, Y. Spectral study of humic substance extract from pressurized oxidizing slag of Carlin-typed gold deposit. J. Phys. Conf. Ser. 2019, 1347, 012027. [Google Scholar] [CrossRef]
- Calza, P.; Vione, D.; Minero, C. The role of humic and fulvic acids in the phototransformation of phenolic compounds in seawater. Sci. Total Environ. 2014, 493, 411–418. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant properties of humic substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, F.; Wang, F.; Zhang, Y.; Shi, Q.; Han, X.; Geng, H. Molecular characteristics of leonardite humic acid and the effect of its fractionations on sulfamethoxazole photodegradation. Chemosphere 2020, 246, 125642. [Google Scholar] [CrossRef]
- Ranga, S. Comparative analysis of homogeneous and heterogeneous catalysis. Int. J. Eng. Technol. Sci. Res. 2017, 4, 1496–1500. [Google Scholar]
- Vekariya, R.L.; Sonigara, K.K.; Fadadu, K.B.; Vaghasiya, J.V.; Soni, S.S. Humic acid as a sensitizer in highly stable dye solar cells: Energy from an abundant natural polymer soil component. ACS Omega 2016, 1, 14–18. [Google Scholar] [CrossRef]
- Porras, J.; Bedoya, C.; Silva-Agredo, J.; Santamaría, A.; Fernández, J.J.; Torres-Palma, R.A. Role of humic substances in the degradation pathways and residual antibacterial activity during the photodecomposition of the antibiotic ciprofloxacin in water. Water Res. 2016, 94, 1–9. [Google Scholar] [CrossRef]
- Porras, J.; Fernández, J.J.; Torres-Palma, R.A.; Richard, C. Humic substances enhance chlorothalonil phototransformation via photoreduction and energy transfer. Environ. Sci. Technol. 2014, 48, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Son, M.H.; Gong, J.; Seo, S.; Yoon, H.; Chang, Y.S. Photosensitized diastereoisomer-specific degradation of hexabromocyclododecane (HBCD) in the presence of humic acid in aquatic systems. J. Hazard. Mater. 2019, 369, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Yamasaki, T.; Yamada, K. ichi Photodecomposition of tetrabromobisphenol A in aqueous humic acid suspension by irradiation with light of various wavelengths. Chemosphere 2016, 147, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Filipe, O.M.S.; Santos, E.B.H.; Otero, M.; Gonçalves, E.A.C.; Neves, M.G.P.M.S. Photodegradation of metoprolol in the presence of aquatic fulvic acids. Kinetic studies, degradation pathways and role of singlet oxygen, OH radicals and fulvic acids triplet states. J. Hazard. Mater. 2020, 385, 121523. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Huang, B.; Yang, B.; Chen, F.; Pan, X.; Dionysiou, D.D. Photobleaching alters the photochemical and biological reactivity of humic acid towards 17α-ethynylestradiol. Environ. Pollut. 2017, 220, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Ren, Z.; Chen, F.; Wang, B.; Huang, B. Predictive role of spectral slope ratio towards 17 α-ethynylestradiol photodegradation sensitized by humic acids. Environ. Pollut. 2019, 254, 112959. [Google Scholar] [CrossRef]
- Trubetskoi, O.A.; Patsaeva, S.V.; Trubetskaya, O.E. Photochemical degradation of organic pollutants in solutions of soil humic acids. Eurasian Soil Sci. 2019, 52, 1075–1080. [Google Scholar] [CrossRef]
- Minella, M.; Merlo, M.P.; Maurino, V.; Minero, C.; Vione, D. Transformation of 2,4,6-trimethylphenol and furfuryl alcohol, photosensitised by Aldrich humic acids subject to different filtration procedures. Chemosphere 2013, 90, 306–311. [Google Scholar] [CrossRef]
- Silva, C.P.; Lima, D.L.D.; Groth, M.B.; Otero, M.; Esteves, V.I. Effect of natural aquatic humic substances on the photodegradation of estrone. Chemosphere 2016, 145, 249–255. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Degradation of diclofenac in aqueous solution by ionizing radiation in the presence of humic acid. Sep. Purif. Technol. 2020, 234, 116079. [Google Scholar] [CrossRef]
- Santoke, H.; Song, W.; Cooper, W.J.; Peake, B.M. Advanced oxidation treatment and photochemical fate of selected antidepressant pharmaceuticals in solutions of Suwannee River humic acid. J. Hazard. Mater. 2012, 217–218, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Koumaki, E.; Mamais, D.; Noutsopoulos, C.; Nika, M.C.; Bletsou, A.A.; Thomaidis, N.S.; Eftaxias, A.; Stratogianni, G. Degradation of emerging contaminants from water under natural sunlight: The effect of season, pH, humic acids and nitrate and identification of photodegradation by-products. Chemosphere 2015, 138, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Amine-Khodja, A.; Trubetskaya, O.; Trubetskoj, O.; Cavani, L.; Ciavatta, C.; Guyot, G.; Richard, C. Humic-like substances extracted from composts can promote the photodegradation of Irgarol 1051 in solar light. Chemosphere 2006, 62, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakov, I.P.; Tyutereva, Y.E.; Parkhats, M.V.; Grivin, V.P.; Fang, Y.; Liu, L.; Wan, D.; Luo, F.; Chen, Y. Mechanistic investigation of humic substances assisted photodegradation of imipramine under simulated sunlight. Sci. Total Environ. 2020, 738, 140298. [Google Scholar] [CrossRef] [PubMed]
- Avetta, P.; Bianco Prevot, A.; Fabbri, D.; Montoneri, E.; Tomasso, L. Photodegradation of naphthalene sulfonic compounds in the presence of a bio-waste derived sensitizer. Chem. Eng. J. 2012, 197, 193–198. [Google Scholar] [CrossRef]
- Avetta, P.; Bella, F.; Bianco Prevot, A.; Laurenti, E.; Montoneri, E.; Arques, A.; Carlos, L. Waste cleaning waste: Photodegradation of monochlorophenols in the presence of waste-derived photosensitizer. ACS Sustain. Chem. Eng. 2013, 1, 1545–1550. [Google Scholar] [CrossRef]
- Aparicio, F.; Escalada, J.P.; De Gerónimo, E.; Aparicio, V.C.; Einschlag, F.S.G.; Magnacca, G.; Carlos, L.; Mártire, D.O. Carbamazepine degradation mediated by light in the presence of humic substances-coated magnetite nanoparticles. Nanomaterials 2019, 9, 1379. [Google Scholar] [CrossRef]
- Calza, P.; Di Sarro, J.; Magnacca, G.; Bianco Prevot, A.; Laurenti, E. Low-cost magnetic materials containing waste derivatives as catalyst for removal of organic pollutants: Insights into the reaction mechanism and odd aspects. In Proceedings of the 6th International Conference on Sustainable Solid Waste Management, Naxos Island, Greece, 13–16 June 2018; pp. 1–12. [Google Scholar]
- Nisticò, R.; Prevot, A.B.; Magnacca, G.; Canone, L.; García-Ballesteros, S.; Arques, A. Sustainable magnetic materials (From chitosan and municipal biowaste) for the removal of diclofenac from water. Nanomaterials 2019, 9, 1091. [Google Scholar] [CrossRef]
- Bertella, S.; Luterbacher, J.S. Lignin functionalization for the production of novel materials. Trends Chem. 2020, 2, 440–453. [Google Scholar] [CrossRef]
- Esquivel-Peña, V.; Guccini, V.; Kumar, S.; Salazar-Alvarez, G.; Rodríguez De San Miguel, E.; De Gyves, J. Hybrids based on borate-functionalized cellulose nanofibers and noble-metal nanoparticles as sustainable catalysts for environmental applications. RSC Adv. 2020, 10, 12460–12468. [Google Scholar] [CrossRef]
- Zhi, Y.; Deng, X.; Ni, Y.; Zhao, W.; Jia, Q.; Shan, S. Cellulosic Cr(salen) complex as an efficient and recyclable catalyst for copolymerization of SO2 with epoxide. Carbohydr. Polym. 2018, 194, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.N.; Al Azad, S.; Deb, H.; Shaun, B.B.; Shen, X.L. Titania-loaded cellulose-based functional hybrid nanomaterial for photocatalytic degradation of toxic aromatic dye in water. J. Water Process Eng. 2020, 33, 101062. [Google Scholar] [CrossRef]
- Akhtar, K.; Ali, F.; Sohni, S.; Kamal, T.; Asiri, A.M.; Bakhsh, E.M.; Khan, S.B. Lignocellulosic biomass supported metal nanoparticles for the catalytic reduction of organic pollutants. Environ. Sci. Pollut. Res. 2020, 27, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Eisa, W.H.; Abdelgawad, A.M.; Rojas, O.J. Solid-state synthesis of metal nanoparticles supported on cellulose nanocrystals and their catalytic activity. ACS Sustain. Chem. Eng. 2018, 6, 3974–3983. [Google Scholar] [CrossRef]
- Gopiraman, M.; Deng, D.; Saravanamoorthy, S.; Chung, I.M.; Kim, I.S. Gold, silver and nickel nanoparticle anchored cellulose nanofiber composites as highly active catalysts for the rapid and selective reduction of nitrophenols in water. RSC Adv. 2018, 8, 3014–3023. [Google Scholar] [CrossRef]
- Tang, J.; Shi, Z.; Berry, R.M.; Tam, K.C. Mussel-inspired green metallization of silver nanoparticles on cellulose nanocrystals and their enhanced catalytic reduction of 4-nitrophenol in the presence of β-cyclodextrin. Ind. Eng. Chem. Res. 2015, 54, 3299–3308. [Google Scholar] [CrossRef]
- Han, Y.; Wu, X.; Zhang, X.; Zhou, Z.; Lu, C. Reductant-free synthesis of silver nanoparticles-doped cellulose microgels for catalyzing and product separation. ACS Sustain. Chem. Eng. 2016, 4, 6322–6331. [Google Scholar] [CrossRef]
- Li, D.D.; Lu, G.P.; Cai, C. Modified cellulose with tunable surface hydrophilicity/hydrophobicity as a novel catalyst support for selective reduction of nitrobenzene. Catal. Commun. 2020, 137, 105949. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Zhang, J.; Yang, L.; Ma, Z.; Wang, L.; Li, H.; Bai, L.; Wei, D.; Wang, W.; et al. Cellulose nanocrystal shelled with poly(ionic liquid)/polyoxometalate hybrid as efficient catalyst for aerobic oxidative desulfurization. J. Colloid Interface Sci. 2019, 554, 572–579. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Shafiei, N.; Nezafat, Z.; Soheili Bidgoli, N.S.; Soleimani, F. Recent progresses in the application of cellulose, starch, alginate, gum, pectin, chitin and chitosan based (nano)catalysts in sustainable and selective oxidation reactions: A review. Carbohydr. Polym. 2020, 241, 116353. [Google Scholar] [CrossRef]
- Kempasiddaiah, M.; Kandathil, V.; Dateer, R.B.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. Immobilizing biogenically synthesized palladium nanoparticles on cellulose support as a green and sustainable dip catalyst for cross-coupling reaction. Cellulose 2020, 27, 3335–3357. [Google Scholar] [CrossRef]
- Kale, D.; Rashinkar, G.; Kumbhar, A.; Salunkhe, R. Facile Suzuki-Miyaura cross coupling using ferrocene tethered N-heterocyclic carbene-Pd complex anchored on cellulose. React. Funct. Polym. 2017, 116, 9–16. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, M.L.; Yusoff, M.M.; Sarkar, S.M. Highly active bio-waste cellulose supported poly(amidoxime) palladium(II) complex for Heck reactions. J. Clean. Prod. 2017, 149, 1045–1050. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, T.; Hu, H.; Cai, X.; Huang, Z.; Liang, X.; Yin, Y.; Qin, Y.; Feng, Z. Effective treatment and utilization of hazardous waste sulfuric acid generated from alkylation by lignocellulose ester-catalyzed oxidative degradation of organic pollutants. J. Hazard. Mater. 2019, 380, 120892. [Google Scholar] [CrossRef]
- Shaabani, A.; Ganji, N.; Seyyedhamzeh, M.; Mofakham, H. Cellulose sulfuric acid: As an efficient bio polymer based catalyst for the selective oxidation of sulfides and thiols by hydrogen peroxide. Iran. J. Chem. Chem. Eng. 2014, 33, 1–7. [Google Scholar]
- Córdova, A.; Afewerki, S.; Alimohammadzadeh, R.; Sanhueza, I.; Tai, C.W.; Osong, S.H.; Engstrand, P.; Ibrahem, I. A sustainable strategy for production and functionalization of nanocelluloses. Pure Appl. Chem. 2019, 91, 865–874. [Google Scholar] [CrossRef]
- Abouzeid, R.E.; Khiari, R.; El-Wakil, N.; Dufresne, A. Current state and new trends in the use of cellulose nanomaterials for wastewater treatment. Biomacromolecules 2019, 20, 573–597. [Google Scholar] [CrossRef]
- Dong, Y.; Lai, Y.; Wang, X.; Gao, M.; Xue, F.; Chen, X.; Ma, Y.; Wei, Y. Design and synthesis of amine-functionalized cellulose with multiple binding sites and their application in C–C bond forming reactions. Int. J. Biol. Macromol. 2019, 130, 778–785. [Google Scholar] [CrossRef]
- Beyki, M.H.; Ghasemi, M.H. Quaternized γ-Fe2O3@cellulose ionomer: An efficient recyclable catalyst for Michael-type addition reaction. Int. J. Biol. Macromol. 2018, 113, 711–718. [Google Scholar] [CrossRef]
- El-Nahas, A.M.; Salaheldin, T.A.; Zaki, T.; El-Maghrabi, H.H.; Marie, A.M.; Morsy, S.M.; Allam, N.K. Functionalized cellulose-magnetite nanocomposite catalysts for efficient biodiesel production. Chem. Eng. J. 2017, 322, 167–180. [Google Scholar] [CrossRef]
- Sabaqian, S.; Nemati, F.; Heravi, M.M.; Nahzomi, H.T. Copper(I) iodide supported on modified cellulose-based nano-magnetite composite as a biodegradable catalyst for the synthesis of 1,2,3-triazoles. Appl. Organomet. Chem. 2017, 31, 1–12. [Google Scholar] [CrossRef]
- De Sá, D.S.; De Andrade Bustamante, R.; Rodrigues Rocha, C.E.; Da Silva, V.D.; Da Rocha Rodrigues, E.J.; Djenne Buarque Müller, C.; Ghavami, K.; Massi, A.; Ginoble Pandoli, O. Fabrication of lignocellulose-based microreactors: Copper-functionalized bamboo for continuous-flow CuAAC click reactions. ACS Sustain. Chem. Eng. 2019, 7, 3267–3273. [Google Scholar] [CrossRef]
- Chen, L.; Cao, W.; Quinlan, P.J.; Berry, R.M.; Tam, K.C. Sustainable catalysts from gold-loaded polyamidoamine dendrimer-cellulose nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 978–985. [Google Scholar] [CrossRef]
- Dhar, P.; Narendren, S.; Gaur, S.S.; Sharma, S.; Kumar, A.; Katiyar, V. Self-propelled cellulose nanocrystal based catalytic nanomotors for targeted hyperthermia and pollutant remediation applications. Int. J. Biol. Macromol. 2020, 158, 1020–1036. [Google Scholar] [CrossRef]




| Treatment Level | Scope | Strategies |
|---|---|---|
| Pretreatment | Enhancement of wastewater compatibility for subsequent treatment processes | Equalization, spill retention, pH neutralization, nutrient addition, toxics, oil and grease removal, and solids removal by flotation, sedimentation, or filtration |
| Primary | Lowering of oxygen-demanding substances by other physical treatments | Screening, grit removal, and sedimentation |
| Secondary | Further abatement of oxygen-demanding substances and disinfection | Biochemical oxidation: action of microorganisms and oxidizing agents as O3, H2O2, MnO4− and chlorine compounds |
| Tertiary | Removal of specific contaminants (especially the recalcitrant ones) to very low residue level | Precipitation, filtration, coagulation, flocculation, air stripping, ion exchange, adsorption, membrane processes, N-regulation and other advanced processes |
| Biomass | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ref. |
|---|---|---|---|---|
| Soybean Hulls | 38–51 | 20–25 | 4–8 | [35] |
| Wheat Straw | 34–40 | 20–25 | 20 | [55] |
| Sugarcane Bagasse | 35–42 | 25–31 | 17–19 | [56] |
| Rice Husks | ca. 38 | ca. 18 | ca. 22 | [57] |
| Fruit peels | 9–23 | 8–17 | 1–9 | [58] |
| Dried fruits shells | 22–51 | 22–32 | 20–52 | [59] |
| Pine Sawdust | ca. 44 | ca. 26 | ca. 26 | [60] |
| Entry | Catalyst Type Material | Used LCB or Derivatives | Reaction Conditions | Main Results and Notes | Ref. |
|---|---|---|---|---|---|
| 1 | Amberlyst® | Spruce wood | Amberlyst®-15, 100 °C, 5 h, ionic liquids | 1 TRS (21%) | [76] |
| 2 | Fructose | Amberlyst®-15, 100 °C, 3 h, 1,4-dioxane | 5-HMF (80%) | [80] | |
| 3 | Fructose | Amberlyst®-15, 135 °C, 5 h, 2 MIBK/H2O | 5-HMF (60%) | [81] | |
| 4 | Avicel-PH101 | Amberlyst®-70, 160 °C, 8 h, water | LA (28%) | [82] | |
| 5 | Paper towel | Amberlyst®-36, 150 °C, 20 min, water | LA (34%) | [83] | |
| 6 | Microcrystalline cellulose (MCC) | Amberlyst®-70, 160 °C, 16 h, water | LA (69%) | [84] | |
| 7 | Heteropolyacids (HP) | MCC | HP based catalysts, 140 °C, 8 h, 2 MIBK/H2O | 5-HMF (75%) | [85] |
| 8 | MCC | HP based catalysts, 140 °C, 12 h, water | LA (63%) | [86] | |
| 9 | Zeolite | Oil palm frond | Fe/HY- Zeolite, 120 °C, 3 h, ionic liquid/water | 1 TRS (27%) | [87] |
| 10 | Fructose | 3 MOR-Zeolite, 135 °C, 5 h, 2 MIBK/H2O | 5-HMF (10%) | [81] | |
| 11 | MCC | Zeolite based catalysts, 200 °C, 6 h, water | LA (91%) | [77] | |
| 12 | Sulfonic oxides | Rice straws | SO3H SBA-15, 180 °C, 1 h, water | Monosaccharide (38%) | [79] |
| 13 | Cellulose | Fe3O4-SBA-SO3H, 150 °C, 3 h, water | Glucose (50%) | [88] | |
| 14 | Cotton Cellulose | SO3H metal oxide, 190 °C, 3.5 h, water | Glucose (27%) | [89] | |
| 15 | Fructose | SO3H MCM41, 190 °C, 3 h, water | 5-HMF (77%) | [90] | |
| 16 | Fructose | SO3H TiO2, 130 °C, 1.5 h, 4 DMSO | 5-HMF (79%) | [91] | |
| 17 | Fructose | SO3H TiO2 based catalysts, 165 °C, 3 h, water | 5-HMF (65%) | [92] | |
| 18 | Cotton straw | SBA-SO3H, 180 °C, 6 h, water | LA (18%) | [88] | |
| 19 | Cellulose | SBA-SO3H, 150 °C, 12 h, water | LA (53%) | [88] |
| Entry | Catalyst Type Material | Used LCB or Derivatives | Reaction Conditions | Main Results and Notes | Ref. |
|---|---|---|---|---|---|
| 1 | Amberlyst® | xylose | Amberlyst®-70, 160 °C, 20 h, toluene/water | FFR (60%) | [97] |
| 2 | xylose | Al-Amberlyst®-15, 100 °C, 8 h, dimethylformamide | FFR (46%) | [98] | |
| 3 | Zeolites | Arabinose | H-β-Zeolite, 160 °C, 40 min, water/GVL (1:9) | FFR (73%) | [99] |
| 4 | Corncob | Al-β-zeolite, 185 °C, 1.5 h, water/GVL (1:9) | FFR (20%) | [100] | |
| 5 | Xylose | H-ZMS-5, 200 °C, 2 h, water | FFR (46%) | [101] | |
| 6 | Xylose | β-Zeolite,130 °C, 1 h, water/isopropanol (1:1) | FAL (75%) | [102] | |
| 7 | FFR | Zr- Al-β-zeolite, 120 °C, 24 h, isopropanol/water (95:5) | GVL (95%) | [103] | |
| 8 | Sulfonic Silica based materials | Xylose | SO3H-MCM41, 140 °C, 24 h, water | FFR (47%) | [104] |
| 9 | Xylose | SO3H-MCM41, 140 °C, 24 h, toluene/water | FFR (96%) | [104] | |
| 10 | Xylose | MethylPropylSulfonic MCM41, 155 °C, 2 h, toluene/H2O (1:1) | FFR (93%) | [105] | |
| 11 | Xylose | SO3H-Ph-SBA15, 160 °C, 20 h, toluene/water | FFR (80%) | [97] | |
| 12 | Xylose | Cu/SO3H-SBA15, 140 °C, 6 h, water/butanol (1:3) | FAL (63%) | [106] | |
| 13 | Xylose | Pt/SO3H-SBA15, 130 °C, 6 h, water/isopropanol (1:1) | FAL (83%) | [107] | |
| 14 | Zirconia based materials | Xylose | Pt/ZrO2-SO4, 130 °C, 1 h, water/isopropanol (1:1) | FAL (27%) | [108] |
| 15 | FFR | ZrO2-SBA15, 170 °C, 7 h, isopropanol | GVL (47%) | [109] | |
| 16 | FFR | Au/ZrO2 + ZMS-5, 120 °C, 24 h, isopropanol | GVL (80%) | [110] |
| Types of HS | Substrate | Reaction Conditions | Main Results and Notes | Ref. |
|---|---|---|---|---|
| Pahokee peat humic acids (5.0 mg L−1) | Ciprofloxacin (6.0 × 10−5 M) | Homogeneous reaction, in aerated medium and pH 7, λmax = 365 nm | 40 min < t1/2 < 60 min and ca. 90% abatement in 120 min | [154] |
| Humic acids from coal (5 mg L−1) | Chlorothalonil (5.0 × 10−6 M) | Homogeneous reaction, in aerated medium and pH 8, 300 < λ < 450 nm | Ca. 45% abatement in 4 h | [155] |
| Commercial humic acids (50 μg mL−1) | α-hexabromocyclododecane (4 ng mL−1) | Homogeneous reaction, neutral pH, λ > 420 nm | Diastereoisomer-specific degradation, 40% in 6 h | [156] |
| Commercial humic acids (1.0 mg mL−1) | Tetrabromobisphenol A (1.0 mM) | Homogeneous reaction, 6.8 < pH < 9, λ > 300 nm and λ > 400 nm | Initial reaction rate: 7.03 × 10−9 mol g−1 HA s−1. The rate increased with pH and light intensity. Time max: 480 s | [157] |
| Fulvic acids from Vouga River (10 mg L−1) | Metoprolol (1.46 × 10−4 M) | Homogeneous reaction, λ > 290 nm | Ca. 80% abatement in 72 h | [158] |
| Humic and fulvic acids from soils and peat (25 mg L−1) | 1-naphtol (2.0 × 10−6 M) | Homogeneous reaction, pH 6.5, λmax = 365 nm | Max. degradation in presence of HA from soil, ca. 90% in 60 min | [146] |
| Humic acids from Dianchi Lake (5.0 mgC L−1) | 17α-ethynylestradiol (1.07 mg L−1) | Homogeneous reaction, pH 8, λ > 290 nm | 5-h removal rate of 30%; detection of HA self-photobleaching | [159] |
| Humic acids from Dianchi Lake (5.0 mgC L−1) | 17α-ethynylestradiol (2 μM) | Homogeneous reaction, pH 7.5, λ > 290 nm | Photodegradation was enhanced by 2.12–7.29 folds with HA | [160] |
| Soil-humic acid fractioned by different molecular sizes (MS) | 2,4,6-Trimethylphenol (0.1 mM) | Homogeneous reaction, pH 6.5, λmax = 365 nm | HA fractions with MS < 5 kDa reached the maximum photodegradation | [161] |
| Commercial humic acids (10 mg L−1) | 2,4,6-Trimethylphenol (TMP,0.3 mM) and furfuryl alcohol (FFA, 0.1 mM) | Homogeneous reaction, 300 < λ < 450 nm | Ca. total abatement in 24 h for TMP and 60% for FFA | [162] |
| Fulvic acids, terrestrial and marine humic acids (50 mg L−1) | Phenol (20 mg L−1) | Homogeneous reaction in different water matrixes, pH 8, λ > 340 nm | Faster phenol degradation in seawater than in pure water, due to Cl− and Br− that scavenge 3HS*, forming higher reactive species. | [149] |
| Humic and fulvic acids from Aveiro Lagoon (20 mg L−1) | Estrone (500 μg mL−1) | Homogeneous reaction, λ > 290 nm | Photodegradation of 66% in presence of HA, 74% for FA, in 6 h | [163] |
| Commercial humic acids (30–80 mg L−1) | Diclofenac (30 mg L−1) | Homogeneous reaction, pH 6.37, γ-radiation (dose 0–1.0 kGy) | HA competing effect: at 0.8 kGy, with 30 mg L−1 of HA, the degradation decreased of 20% | [164] |
| Commercial humic and fulvic acids (10 mg L−1) | Naproxen (5.0 μM) | Homogeneous reaction, variable pH values, λ > 300 nm | Photodegradation rates decreased with increasing pH. HA inhibited the degradation; FA accelerated it at lower substrate concentration and light intensity | [145] |
| Humic acids from Suwannee River (25 mg L−1) | Duloxetine, venlafaxine and bupropion (1 mM) | Homogeneous reaction, λ = 350 nm | Higher degradation by HA (+10–25%) than direct photolysis. Time max: 7 h | [165] |
| Leonardite humic acids (0.5–20 mg L−1), pristine or fractioned by molecular weight (MW) | Sulfamethoxazole (5 mg L−1) | Homogeneous reaction, pH 6.8, solar light simulated by xenon lamp | At the lowest concentration (0.5 mg L−1), only the HA fraction with 25,000–100,000 MW increased the degradation rate | [151] |
| Commercial Humic Acids (20 mg L−1) | Mix of nonylphenol (NP), NP ethoxylates (NPEs), diclofenac, bisphenol-A (BPA), and others (2.0 μg L−1 each) | Homogeneous reaction, pH 7, under daily sunlight | NP, NPEs and BPA decreased by 70%, 15%, 55%, after 15 h; for more photo-sensitive pollutants, HA inhibited the degradation | [166] |
| Humic acids from sewage sludge/trimmings (25 mg L−1) | Irgarol 1051 (1.0 × 10−5 M) | Homogeneous reaction, pH 6.5, λmax = 365 nm | The degradation rate increased with HA extracted after higher composting times | [167] |
| Commercial humic and fulvic acids (10 mg L−1) | Imipramine (10 μM) | Homogeneous reaction, pH 8, λ > 300 nm | The photodegradation increased with increasing pH and with deoxygenation. | [168] |
| Humic acids from composted food/green waste (150 mg L−1) | Naphthalene sulfonates (20 mg L−1) | Homogeneous reaction, λ > 340 nm | Depletion of 70–80% of mono- and disulfonated substrates after 24 h | [169] |
| Green waste-derived substances (500 mg L−1) | 2-, 3- and 4-chlorophenol (CP, 1.0 × 10–4 M) | Homogeneous reaction, pH 9.8, λ > 340 nm | Degradation rate 2-CP > 3-CP > 4-CP. Total degradation of 2-CP after 5 h. | [170] |
| Leonardite humic acids on magnetite nanoparticles (500 mg L−1) | Carbamazepine (2 mg L−1) | Heterogeneous reaction, pH 6, λmax = 350 nm | 16% substrate removal after 6 h | [171] |
| Green waste-derived substances on magnetite nanoparticles (100–1000 mg L−1) | Phenol (10 mg L−1) | Heterogeneous reaction, pH 3.5, λmax = 365 nm | Ca. total abatement in 24 h with 1000 mg L−1 | [172] |
| Green waste-derived substances on magnetite nanoparticles (500 mg L−1) | Diclofenac (10 mg L−1) | Heterogeneous reaction, λ > 300 nm | Substrate decomposition of ca. 80% after 90 min | [173] |
| Green waste-derived substances on three different silicas (800 mg L−1) | 4-methylphenol (10 mg L−1) | Heterogeneous reaction, λ > 340 nm | 70–80% abatement in 8 h, completed in 15 h; higher stability achieved by the SBA hybrid | [138] |
| Green waste-derived substances on SBA-15 silica (800 mg L−1) | Orange II and Rhodamine B (OII and RH, 10 mg L−1) | Heterogeneous reaction, pH 5.5, λ > 340 nm | Initial adsorption followed by degradation: 45% for OII and 30% for RH. Time max: 6 h | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, M.L.; Tummino, M.L. Lignocellulose Biomass as a Multifunctional Tool for Sustainable Catalysis and Chemicals: An Overview. Catalysts 2021, 11, 125. https://doi.org/10.3390/catal11010125
Testa ML, Tummino ML. Lignocellulose Biomass as a Multifunctional Tool for Sustainable Catalysis and Chemicals: An Overview. Catalysts. 2021; 11(1):125. https://doi.org/10.3390/catal11010125
Chicago/Turabian StyleTesta, Maria Luisa, and Maria Laura Tummino. 2021. "Lignocellulose Biomass as a Multifunctional Tool for Sustainable Catalysis and Chemicals: An Overview" Catalysts 11, no. 1: 125. https://doi.org/10.3390/catal11010125
APA StyleTesta, M. L., & Tummino, M. L. (2021). Lignocellulose Biomass as a Multifunctional Tool for Sustainable Catalysis and Chemicals: An Overview. Catalysts, 11(1), 125. https://doi.org/10.3390/catal11010125