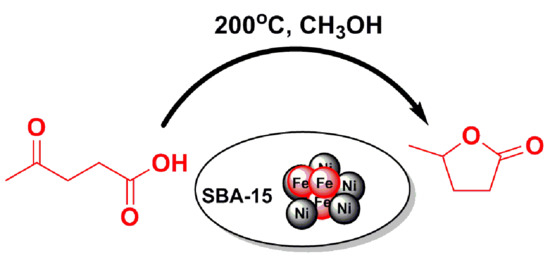
Hydrogenative Cyclization of Levulinic Acid to γ-Valerolactone with Methanol and Ni-Fe Bimetallic Catalysts
Abstract
1. Introduction
2. Results and Discussion
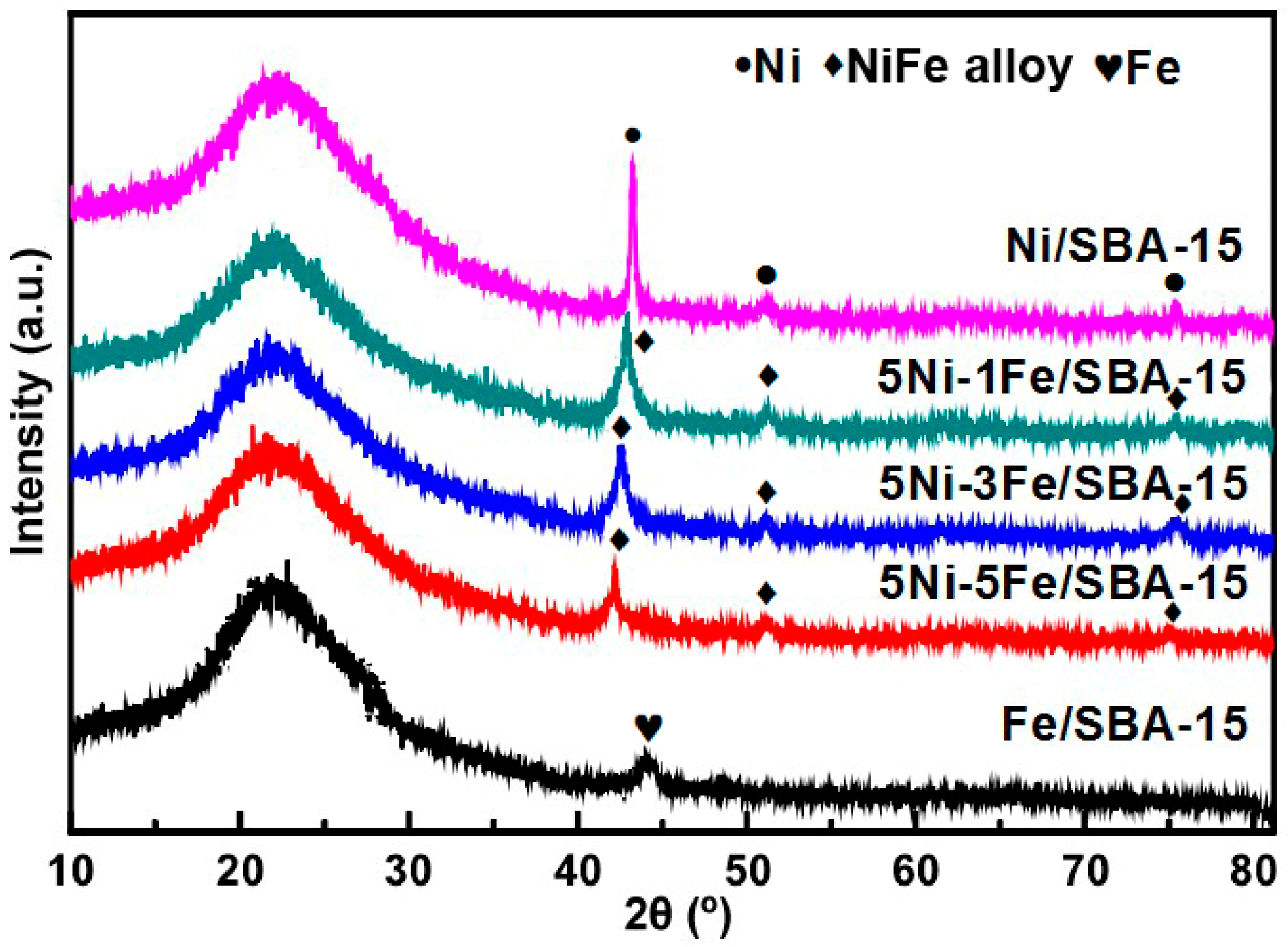
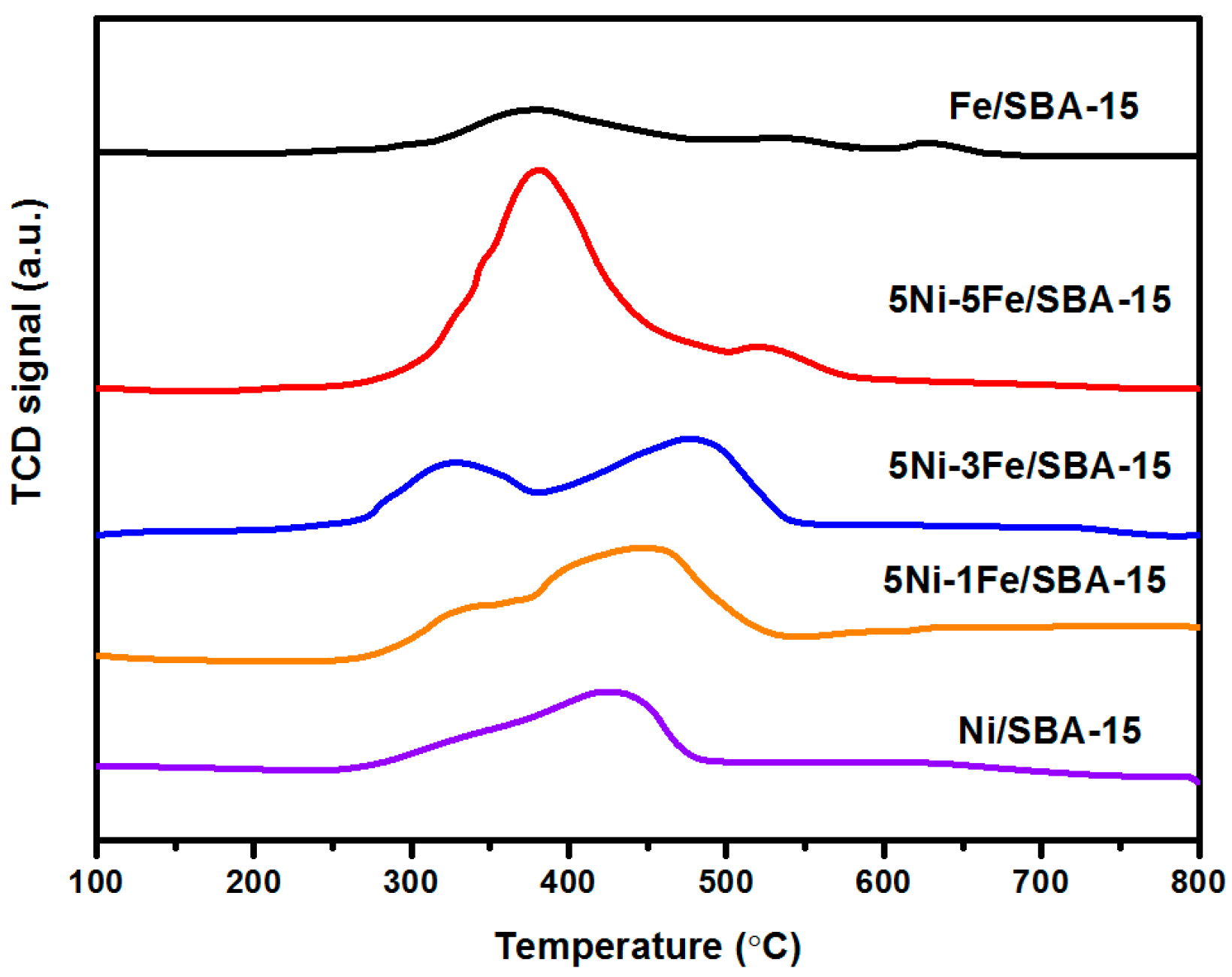
2.1. Characterization of Catalysts
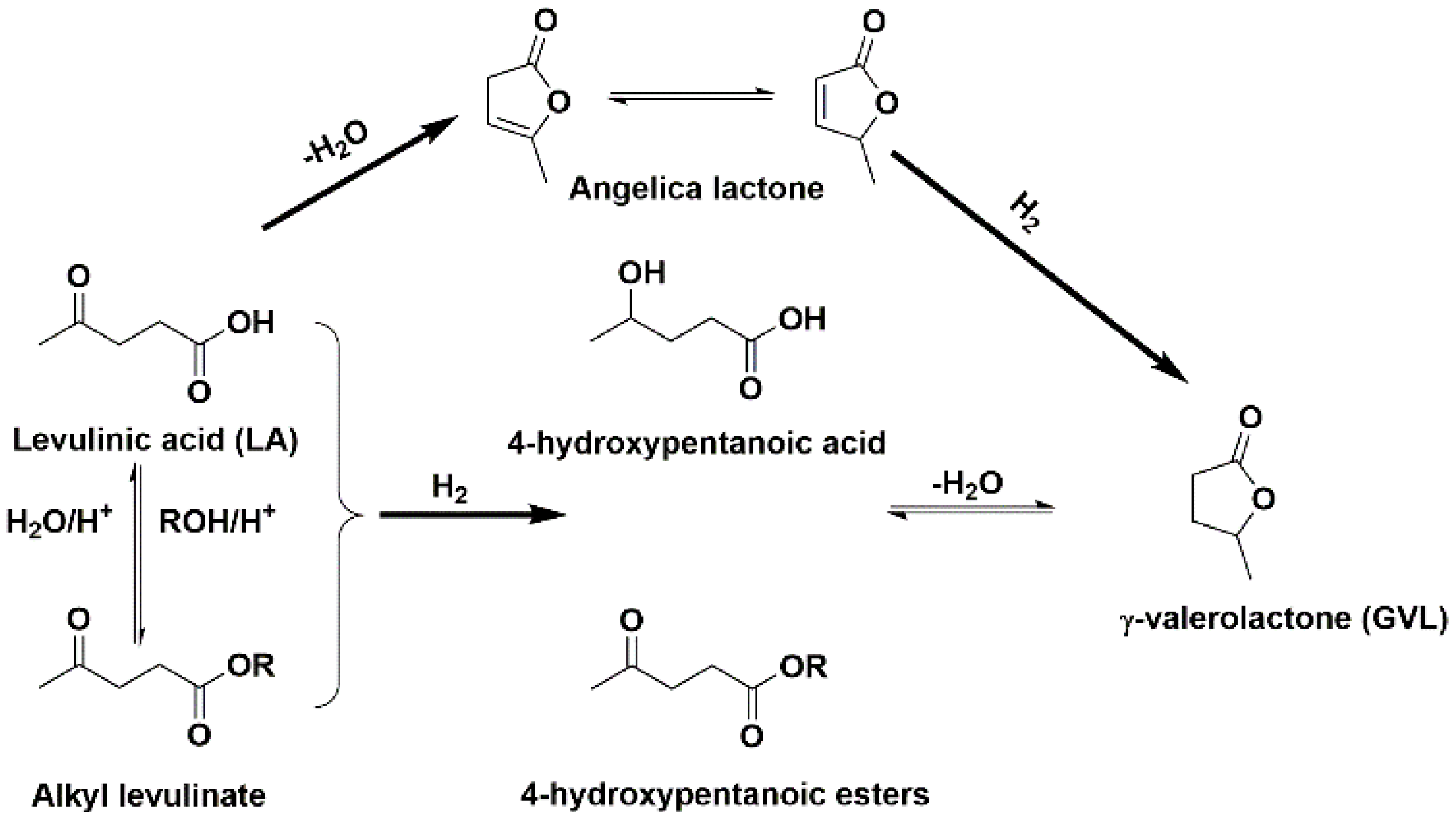
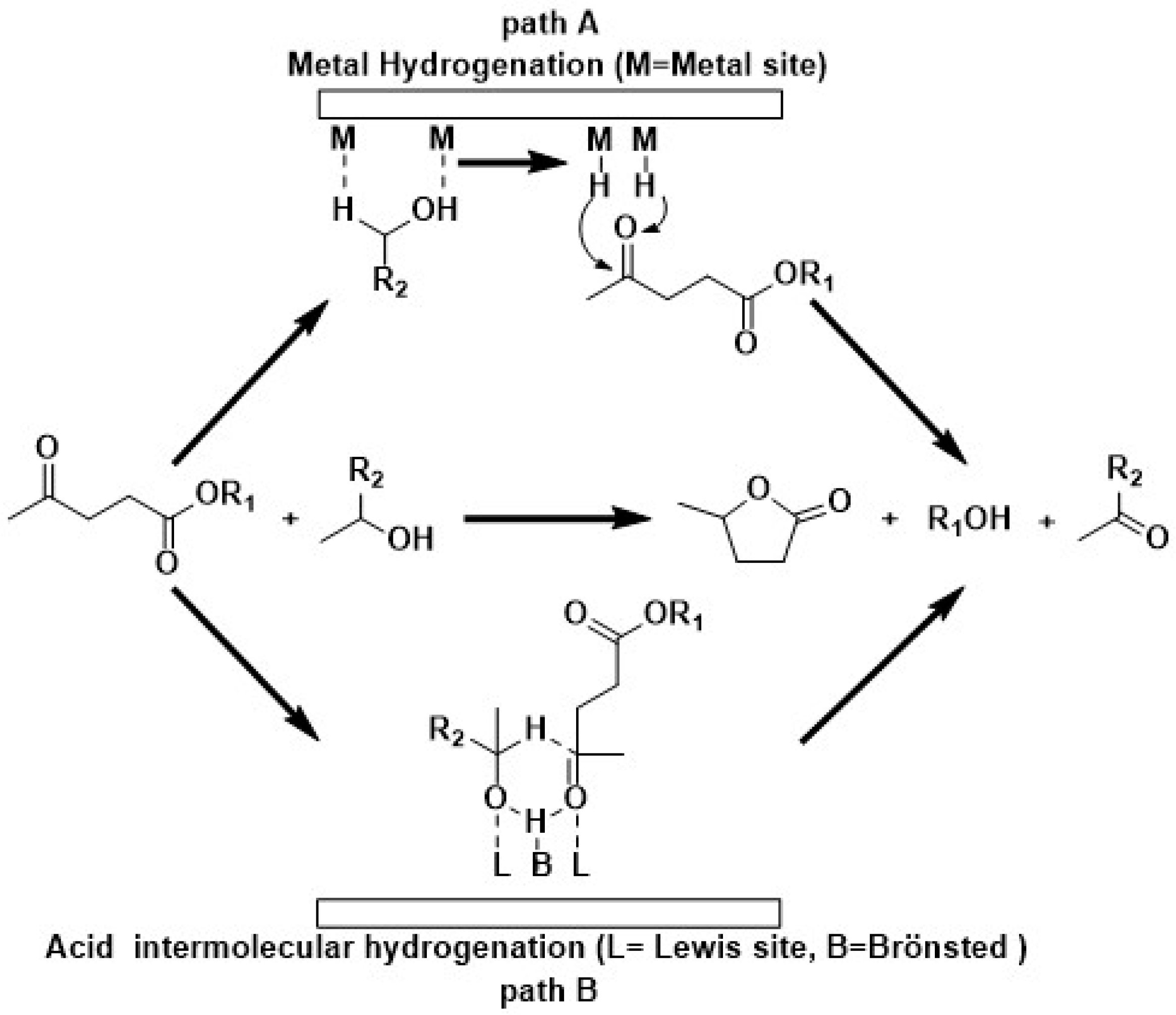
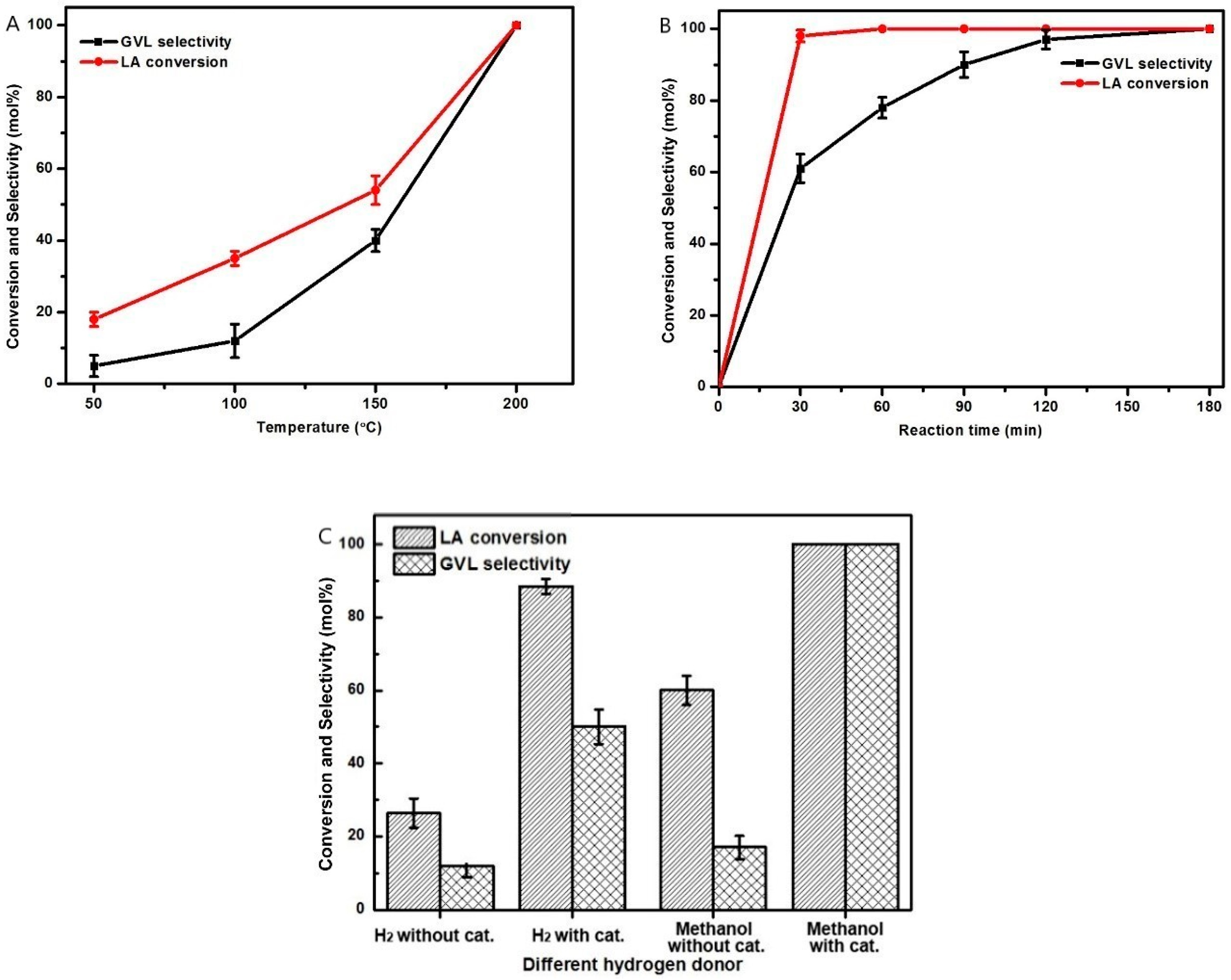
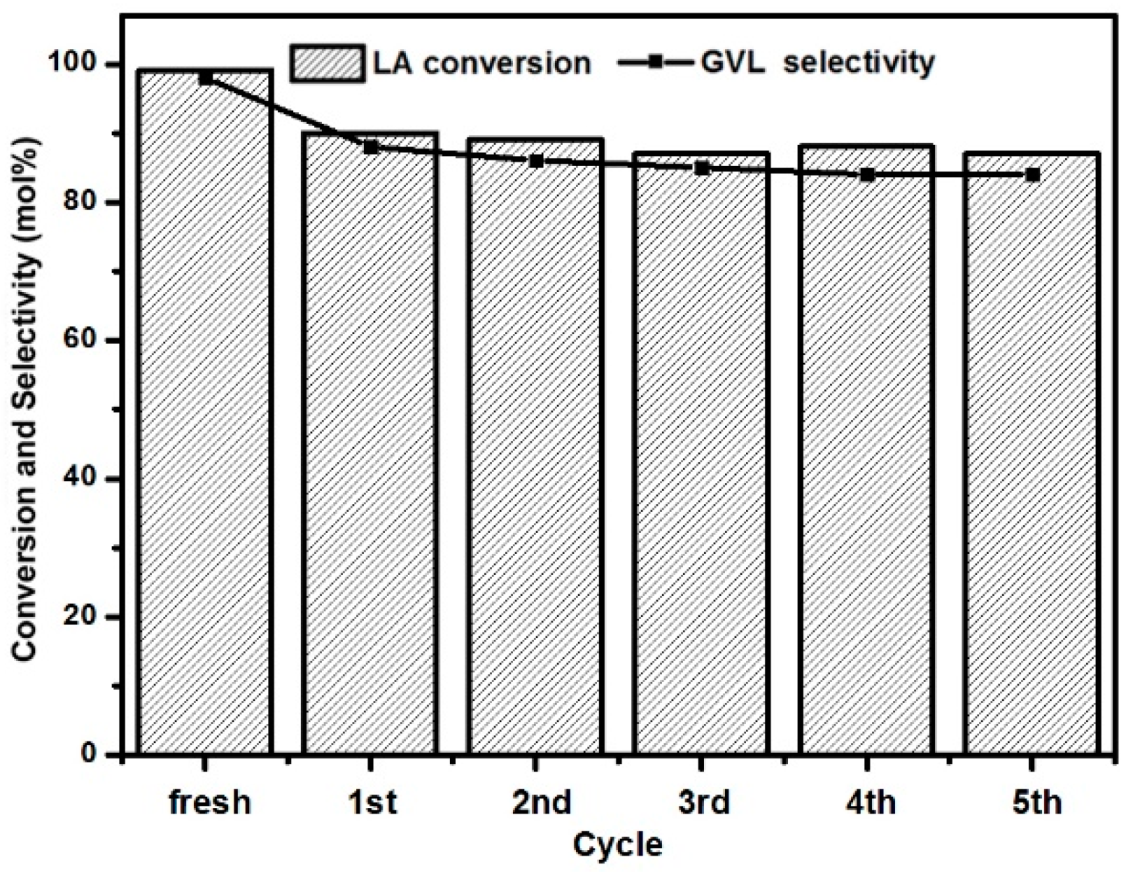
2.2. LA to GVL Catalytic Tests
3. Experimental Section
3.1. Materials
3.2. Preparation of Catalyst
3.3. Characterization of the Catalysts
3.4. Catalytic Tests
3.5. Analysis of the Liquid Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, H.L.Q.; Wang, C.; Xu, Y.; Zhang, Q.; Ma, L. Chapter 3-5-hydroxymethyl furfural-a C6 precursor for fuels and chemicals. In Biomass, Biofuels, Biochemicals; Saravanamurugan, S., Pandey, A., Li, H., Riisager, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–94. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Molinari, V.; Antonietti, M.; Esposito, D. An integrated strategy for the conversion of cellulosic biomass into γ-valerolactone. Catal. Sci. Technol. 2014, 4, 3626–3630. [Google Scholar] [CrossRef]
- Tang, X.; Sun, Y.; Zeng, X.; Lei, T.; Li, H.; Lin, L. γ-Valerolactone—An excellent solvent and a promising building block. In Biomass, Biofuels, Biochemicals; Saravanamurugan, S., Pandey, A., Li, H., Riisager, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–226. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.; Feng, J.; Lu, X. A review about GVL production from lignocellulose: Focusing on the full components utilization. Ind. Crops Prop. 2020, 144, 112031. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Yan, Z.; Lin, L.; Liu, S. Synthesis of γ-valerolactone by hydrogenation of biomass-derived levulinic acid over Ru/C catalyst. Energy Fuel 2009, 23, 3853–3858. [Google Scholar] [CrossRef]
- Manzer, L.E. Catalytic synthesis of α-methylene-γ-valerolactone: A biomass-derived acrylic monomer. Appl. Catal. A Gen. 2004, 272, 249–256. [Google Scholar] [CrossRef]
- Son, P.A.; Nishimura, S.; Ebitani, K. Production of γ-valerolactone from biomass derived compounds using formic acid as a hydrogen source over supported metal catalysts in water solvent. RSC Adv. 2014, 4, 10525. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.; Hwang, D.; Halligudi, S.B.; Hwang, Y.; Chang, J. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, Y.; Li, J.; Fu, Y.; Liao, B.; Guo, Q. Conversion of levulinic acid and formic acid into γ-valerolactone over heterogeneous catalysts. ChemSusChem 2010, 3, 1172–1175. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Bai, H.; Xiong, J.; Feng, W.; Ji, N. Effects of solid acid supports on the bifunctional catalysis of levulinic acid to γ-valerolactone: Catalytic activity and stability. Chem. Asian J. 2020, 15, 1182–1201. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Mohan, V.; Venkateshwarlu, V.; Pramod, C.V.; Raju, B.D.; Rao, K.S.R. Vapour phase hydrocyclisation of levulinic acid to γ-valerolactone over supported Ni catalysts. Catal. Sci. Technol. 2014, 4, 1253–1259. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; De Luise, V.; Martinelli, M. A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-derived levulinic acid. Green Chem. 2012, 14, 688–694. [Google Scholar] [CrossRef]
- Shimizu, K.; Kanno, S.; Kon, K. Hydrogenation of levulinic acid to γ-valerolactone by Ni and MoOx co-loaded carbon catalysts. Green Chem. 2014, 16, 3899–3903. [Google Scholar] [CrossRef]
- Obregón, I.; Corro, E.; Izquierdo, U.; Requies, J.; Arias, P.L. Levulinic acid hydrogenolysis on Al2O3-based Ni-Cu bimetallic catalysts. Chin. J. Catal. 2014, 35, 656–662. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, Y.; Xing, W.; Liu, C.; Liao, J.; Lu, T. High-quality hydrogen from the catalyzed decomposition of formic acid by Pd- Au/C and Pd-Ag/C. Chem. Commun. 2008, 3540–3542. [Google Scholar] [CrossRef] [PubMed]
- Fellay, C.; Yan, N.; Dyson, P.J.; Laurenczy, G. Selective formic acid decomposition for high-pressure hydrogen generation: A mechanistic study. Chem. Eur. J. 2009, 15, 3752–3760. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Sneka-Płatek, O.; Jędrzejczyk, M.; Keller, N.; Dumon, A.S.; Michel, C.; Sautet, P.; Grams, J. Ru catalysts for levulinic acid hydrogenation with formic acid as a hydrogen source. Green Chem. 2016, 18, 2014–2028. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Bai, H.; Xiong, J.; Feng, W.; Ji, N. Heterogeneous catalytic hydrogenation of levulinic acid to γ-valerolactone with formic acid as internal hydrogen source: Key issues and their effects. Chemsuschem 2020, 13, 2916–2930. [Google Scholar] [CrossRef]
- Chia, M.; Dumesic, J.A. Liquid-phase catalytic transfer hydrogenation and cyclization of levulinic acid and its esters to gamma-valerolactone over metal oxide catalysts. Chem. Commun. 2011, 47, 12233–12235. [Google Scholar] [CrossRef] [PubMed]
- Gilkey, M.J.; Xu, B. Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Luo, H.Y.; Consoli, D.F.; Gunther, W.R.; Román-Leshkov, Y. Investigation of the reaction kinetics of isolated Lewis acid sites in Beta zeolites for the Meerwein-Ponndorf-Verley reduction of methyl levulinate to γ-valerolactone. J. Catal. 2014, 320, 198–207. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, X.; Li, Z.; Li, W.; Jiang, Y.; Hu, L.; Liu, S.; Sun, Y.; Lin, L. In Situ generated catalyst system to convert biomass-derived levulinic acid to γ-Valerolactone. Chemcatchem 2015, 7, 1372–1379. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, H.; Xiao, L.; Wang, B.; Song, G. Highly efficient hydrogenation of levulinic acid into γ-valerolactone using an iron pincer complex. Chemsuschem 2018, 9, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, J.; Yan, G.; Liu, H.; Tang, X.; Sun, Y.; Zeng, X.; Lei, T.; Lin, L. Cascade conversion of furfural to fuel bioadditive ethyl levulinate over bifunctional zirconium-based catalysts. Renew. Energy 2020, 147, 916–923. [Google Scholar] [CrossRef]
- Norhasyimi, R.; Ahmad, Z.A.; Abdullah, R.M. A Review: Mesoporous santa barbara amorphous-15, types, synthesis and its applications towards biorefinery production. Am. J. Appl. Sci. 2010, 7, 1579–1586. [Google Scholar] [CrossRef]
- Oyama, S.T.; Zhao, H.; Freund, H.J.; Asakura, K.; Włodarzyk, R.; Sierka, M. Unprecedented selectivity to the direct desulfurization (DDS) pathway in a highly active FeNi bimetallic phosphide catalyst. J. Catal. 2012, 285, 1–5. [Google Scholar] [CrossRef]
- Jie, F.; Dong, S.; Lu, X. Hydrogenation of levulinic acid over nickel catalysts supported on aluminum oxide to prepare γ-valerolactone. Catalysts 2016, 6, 6. [Google Scholar] [CrossRef]
- Hsu, P.; Jiang, J.; Lin, Y. Does a strong oxophilic promoter enhance direct deoxygenation? A study of NiFe, NiMo, and NiW catalysts in p-Cresol conversion. ACS Sustain. Chem. Eng. 2018, 6, 660–667. [Google Scholar] [CrossRef]
- Ren, J.; Qin, X.; Yang, J.; Qin, Z.; Guo, H.; Lin, J.; Li, Z. Methanation of carbon dioxide over Ni-M/ZrO2 (M=Fe, Co, Cu) catalysts: Effect of addition of a second metal. Fuel Process. Technol. 2015, 137, 204–211. [Google Scholar] [CrossRef]
- Daniel, T.; José, L.P.; Isabel, S. Co-, Cu- and Fe-doped Ni/Al2O3 catalysts for the catalytic decomposition of methane into hydrogen and carbon nanofibers. Catalysts 2018, 8, 300. [Google Scholar] [CrossRef]
- Nie, L.; Souza, P.M.D.; Noronha, F.B.; An, W.; Sooknoi, T.; Resasco, D.E. Selective conversion of m-cresol to toluene over bimetallic Ni-Fe catalysts. J. Mol. Catal. A Chem. 2014, 388–389, 47–55. [Google Scholar] [CrossRef]
- Kumar, V.V.; Naresh, G.; Sudhakar, M.; Tardio, J.; Bhargava, S.K.; Venugopal, A. Role of brönsted and lewis acid sites on Ni/TiO2 catalyst for vapour phase hydrogenation of levulinic acid: Kinetic and mechanistic study. Appl. Catal. A Gen. 2015, 505, 217–223. [Google Scholar] [CrossRef]
- Kordulis, C.; Bourikas, K.; Gousi, M. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: A critical review. Appl. Catal. B Environ. 2016, 181, 156–196. [Google Scholar] [CrossRef]
- Ali, A.; Li, B.; Lu, Y.; Zhao, C. Highly selective and low-temperature hydrothermal conversion of natural oils to fatty alcohols. Green Chem. 2019, 21, 3059–3064. [Google Scholar] [CrossRef]
- Nadgeri, J.M.; Hiyoshi, N.; Yamaguchi, A.; Sato, O.; Shirai, M. Liquid phase hydrogenation of methyl levulinate over the mixture of supported ruthenium catalyst and zeolite in water. Appl. Catal. A Gen. 2014, 470, 215–220. [Google Scholar] [CrossRef]
- Xiao, C.; Goh, T.W.; Qil, Z.; Goes, S.; Brashler, K.; Perez, C.; Huang, W. Conversion of levulinic acid to γ-valerolactone over few-layer graphene-supported ruthenium catalysts. ACS Catal. 2016, 6, 593–599. [Google Scholar] [CrossRef]
- Piskun, A.; Winkelman, J.G.M.; Tang, Z.; Heeres, H.J. Support screening studies on the hydrogenation of levulinic acid to γ-valerolactone in water using Ru catalysts. Catalysts 2016, 6, 131. [Google Scholar] [CrossRef]
- Luo, W.; Deka, U.; Beale, A.M.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Ruthenium-catalyzed hydrogenation of levulinic acid: Influence of the support and solvent on catalyst selectivity and stability. J. Catal. 2013, 301, 175–186. [Google Scholar] [CrossRef]


| Catalyst | Ni (mmol/g) | Fe (mmol/g) | H2 (μmol/g) a | Particle Dispersion (%) b | Total Acidity (μmol/g) c |
|---|---|---|---|---|---|
| Ni/SBA-15 | 0.85 | 0 | 6.3 | 1.45 | 28.3 |
| 5Ni-1Fe/SBA-15 | 0.85 | 0.17 | 17.1 | 3.34 | 30.6 |
| 5Ni-3Fe/SBA-15 | 0.85 | 0.50 | 23.6 | 3.49 | 65.2 |
| 5Ni-5Fe/SBA-15 | 0.85 | 0.86 | 23.8 | 2.76 | 61.5 |
| Fe/SBA-15 | 0 | 0.86 | 14.8 | - | 78.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Han, X.; Zeng, Q. Hydrogenative Cyclization of Levulinic Acid to γ-Valerolactone with Methanol and Ni-Fe Bimetallic Catalysts. Catalysts 2020, 10, 1096. https://doi.org/10.3390/catal10091096
Luo L, Han X, Zeng Q. Hydrogenative Cyclization of Levulinic Acid to γ-Valerolactone with Methanol and Ni-Fe Bimetallic Catalysts. Catalysts. 2020; 10(9):1096. https://doi.org/10.3390/catal10091096
Chicago/Turabian StyleLuo, Ligang, Xiao Han, and Qin Zeng. 2020. "Hydrogenative Cyclization of Levulinic Acid to γ-Valerolactone with Methanol and Ni-Fe Bimetallic Catalysts" Catalysts 10, no. 9: 1096. https://doi.org/10.3390/catal10091096
APA StyleLuo, L., Han, X., & Zeng, Q. (2020). Hydrogenative Cyclization of Levulinic Acid to γ-Valerolactone with Methanol and Ni-Fe Bimetallic Catalysts. Catalysts, 10(9), 1096. https://doi.org/10.3390/catal10091096