Titanium-Modified MIL-101(Cr) Derived Titanium-Chromium-Oxide as Highly Efficient Oxidative Desulfurization Catalyst
Abstract
1. Introduction
2. Results and Discussion
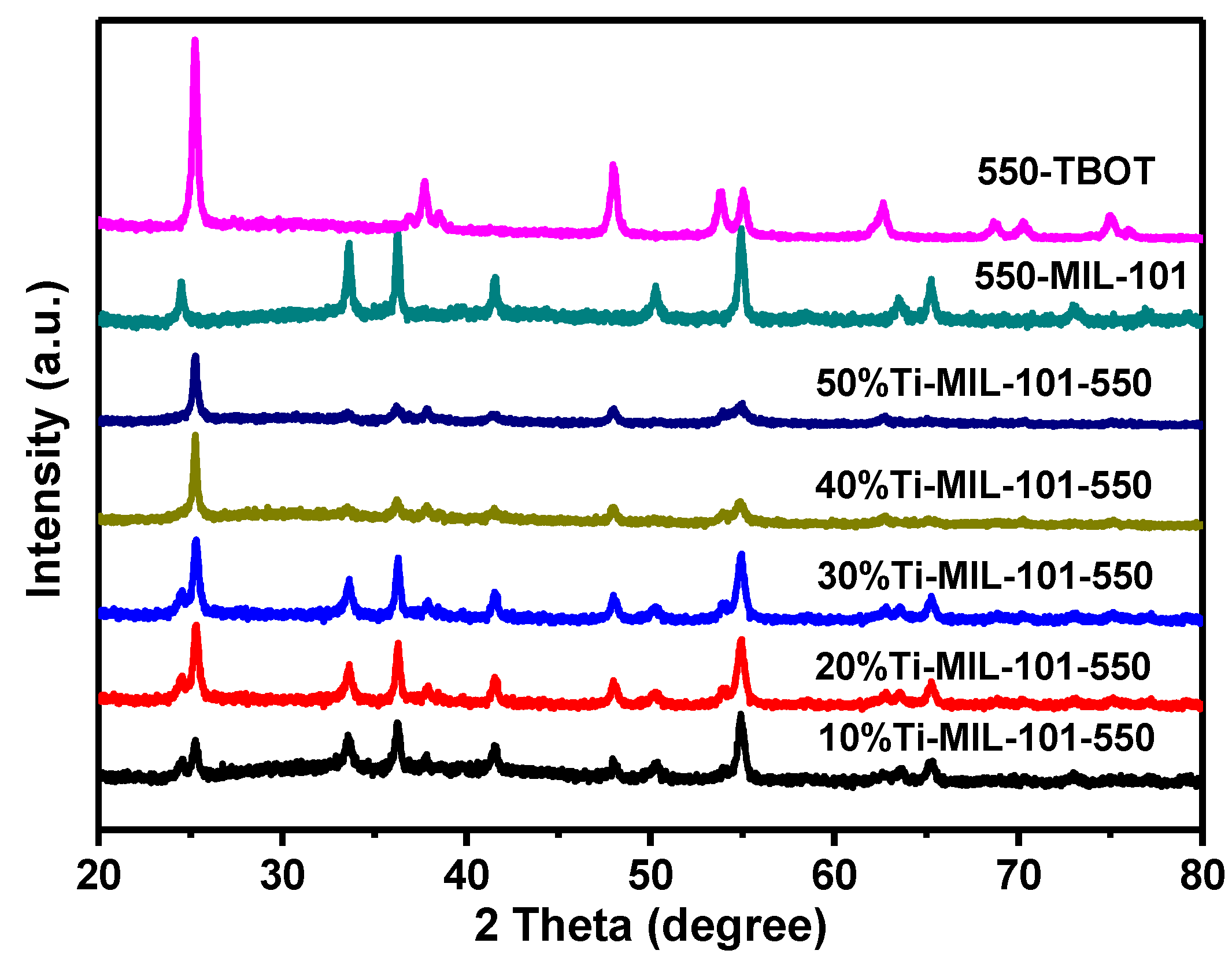
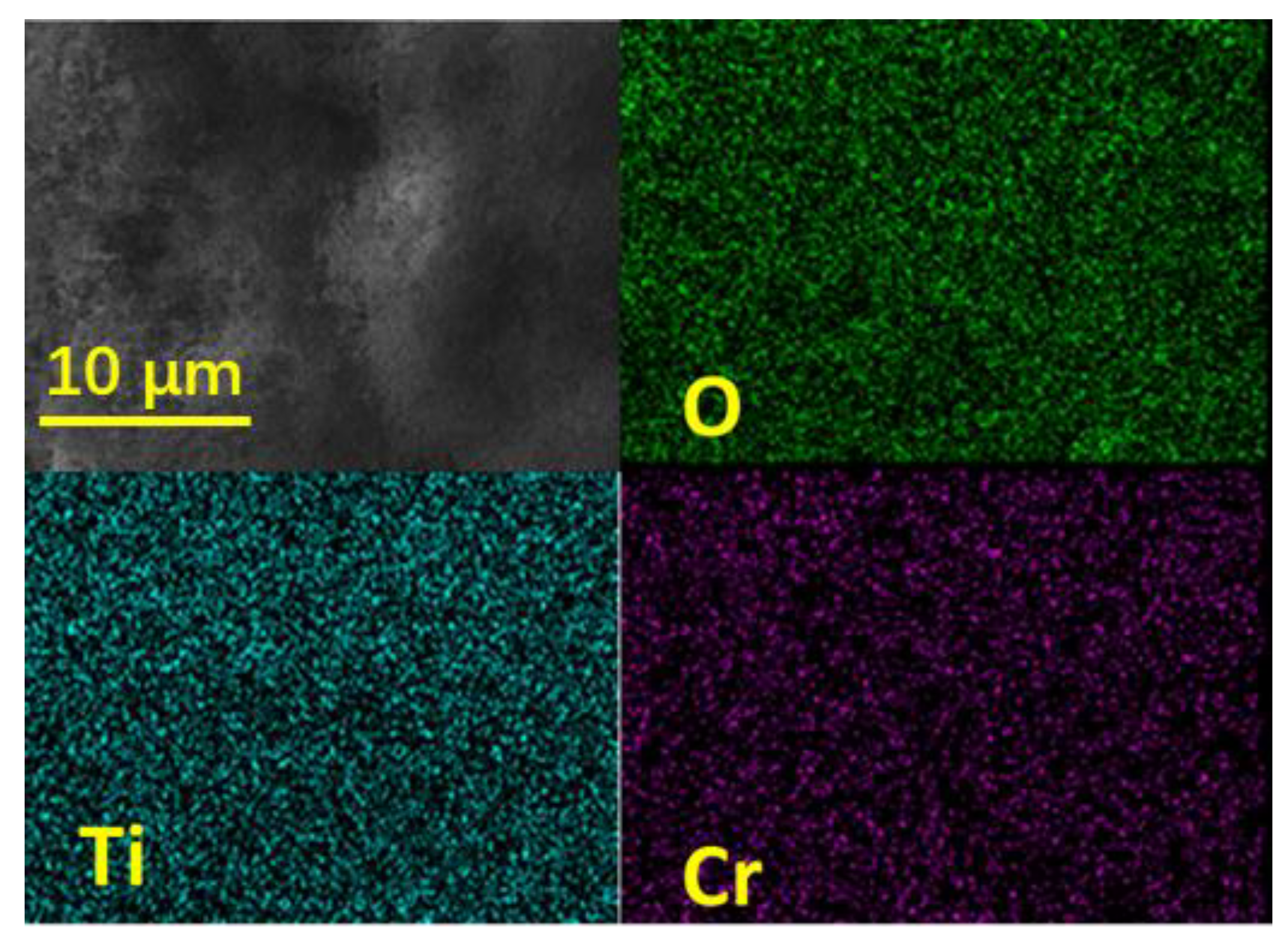
2.1. Characterization of Catalyst
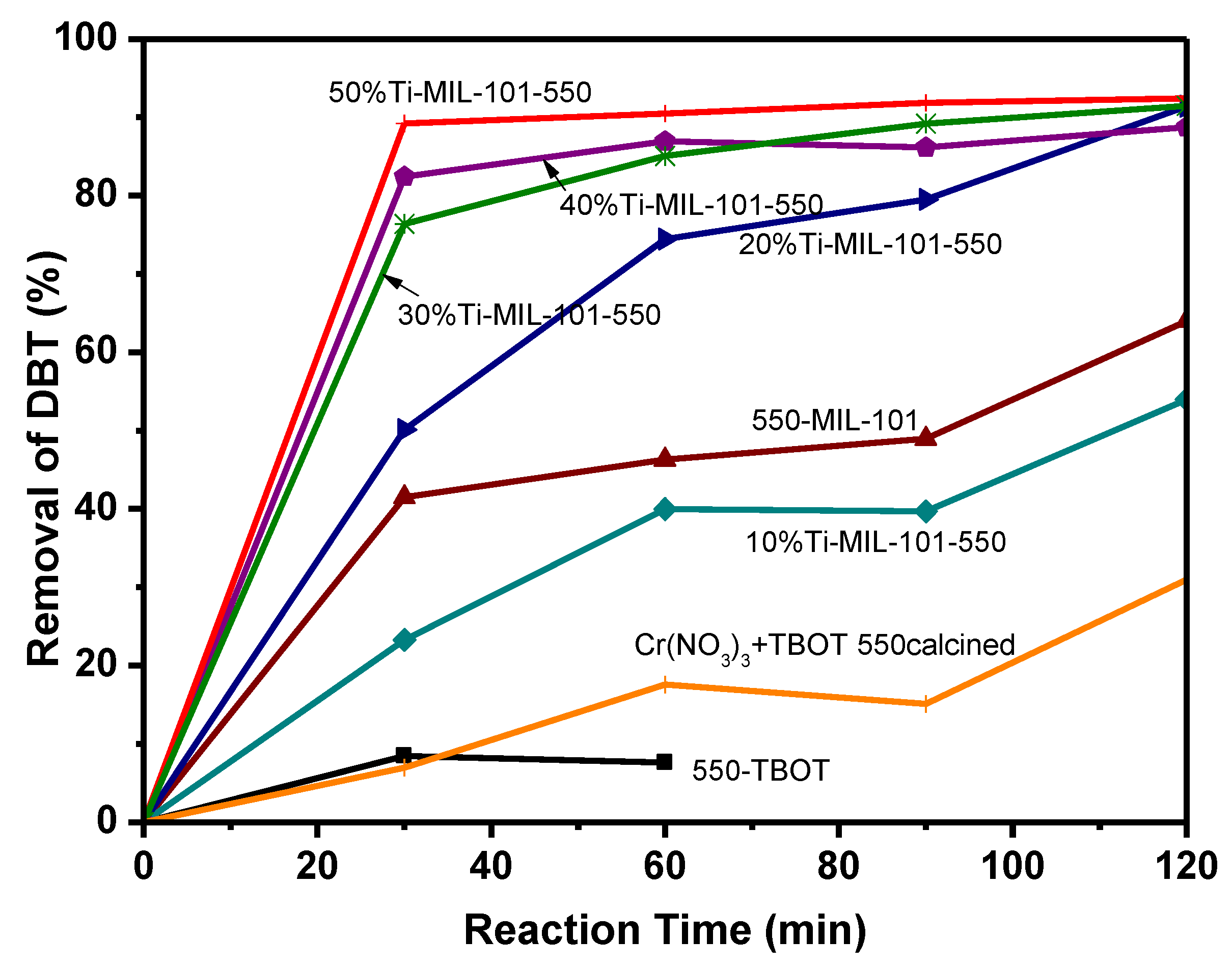
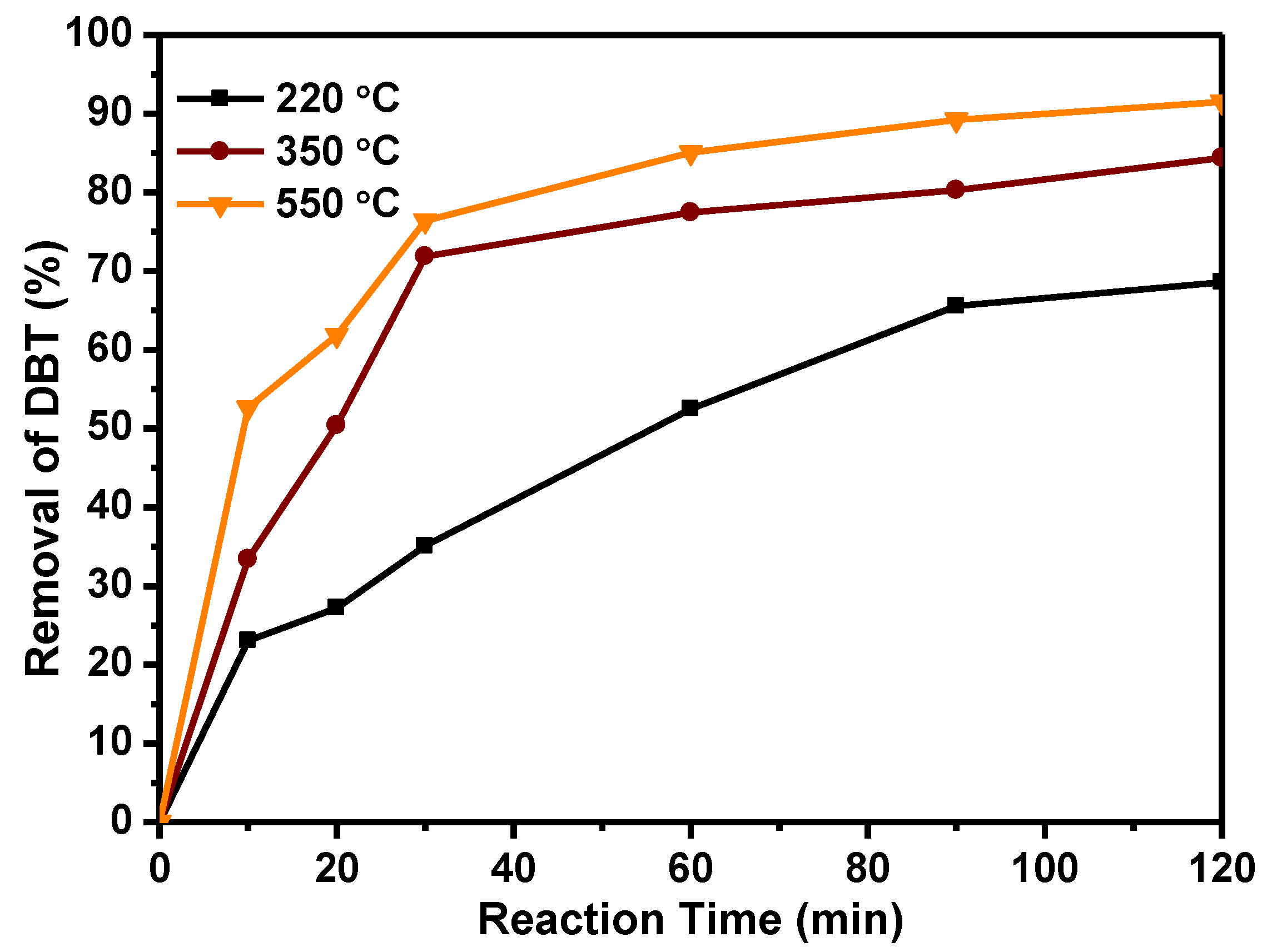
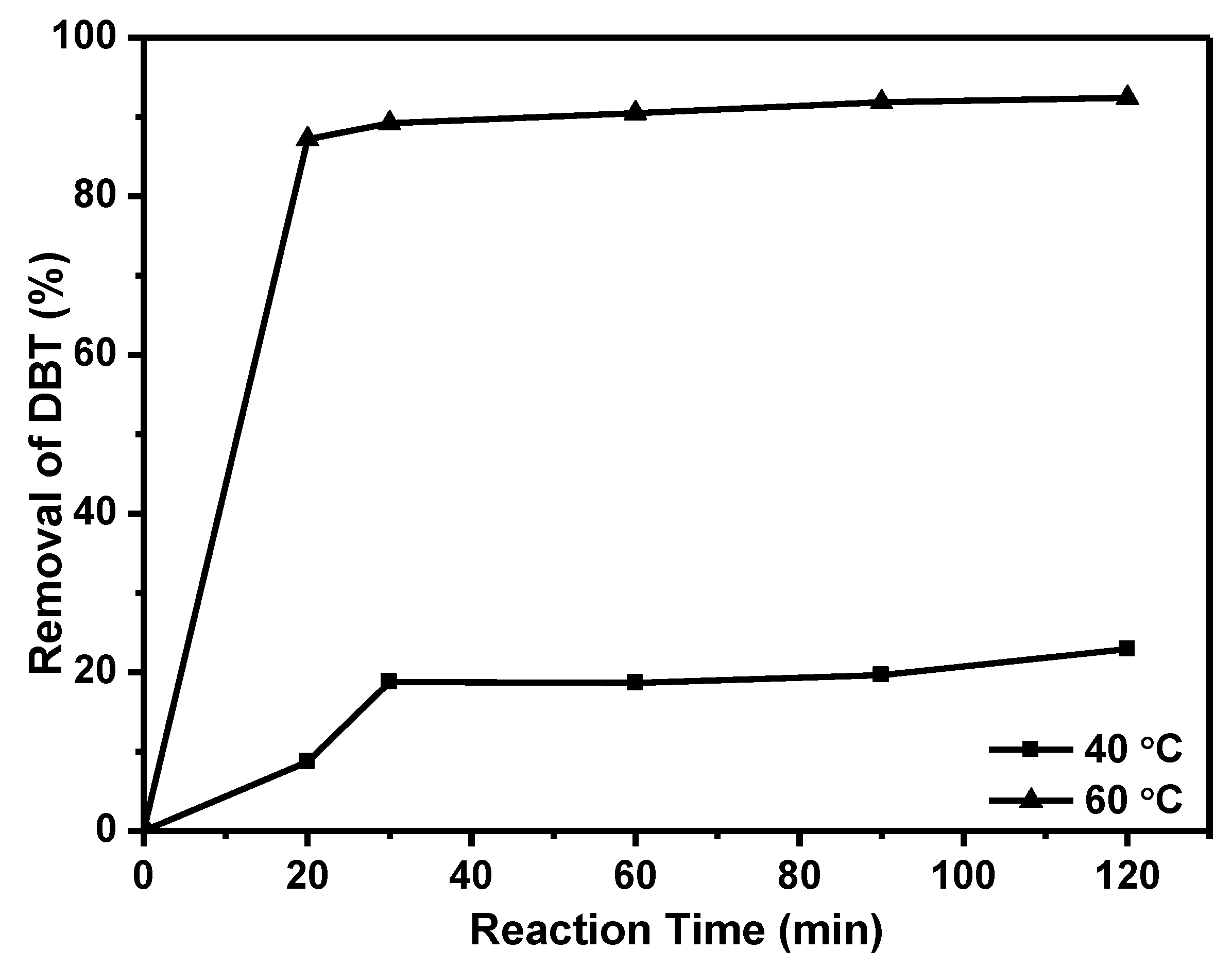
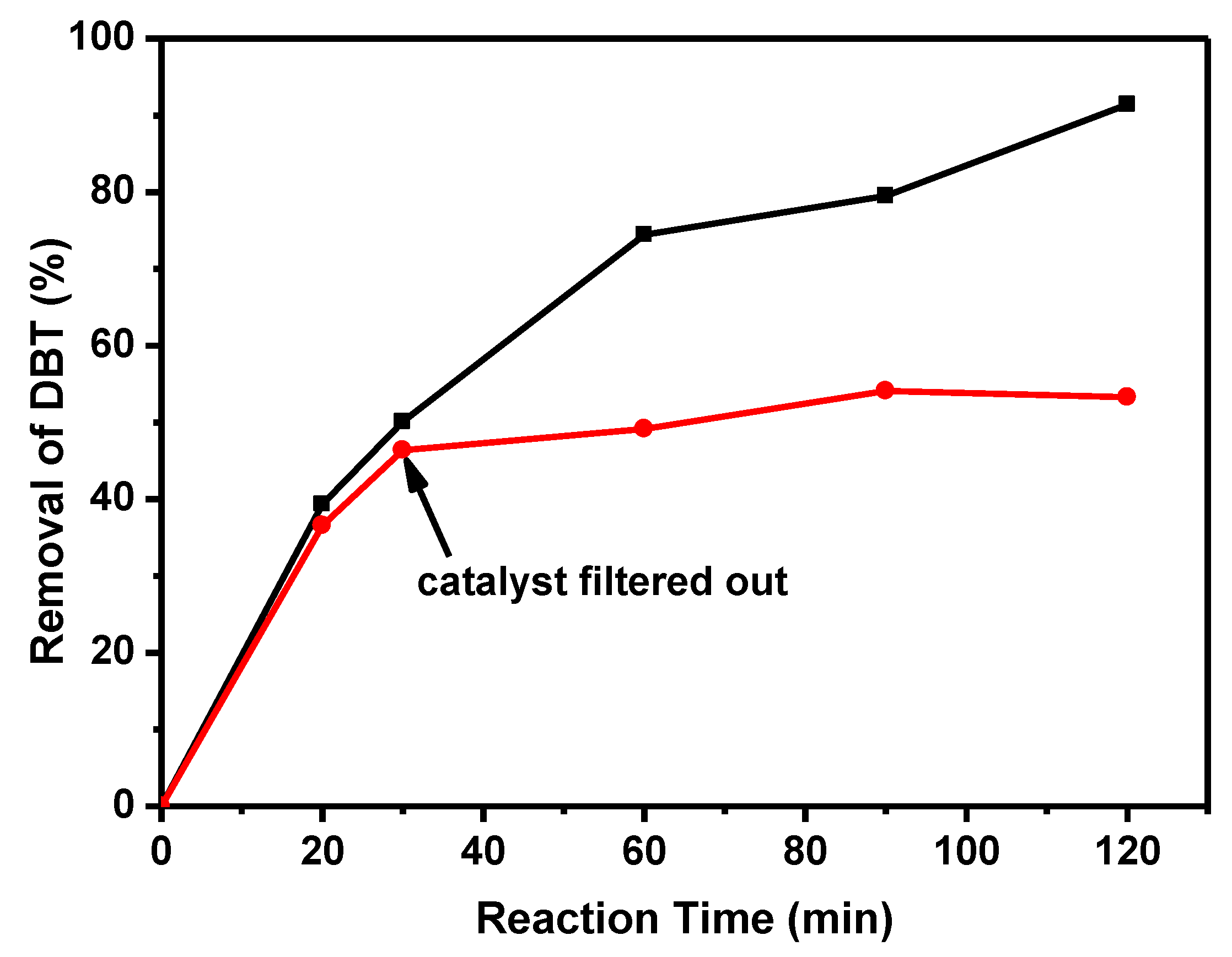
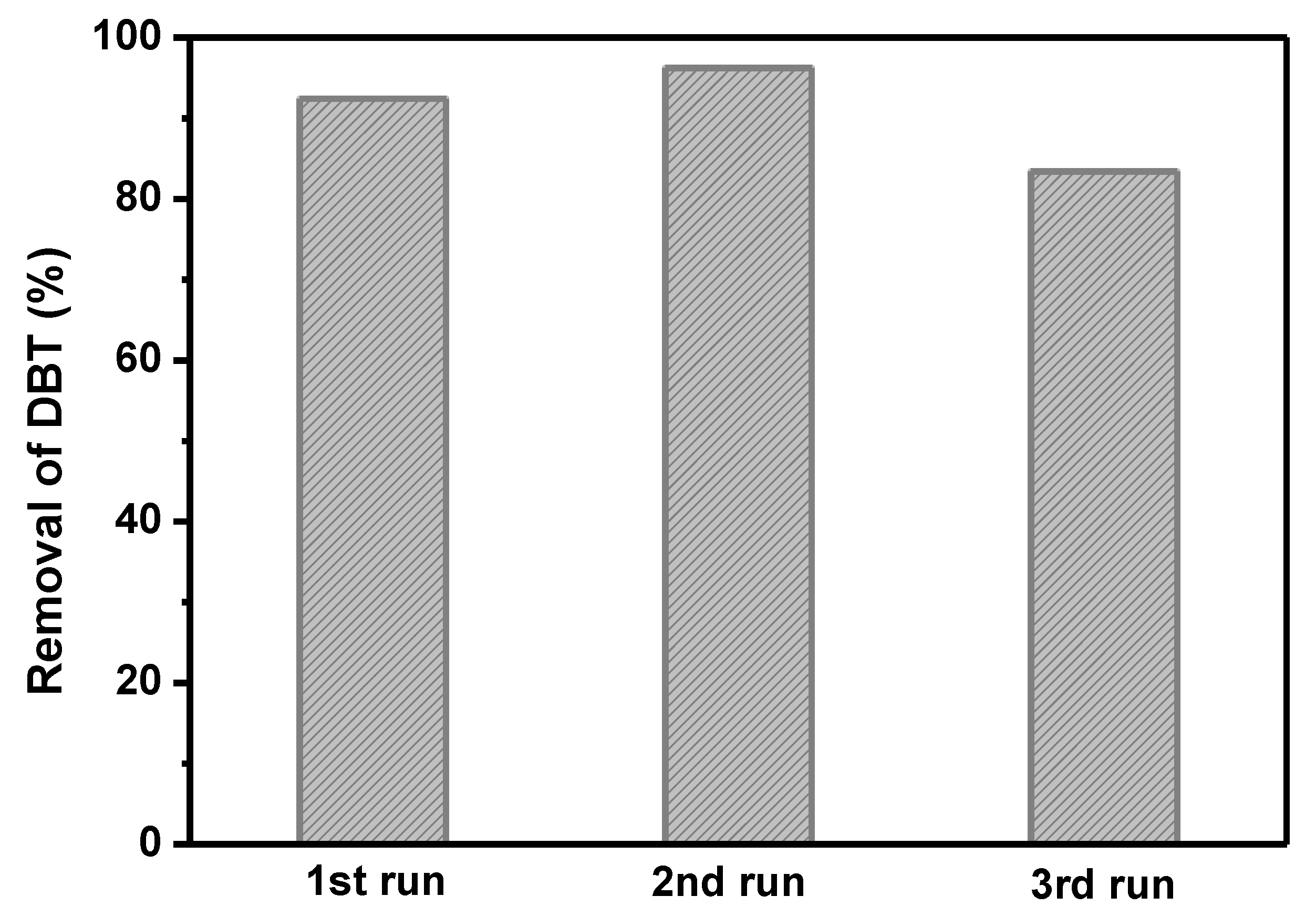
2.2. Catalytic Activity of Catalyst
3. Experimental
3.1. Materials
3.2. Preparation of Catalyst
3.3. Catalyst Characterization
3.4. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Campos-Martin, J.M.; Capel-Sanchez, M.; Perez-Presas, P.; Fierro, J.L.G. Oxidative processes of desulfurization of liquid fuels. J. Chem. Technol. 2010, 85, 879–890. [Google Scholar] [CrossRef]
- Chandra Srivastava, V. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Ismagilov, Z.; Yashnik, S.; Kerzhentsev, M.; Parmon, V. Oxidative desulfurization of hydrocarbon fuels. Catal. Rev. 2011, 53, 199–255. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Capel-Sanchez, M.C.; Fierro, J.L.G. Highly efficient deep desulfurization of fuels by chemical oxidation. Green Chem. 2004, 6, 557–562. [Google Scholar] [CrossRef]
- Gómez-Paricio, A.; Santiago-Portillo, A.; Navalón, S.; Concepción, P.; Alvaroa, M.; Garcia, H. MIL-101 promotes the efficient aerobic oxidative desulfurization of dibenzothiophenes. Green Chem. 2016, 18, 508–515. [Google Scholar] [CrossRef]
- Bakar, W.A.W.A.; Ali, R.; Kadir, A.A.A.; Mokhtar, W.N.A.W. Effect of transition metal oxides catalysts on oxidative desulfurization of model diesel. Fuel Process. Technol. 2012, 101, 78–84. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.; Fan, H.; Yang, Z.; Feng, J.; Li, W. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Fraile, J.M.; Gila, C.; Mayoral, J.A.; Muel, B.; Roldána, L.; Vispe, E.; Calderón, S.; Puente, F. Heterogeneous titanium catalysts for oxidation of dibenzothiophene in hydrocarbon solutions with hydrogen peroxide: On the road to oxidative desulfurization. Appl. Catal. B Environ. 2016, 180, 680–686. [Google Scholar] [CrossRef]
- Chica, A.; Corma, A.; Dómine, M.E. Catalytic oxidative desulfurization (ODS) of diesel fuel on a continuous fixed-bed reactor. J. Catal. 2006, 242, 299–308. [Google Scholar] [CrossRef]
- Ramos-Luna, M.A.; Cedeno-Caero, L. Effect of sulfates and reduced-vanadium species on oxidative desulfurization (ODS) with V2O5/TiO2 catalysts. Ind. Eng. Chem. Res. 2010, 50, 2641–2649. [Google Scholar] [CrossRef]
- Zheng, D.; Zhu, W.; Xun, S.; Zhou, M.; Zhang, M.; Jiang, W.; Qin, Y.; Li, H. Deep oxidative desulfurization of dibenzothiophene using low-temperature-mediated titanium dioxide catalyst in ionic liquids. Fuel 2015, 159, 446–453. [Google Scholar] [CrossRef]
- Bazyari, A.; Khodadadi, A.A.; Mamaghani, A.H.; Beheshtian, J.; Thompson, L.T.; Mortazavi, Y. Microporous titania-silica nanocomposite catalyst-adsorbent for ultra-deep oxidative desulfurization. Appl. Catal. B Environ. 2016, 180, 65–77. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Shen, C.; Wang, Y.; Luo, G. Oxidative desulfurization of model fuels with pure nano-TiO2 as catalyst directly without UV irradiation. Fuel 2016, 167, 9–16. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Y.; Xu, J.; Luo, G. Oxidative desulfurization of DBT with H2O2 catalysed by TiO2/porous glass. Green Chem. 2016, 18, 771–781. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.T. Gas adsorption and storage in metal-organic framework MOF-177. Langmuir 2007, 23, 12937–12944. [Google Scholar]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF positioning technology and device fabrication. Chem. Soc. Rev. 2014, 43, 5513–5560. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.F.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Tang, J.; Kamachi, J.; Nakato, T.; Kim, J.H.; Yamauchi, Y. Asymmetric supercapacitors using 3D nanoporous carbon and cobalt oxide electrodes synthesized from a single metal-organic framework. ACS Nano 2015, 9, 6288–6296. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Tang, J.; Salunkhe, R.R.; Jiang, X.; Yu, A.; Wu, K.C.-W.; Yamauchi, Y. Nanoarchitectured design of porous materials and nanocomposites from metal-organic frameworks. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. Oxidative desulfurization and denitrogenation of fuels using metal-organic framework-based/-derived catalysts. Appl. Catal. B Environ. 2019, 259, 118021. [Google Scholar] [CrossRef]
- Kim, J.; McNamara, N.D.; Her, T.H.; Hicks, J.C. Carbothermal reduction of Ti-modified IRMOF-3: An adaptable synthetic method to support catalytic nanoparticles on carbon. ACS Appl. Mater. Interfaces 2013, 5, 479–11487. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Song, J.Y.; Khan, N.A.; Jhung, S.H. TiO2-containing carbon derived from a metal-organic framework composite: A highly active catalyst for oxidative desulfurization. ACS Appl. Mater. Interfaces 2017, 9, 31192–31202. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- McNamara, N.D.; Neumann, G.T.; Masko, E.T.; Urban, J.A.; Hicks, J.C. Catalytic performance and stability of (V) MIL-47 and (Ti) MIL-125 in the oxidative desulfurization of heterocyclic aromatic sulfur compounds. J. Catal. 2013, 305, 217–226. [Google Scholar] [CrossRef]
- McNamara, N.D.; Hicks, J.C. Chelating Agent-Free, Vapor-Assisted Crystallization Method to Synthesize Hierarchical Microporous/Mesoporous MIL-125 (Ti). ACS Appl. Mater. Interfaces 2017, 7, 5338–5346. [Google Scholar] [CrossRef]
- Bromberg, L.; Diao, Y.; Wu, H.; Speakman, S.A.; Hatton, T.A. Chromium(III) Terephthalate Metal Organic Framework (MIL-101): HF-Free Synthesis, Structure, Polyoxometalate Composites, and Catalytic Properties. Chem. Mater. 2012, 24, 1664–1675. [Google Scholar] [CrossRef]

| x%Ti-MIL-101-550 | 10 | 20 | 30 | 40 | 50 |
|---|---|---|---|---|---|
| D/nm (Cr2O3) | 24.5 | 20.5 | 18.9 | 11.5 | 15.0 |
| D/nm (TiO2) | 23.8 | 22.9 | 20.3 | 14.4 | 28.9 |
| Material | SBET (m2/g) | Mesopore Volume (mL/g) | Pore Size (nm) |
|---|---|---|---|
| 10%Ti-MIL-101-Cr-550 | 62 | 0.47 | 24 |
| 20%Ti-MIL-101-Cr-550 | 52 | 0.44 | 21 |
| 30%Ti-MIL-101-Cr-550 | 49 | 0.48 | 19 |
| 40%Ti-MIL-101-Cr-550 | 51 | 0.33 | 17 |
| 50%Ti-MIL-101-Cr-550 | 44 | 0.26 | 17 |
| 550-MIL-101-Cr | 52 | 0.33 | 20 |
| Catalyst | SBET (m2/g) | Initial S Content (mmol) | Reaction Temp. (°C) | Reaction Time (min) | Catalyst Amount (g) | Conversion (%) | Specific Activity (μmol S/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| MIL-125(Ti) | 935 | 3.5 | 60 | 30 | 0.017 | 5 a | 11.0 | [26] |
| Meso-MIL-125(Ti) | 975 | 0.35 | 80 | 30 | 0.017 | 47 a | 9.92 | [27] |
| Ti/NC | 1060 | 3.5 | 100 | 30 | 0.04 | 22 a | 18.2 | [23] |
| MDC-C | 374 | 0.22 | 80 | 30 | 0.02 | 90 | 26.5 | [24] |
| TiO2/porous glass | 116.9 | 0.03 | 60 | 8 | 1 | 100 | 0.26 | [14] |
| Anatase TiO2 | 24.5 | 0.027 | 70 | 30 | 0.3 | 80 | 2.94 | [13] |
| TS-50 | 300 | 0.31 | 80 | 30 | 0.2 | 100 | 5.17 | [12] |
| 10%Ti-MIL-101-550 | 62 | 0.63 | 60 | 30 | 0.1 | 23 | 23 | This work |
| 20%Ti-MIL-101-550 | 52 | 0.63 | 60 | 30 | 0.1 | 50 | 61 | This work |
| 30%Ti-MIL-101-550 | 49 | 0.63 | 60 | 30 | 0.1 | 76 | 98 | This work |
| 40%Ti-MIL-101-550 | 51 | 0.63 | 60 | 30 | 0.1 | 82 | 101 | This work |
| 50%Ti-MIL-101-550 | 44 | 0.63 | 60 | 30 | 0.1 | 90 | 129 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, L.; Sun, Y. Titanium-Modified MIL-101(Cr) Derived Titanium-Chromium-Oxide as Highly Efficient Oxidative Desulfurization Catalyst. Catalysts 2020, 10, 1091. https://doi.org/10.3390/catal10091091
Li X, Zhang L, Sun Y. Titanium-Modified MIL-101(Cr) Derived Titanium-Chromium-Oxide as Highly Efficient Oxidative Desulfurization Catalyst. Catalysts. 2020; 10(9):1091. https://doi.org/10.3390/catal10091091
Chicago/Turabian StyleLi, Xiaolin, Liang Zhang, and Yinyong Sun. 2020. "Titanium-Modified MIL-101(Cr) Derived Titanium-Chromium-Oxide as Highly Efficient Oxidative Desulfurization Catalyst" Catalysts 10, no. 9: 1091. https://doi.org/10.3390/catal10091091
APA StyleLi, X., Zhang, L., & Sun, Y. (2020). Titanium-Modified MIL-101(Cr) Derived Titanium-Chromium-Oxide as Highly Efficient Oxidative Desulfurization Catalyst. Catalysts, 10(9), 1091. https://doi.org/10.3390/catal10091091






