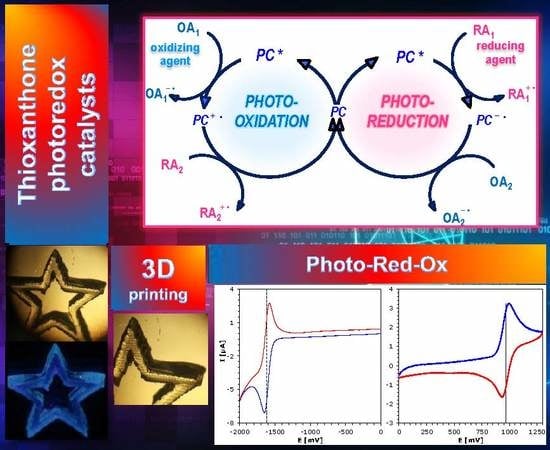
Thioxanthone Derivatives as a New Class of Organic Photocatalysts for Photopolymerisation Processes and the 3D Printing of Photocurable Resins under Visible Light
Abstract
1. Introduction
- (i)
- Photosensitizers in bimolecular photoinitiating systems for cationic polymerisation;
- (ii)
- Photosensitizers in bimolecular photoinitiating systems for free-radical polymerisation;
- (iii)
- One-component free-radical photoinitiators for polymerisation of acrylate monomer;
- (iv)
- Type II photoinitiators for polymerisation of acrylate monomer.
2. Results and Discussion
2.1. Light Absorption Properties of 2,4-Diethyl-thioxanthen-9-one Derivatives
2.2. Performance of 2,4-Diethyl-thioxanthen-9-one Derivatives as Photosensitizers in Bimolecular Photoinitiating Systems for Cationic Photopolymerisation
2.3. Free-Radical Photopolymerisation of Acrylates in Thin Film Laminate
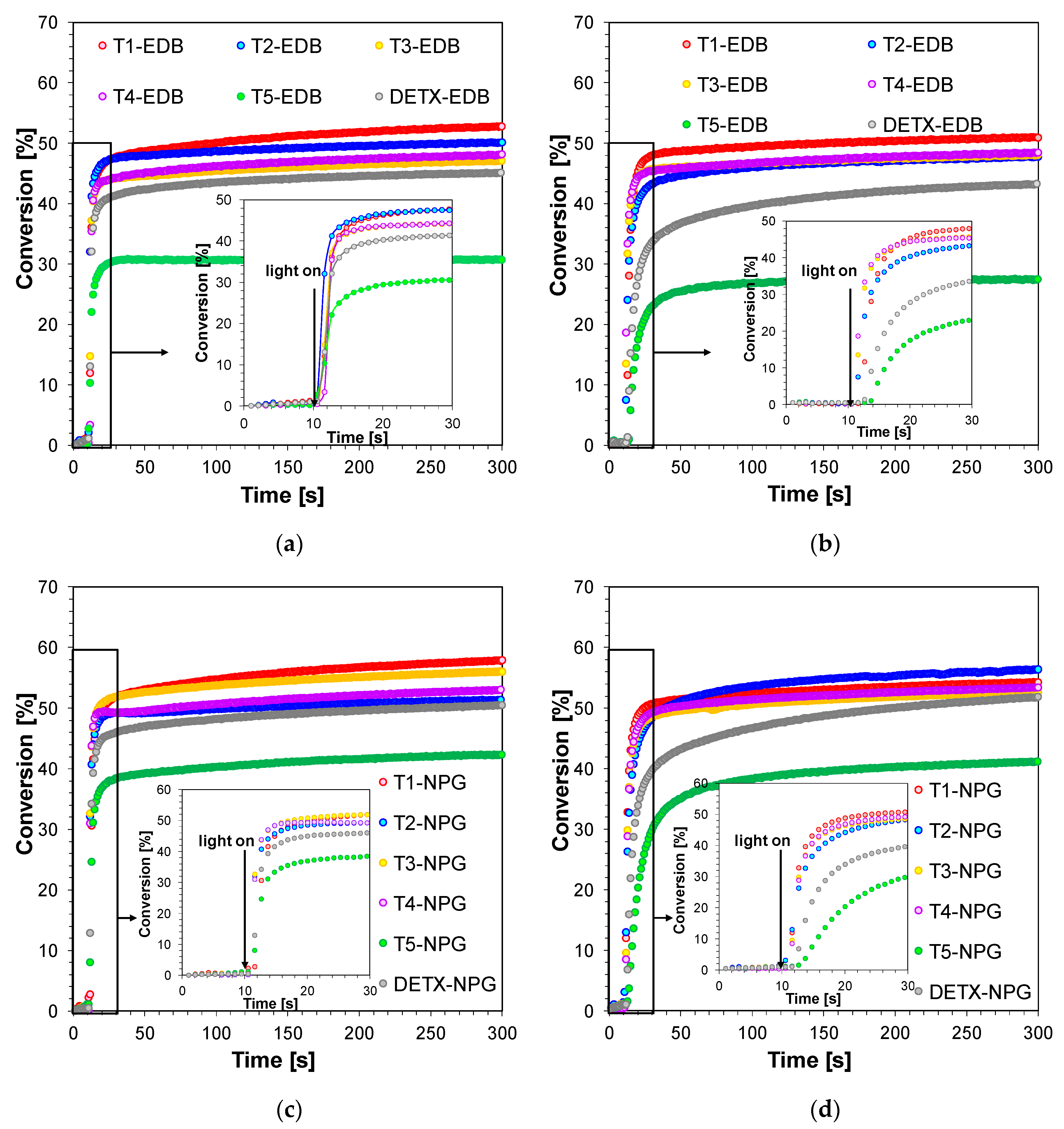
2.3.1. Performance of 2,4-Diethyl-thioxanthen-9-one Derivatives as Photosensitizers in Bimolecular Photoinitiating Systems for Free-Radical Photopolymerisation
2.3.2. 2,4-Diethyl-thioxanthen-9-one Derivatives as One Component Free-Radical Photoinitiators during Photopolymerisation of Acrylate Monomers
2.3.3. 2,4-Diethyl-thioxanthen-9-one Derivatives as Type II Photoinitiators during Photopolymerisation of Acrylate Monomers
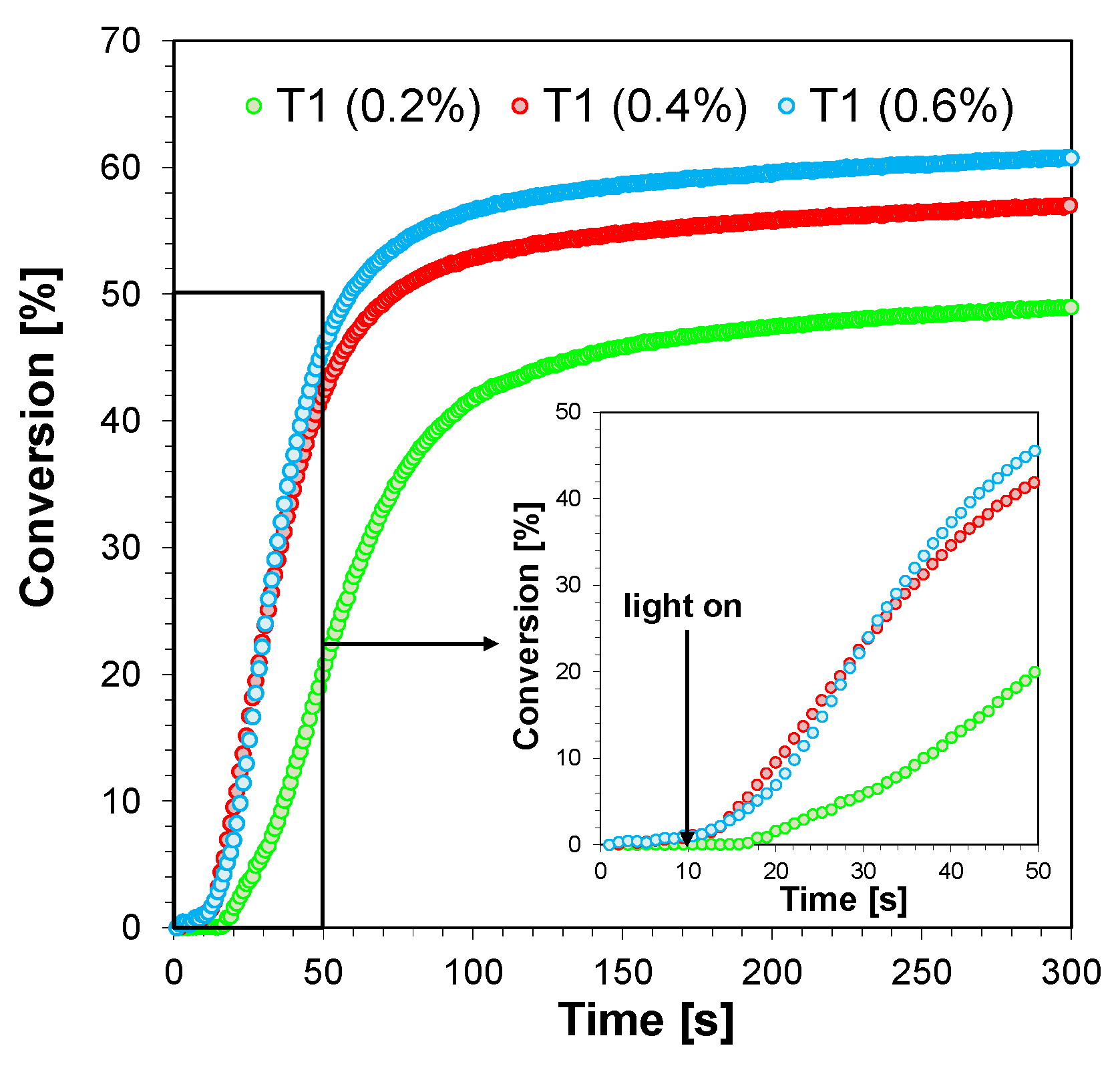
2.3.4. Three-Component Photoinitiating Systems—Photocatalyst Behaviour of T1
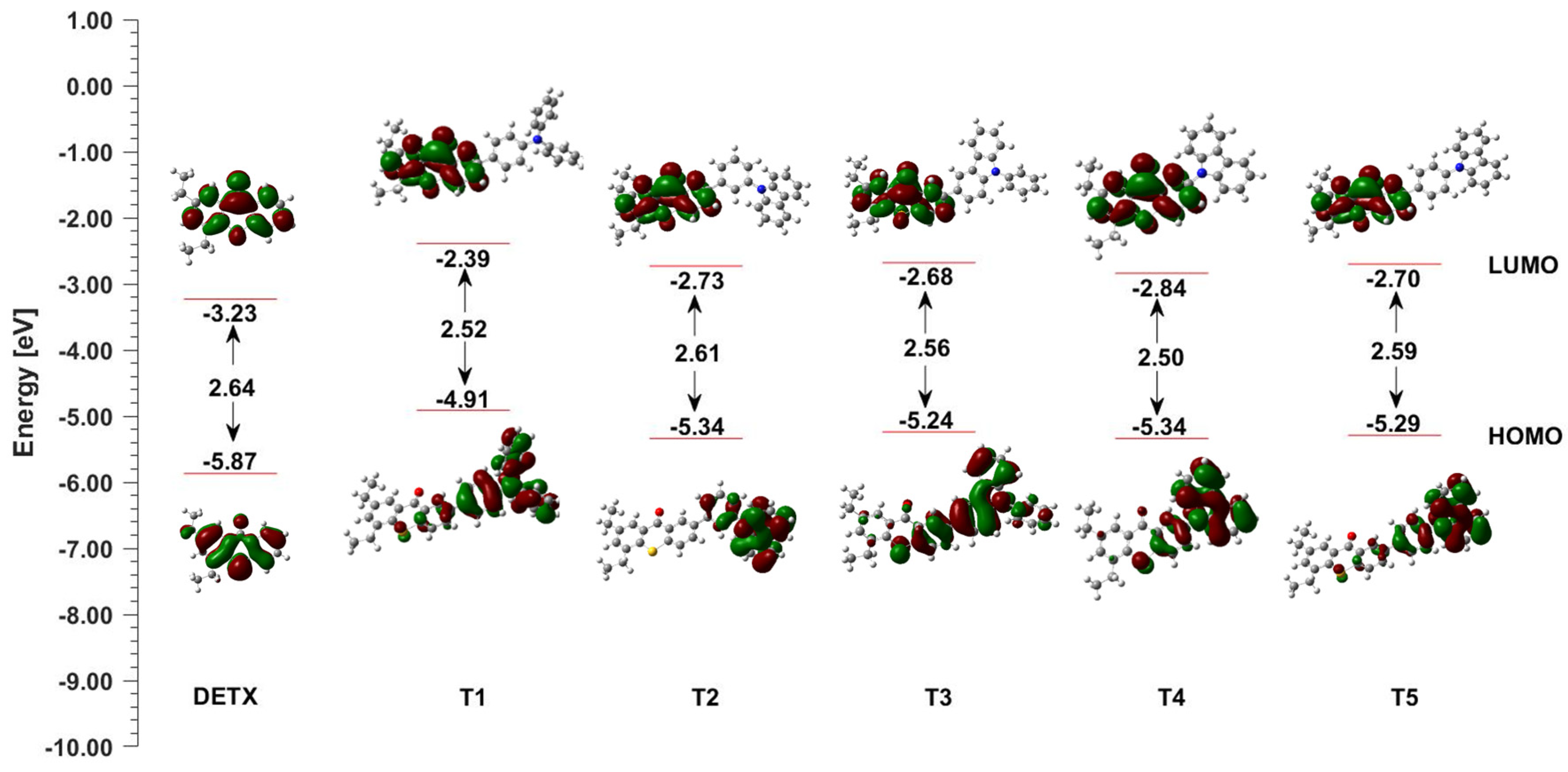
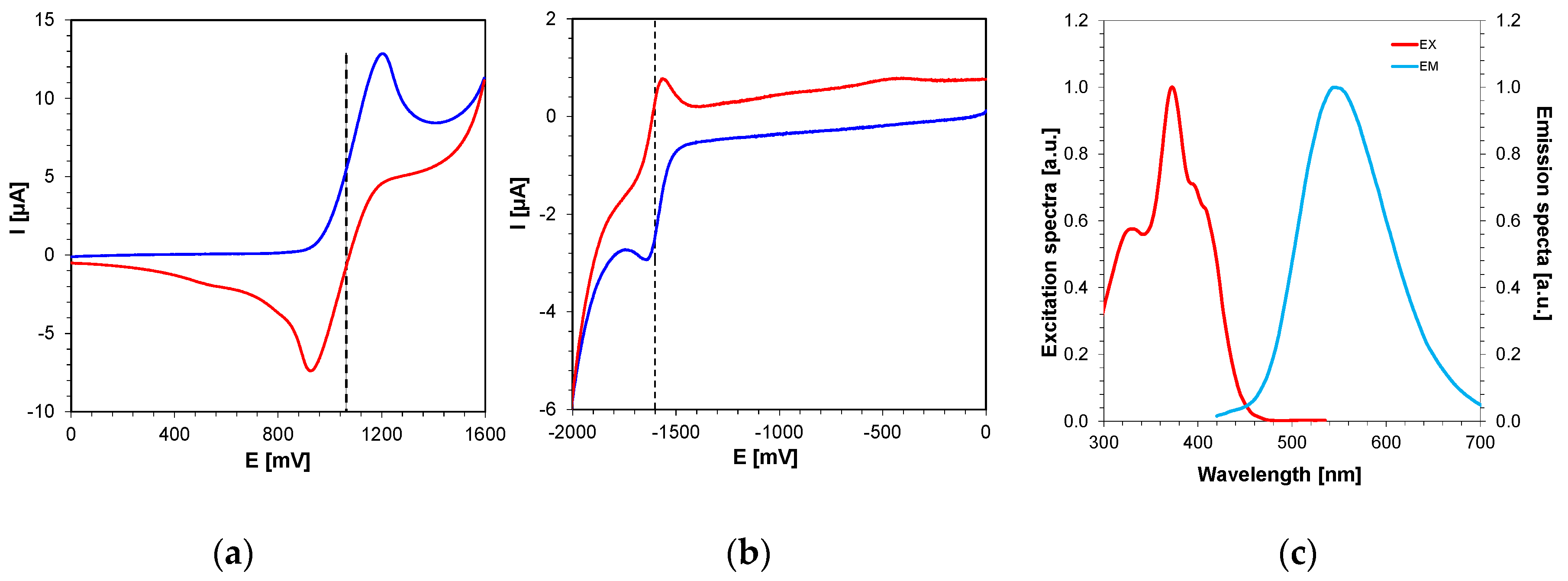
2.4. Photochemical Mechanism-Dual Photochemical Behaviour of 2,4-Diethyl-thioxanthen-9-one Derivatives during Photopolymerisation by Photoreduction and Photooxidation Processes
2.4.1. 2,4-Diethyl-thioxanthen-9-one Derivatives as Electron Donors in Two-Component Systems
2.4.2. 2,4-Diethyl-thioxanthen-9-one Derivatives as Electron Acceptors in Two-Component Systems with EDB or NPG
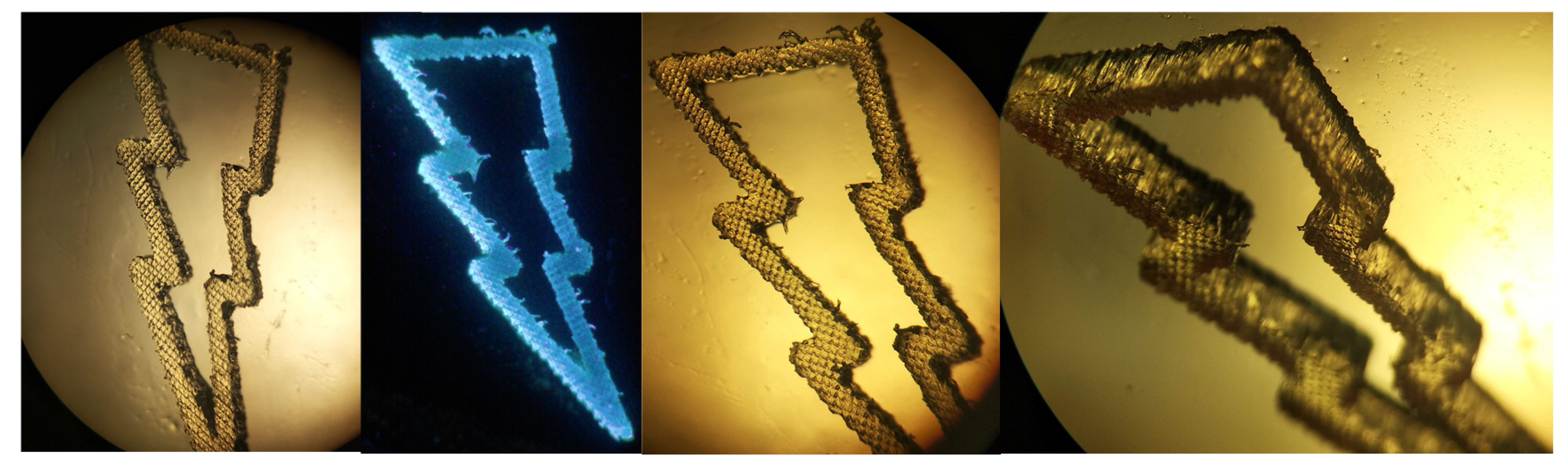
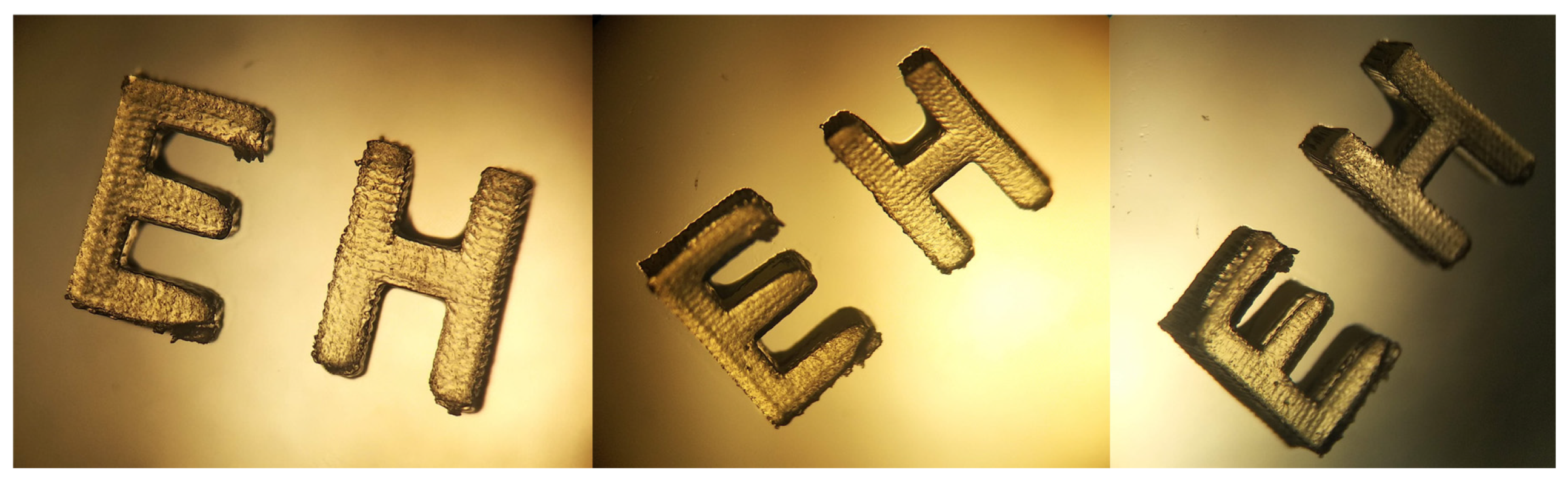
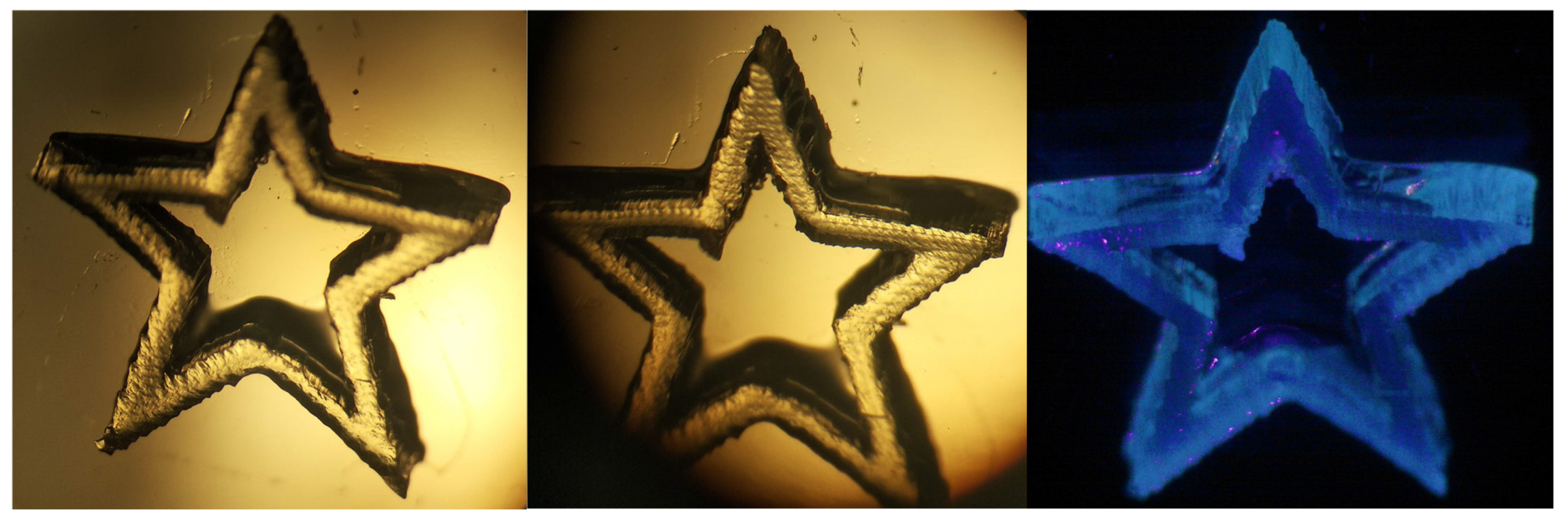
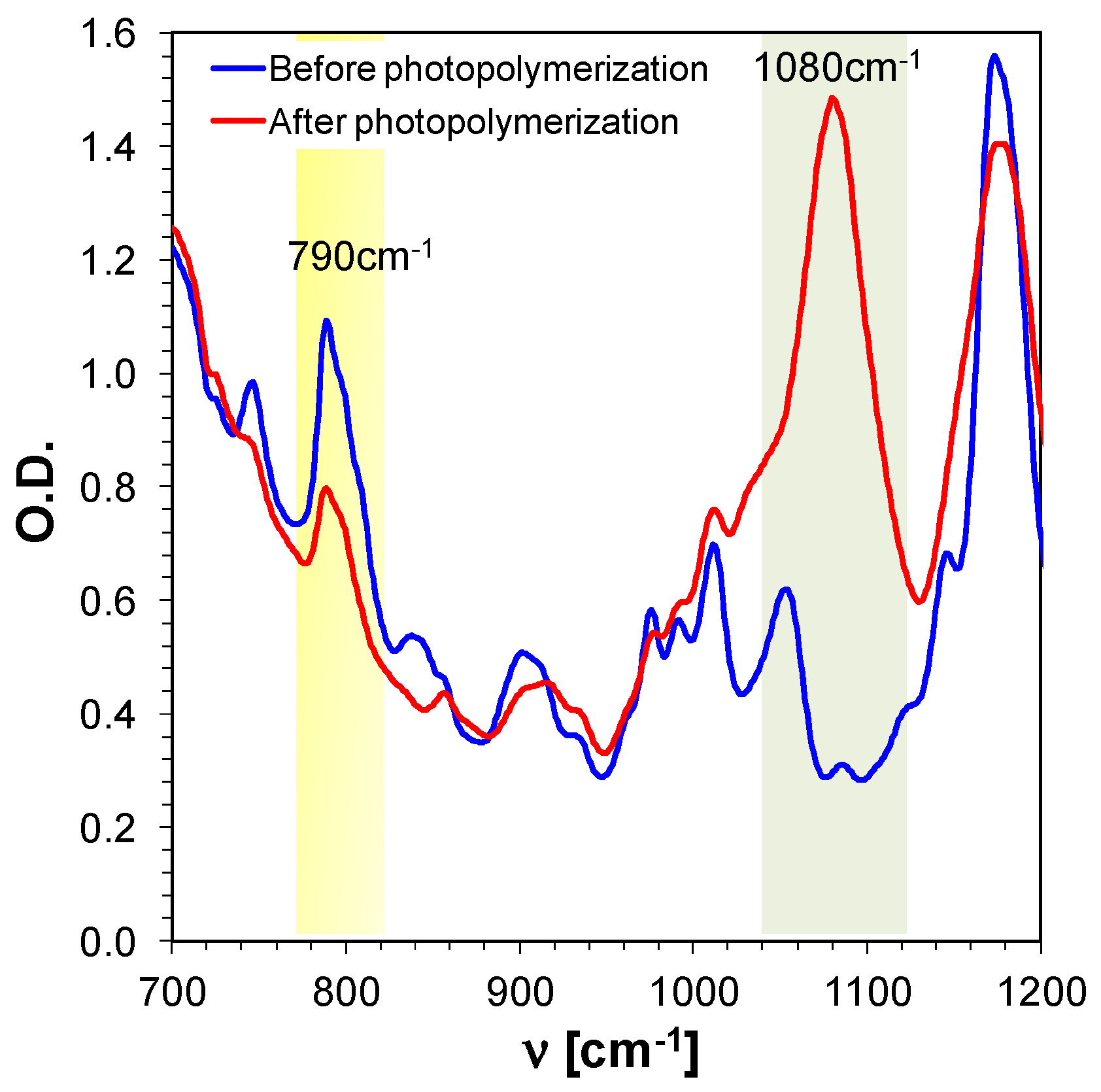
2.5. 3D Printing Experiments (Laser Writing)
3. Materials and Methods
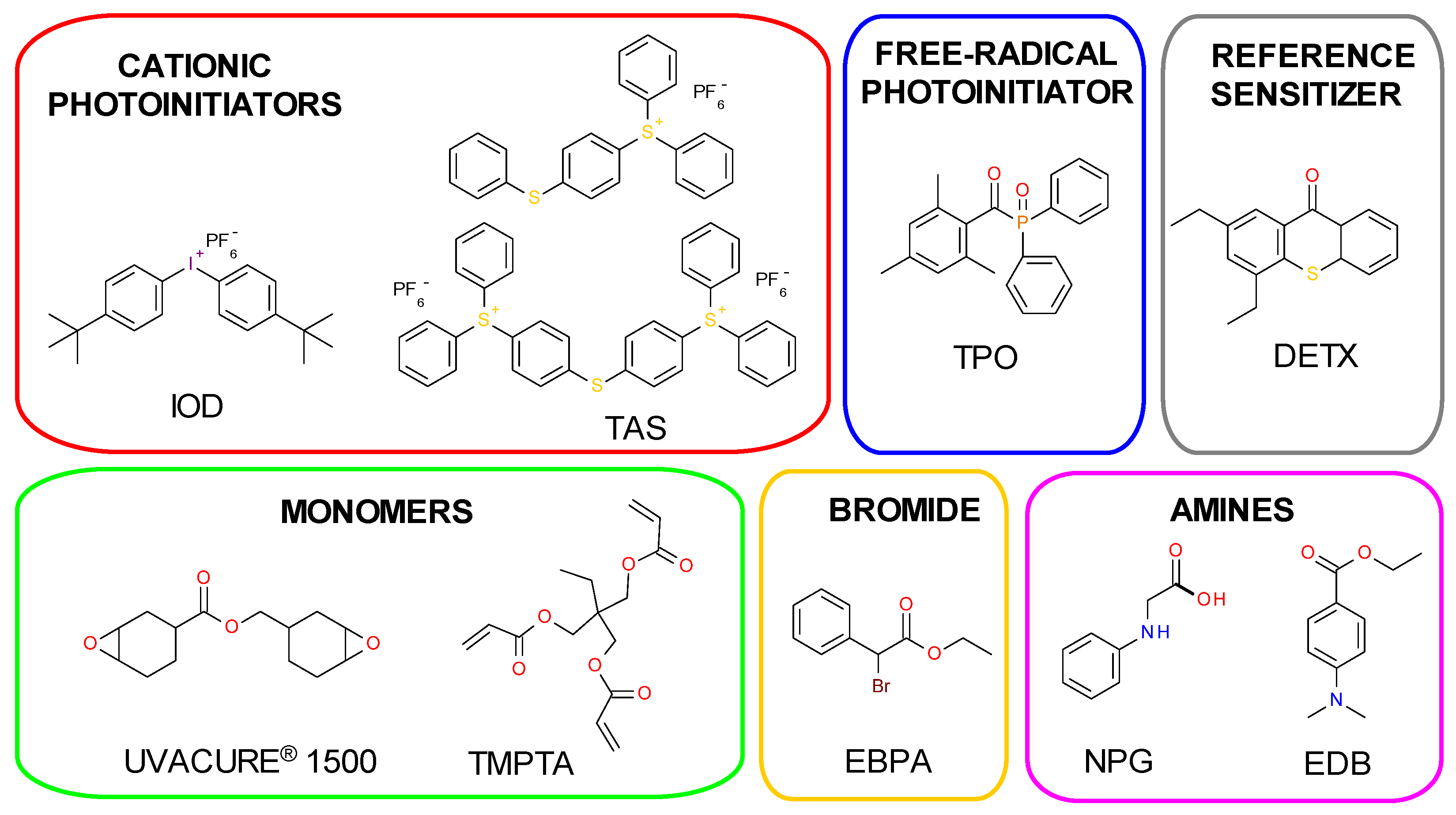
3.1. Materials
3.2. UV-Visible Absorption, Excitation and Emission Spectra
3.3. Electrochemical Characteristics Determination of Oxidation Potentials
3.4. Computational Procedure
3.5. Photopolymerisation Processes
3.5.1. Cationic Photopolymerisation of Epoxy Monomer UVACURE®1500
3.5.2. Free Radical Photopolymerisation of Acrylate TMPTA
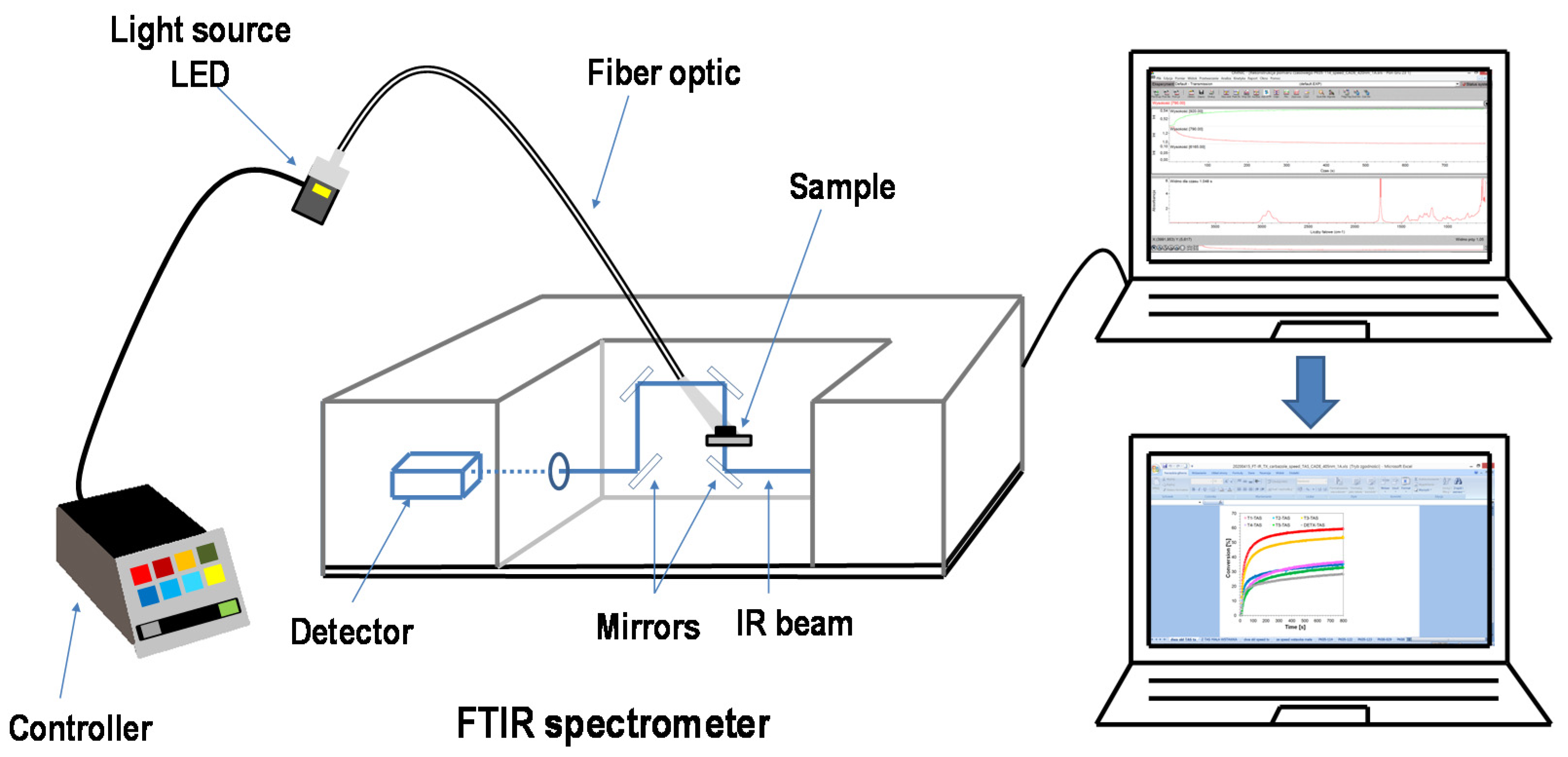
3.5.3. Sources of Light for Real-Time Experiments of Photopolymerisation Processes
3.6. 3D Printing
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chatani, S.; Kloxin, C.J.; Bowman, C.N. The power of light in polymer science: Photochemical processes to manipulate polymer formation, structure, and properties. Polym. Chem. 2014, 5, 2187–2201. [Google Scholar] [CrossRef]
- Javadi, A.; Mehr, H.S.; Sobani, M.; Soucek, M.D. Cure-on-command technology: A review of the current state of the art. Prog. Org. Coat. 2016, 100, 2–31. [Google Scholar] [CrossRef]
- Hola, E.; Pilch, M.; Galek, M.; Ortyl, J. New versatile bimolecular photoinitiating systems based on amino-: M-terphenyl derivatives for cationic, free-radical and thiol-ene photopolymerization under low intensity UV-A and visible light sources. Polym. Chem. 2020, 11, 480–495. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers: Part I: Comparison of the performance of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2017, 64, 313–320. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers, Part II: Determination of relative quantum efficiency of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2018, 67, 144–150. [Google Scholar] [CrossRef]
- Ortyl, J.; Wilamowski, J.; Milart, P.; Galek, M.; Popielarz, R. Relative sensitization efficiency of fluorescent probes/sensitizers for monitoring and acceleration of cationic photopolymerization of monomers. Polym. Test. 2015, 48, 151–159. [Google Scholar] [CrossRef]
- Ortyl, J.; Fiedor, P.; Chachaj-Brekiesz, A.; Pilch, M.; Hola, E.; Galek, M. The applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile sensors for monitoring different types of photopolymerization processes and acceleration of cationic and free-radical photopolymerization under near UV light. Sensors 2019, 19, 1668. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Applicability of quinolizino-coumarins for monitoring free radical photopolymerization by fluorescence spectroscopy. Polym. Test. 2015, 42, 99–107. [Google Scholar] [CrossRef]
- Kostrzewska, K.; Ortyl, J.; Dobosz, R.; Kabatc, J. Squarylium dye and onium salts as highly sensitive photoradical generators for blue light. Polym. Chem. 2017, 8, 3464–3474. [Google Scholar] [CrossRef]
- Hola, E.; Topa, M.; Chachaj-Brekiesz, A.; Pilch, M.; Fiedor, P.; Galek, M.; Ortyl, J. New, highly versatile bimolecular photoinitiating systems for free-radical, cationic and thiol-ene photopolymerization processes under low light intensity UV and visible LEDs for 3D printing application. RSC Adv. 2020, 10, 7509–7522. [Google Scholar] [CrossRef]
- Fiedor, P.; Pilch, M.; Szymaszek, P.; Chachaj-Brekiesz, A.; Galek, M.; Ortyl, J. Photochemical study of a new bimolecular photoinitiating system for vat photopolymerization 3D printing techniques under visible light. Catalysts 2020, 10, 284. [Google Scholar] [CrossRef]
- Lalevée, J.; Fouassier, J.P. (Eds.) Photopolymerisation Initiating Systems, 1st ed.; Royal Society of Chemistry: Croydon, UK, 2018. [Google Scholar]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Nair, G.B.; Dhoble, S.J. A perspective perception on the applications of light-emitting diodes. Luminescence 2015, 30, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, H.; Toufaily, J.; Hamieh, T.; Dumur, F.; Campolo, D.; Gigmes, D.; Pierre Fouassier, J.; Ortyl, J.; Lalevée, J. Specific cationic photoinitiators for near UV and visible LEDs: Iodonium versus ferrocenium structures. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Ortyl, J.; Popielarz, R. New photoinitiators for cationic polymerization. Polimery/Polymers 2012, 57, 510–517. [Google Scholar] [CrossRef]
- Crivello, J.V. Cationic polymerization—Iodonium and sulfonium salt photoinitiators. In Initiators—Poly-Reactions—Optical Activity; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–48. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced Electron Transfer Reactions for Macromolecular Syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef]
- Crivello, J.P. Photoinitiated cationic polymerization. Ann. Rev. Mater. Sci. 1983, 13, 173–190. [Google Scholar] [CrossRef]
- Lewis, F.D.; Lauterbach, R.T.; Heine, H.G.; Hartmann, W.; Rudolph, H. Photochemical alpha Cleavage of Benzoin Derivatives. Polar Transition States for Free-Radical Formation. J. Am. Chem. Soc. 1975, 97, 1519–1525. [Google Scholar] [CrossRef]
- Günzler, F.; Wong, E.H.H.; Koo, S.P.S.; Junkers, T.; Christopher, B.K. Quantifying the eficiency of photoinitiation processes in methyl methacrylate free radical polymerization via electrospray ionization mass spectrometry. Macromolecules 2009, 42, 1488–1493. [Google Scholar] [CrossRef]
- Voll, D.; Hufendiek, A.; Junkers, T.; Barner-Kowollik, C. Quantifying photoinitiation efficiencies in a multiphotoinitiated free-radical polymerization. Macromol. Rapid Commun. 2012, 33, 47–53. [Google Scholar] [CrossRef]
- Stocker, T.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P. Summary for policymakers. In Climate Change 2013—The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 1–30. [Google Scholar] [CrossRef]
- Allen, N.S.; Catalina, F.; Green, P.N.; Green, W.A. Photochemistry of carbonyl photoinitiators. Photopolymerisation, flash photolysis and spectroscopic study. Eur. Polym. J. 1986, 22, 49–56. [Google Scholar] [CrossRef]
- Gruber, H.F. Photoinitiators for free radical polymerization. Prog. Polym. Sci. 1992, 17, 953–1044. [Google Scholar] [CrossRef]
- Temel, G.; Enginol, B.; Aydin, M.; Balta, D.K.; Arsu, N. Photopolymerization and photophysical properties of amine linked benzophenone photoinitiator for free radical polymerization. J. Photochem. Photobiol. A Chem. 2011, 219, 26–31. [Google Scholar] [CrossRef]
- Yu, Q.; Nauman, S.; Santerre, J.P.; Zhu, S. UV photopolymerization behavior of dimethacrylate oligomers with camphorquinone/amine initiator system. J. Appl. Polym. Sci. 2001, 82, 1107–1117. [Google Scholar] [CrossRef]
- Anderson, D.G.; Davidson, R.S.; Elvery, J.J. Thioxanthones: Their fate when used as photoinitiators. Polymer 1996, 37, 2477–2484. [Google Scholar] [CrossRef]
- Fouassier, J.P. Photoinitiation, photopolymerization, and photocuring (fundamentals and applications). IEEE Electr. Insul. Mag. 1996, 12, 36. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a light on an adaptable photoinitiator: Advances in photopolymerizations initiated by thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Temel, G.; Arsu, N. 2-Methylol-thioxanthone as a free radical polymerization initiator. J. Photochem. Photobiol. A Chem. 2007, 191, 149–152. [Google Scholar] [CrossRef]
- Doǧruyol, S.K.; Doǧruyol, Z.; Arsu, N. Thioxanthone based 9-[2-(methyl-phenyl-amino)-acetyl]-thia-naphthacene-12- one as a visible photoinitiator. J. Lumin. 2013, 138, 98–104. [Google Scholar] [CrossRef]
- Balta, D.K.; Arsu, N.; Yagci, Y.; Sundaresan, A.K.; Jockusch, S.; Turro, N.J. Mechanism of photoinitiated free radical polymerization by thioxanthone-anthracene in the presence of air. Macromolecules 2011, 44, 2531–2535. [Google Scholar] [CrossRef]
- Mutlu, S.; Watanabe, K.; Takahara, S.; Arsu, N. Thioxanthone–anthracene-9-carboxylic acid as radical photoinitiator in the presence of atmospheric air. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1878–1883. [Google Scholar] [CrossRef]
- Balta, D.K.; Temel, G.; Goksu, G.; Ocal, N.; Arsu, N. Thioxanthone-diphenyl anthracene: Visible light photoinitiator. Macromolecules 2012, 45, 119–125. [Google Scholar] [CrossRef]
- Karaca, N.; Ocal, N.; Arsu, N.; Jockusch, S. Thioxanthone-benzothiophenes as photoinitiator for free radical polymerization. J. Photochem. Photobiol. A Chem. 2016, 331, 22–28. [Google Scholar] [CrossRef]
- Sevinc, D.; Karasu, F.; Arsu, N. Thioxanthone-benzotriazole: Initiator and stabilizer properties in one component. J. Photochem. Photobiol. A Chem. 2009, 203, 81–84. [Google Scholar] [CrossRef]
- Ścigalski, F.; Jankowski, K. Mercaptoalkoxy-thioxanthones as a novel photoinitiator for free radical polymerization. Polym. Bull. 2015, 72, 255–263. [Google Scholar] [CrossRef]
- Esen, D.S.; Temel, G.; Balta, D.K.; Allonas, X.; Arsu, N. One-component thioxanthone acetic acid derivative photoinitiator for free radical polymerization. Photochem. Photobiol. 2014, 90, 463–469. [Google Scholar] [CrossRef]
- Keskin, S.; Jockusch, S.; Turro, N.J.; Arsu, N. 2-Mercaptothioxanthone as sensitizer and coinitiator for acylphosphine oxide photoinitiators for free radical polymerization. Macromolecules 2008, 41, 4631–4634. [Google Scholar] [CrossRef]
- Tar, H.; Sevinc Esen, D.; Aydin, M.; Ley, C.; Arsu, N.; Allonas, X. Panchromatic type II photoinitiator for free radical polymerization based on thioxanthone derivative. Macromolecules 2013, 46, 3266–3272. [Google Scholar] [CrossRef]
- Wu, Q.; Xiong, Y.; Liang, Q.; Tang, H. Developing thioxanthone based visible photoinitiators for radical polymerization. RSC Adv. 2014, 4, 52324–52331. [Google Scholar] [CrossRef]
- Dogruyol, S.K.; Dogruyol, Z.; Arsu, N. A thioxanthone-based visible photoinitiator. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4037–4043. [Google Scholar] [CrossRef]
- Jiang, X.; Yin, J. Polymeric photoinitiator containing in-chain thioxanthone and coinitiator amines. Macromol. Rapid Commun. 2004, 25, 748–752. [Google Scholar] [CrossRef]
- Jiang, X.; Yin, J. Study of macrophotoinitiator containing in-chain thioxanthone and coinitiator amines. Polymer (Guildf.) 2004, 45, 5057–5063. [Google Scholar] [CrossRef]
- Yilmaz, G.; Tuzun, A.; Yagci, Y. Thioxanthone-carbazole as a visible light photoinitiator for free radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5120–5125. [Google Scholar] [CrossRef]
- Karaca, N.; Karaca, B.D.; Ocal, N.; Arsu, N. Mechanistic studies of thioxanthone-carbazole as a one-component type II photoinitiator. J. Lumin. 2014, 146, 424–429. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Stability and Utility of Pyridyl Disulfide Functionality in RAFT and Conventional Radical Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Ali Tehfe, M.; Fries, C.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. New thioxanthone and xanthone photoinitiators based on silyl radical chemistry. Polym. Chem. 2011, 2, 1077–1084. [Google Scholar] [CrossRef]
- Alonso, R.; Bach, T. A chiral thioxanthone as an organocatalyst for enantioselective [2+2] photocycloaddition reactions induced by visible light. Angew. Chem. Int. Ed. 2014, 53, 4368–4371. [Google Scholar] [CrossRef]
- Dansholm, C.N.; Junker, A.K.R.; Nielsen, L.G.; Kofod, N.; Pal, R.; Sørensen, T.J. π-Expanded Thioxanthones—Engineering the Triplet Level of Thioxanthone Sensitizers for Lanthanide-Based Luminescent Probes with Visible Excitation. Chempluschem 2019, 84, 1778–1788. [Google Scholar] [CrossRef]
- Xiao, P.; Lalevée, J.; Zhao, J.; Stenzel, M.H. N-Vinylcarbazole as Versatile Photoinaddimer of Photopolymerization under Household UV LED Bulb (392 nm). Macromol. Rapid Commun. 2015, 36, 1675–1680. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Guo, C.; Li, Y.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Panchromatic photoinitiators for radical, cationic and thiol-ene polymerization reactions: A search in the diketopyrrolopyrrole or indigo dye series. Mater. Today Commun. 2015, 4, 101–108. [Google Scholar] [CrossRef]
- Mousawi, A.A.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.P.; Chachaj-Brekiesz, A.; Ortyl, J.; Lalevée, J. Meta-Terphenyl Derivative/Iodonium Salt/9H-Carbazole-9-ethanol Photoinitiating Systems for Free Radical Promoted Cationic Polymerization upon Visible Lights. Macromol. Chem. Phys. 2016, 217, 1955–1965. [Google Scholar] [CrossRef]
- Al, M.A.; Kermagoret, A.; Versace, D.L.; Toufaily, J.; Hamieh, T.; Graff, B.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper photoredox catalysts for polymerization upon near UV or visible light: Structure/reactivity/efficiency relationships and use in LED projector 3D printing resins. Polym. Chem. 2017, 8, 568–580. [Google Scholar] [CrossRef]
- Hola, E.; Ortyl, J.; Jankowska, M.; Pilch, M.; Galek, M.; Morlet-Savary, F.; Graff, B.; Dietlin, C.; Lalevée, J. New bimolecular photoinitiating systems based on terphenyl derivatives as highly efficient photosensitizers for 3D printing application. Polym. Chem. 2020, 11, 922–935. [Google Scholar] [CrossRef]
- Mousawi, A.A.; Garra, P.; Dumur, F.; Bui, T.T.; Goubard, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; et al. Novel carbazole skeleton-based photoinitiators for led polymerization and LED projector 3D printing. Molecules 2017, 22, 2143. [Google Scholar] [CrossRef]
- Sundell, P.-E.; Jösson, S.; Hult, A. Photo-redox induced cationic polymerization of divinyl ethers. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 1525–1533. [Google Scholar] [CrossRef]
- Popal, M.; Volk, J.; Leyhausen, G.; Geurtsen, W. Cytotoxic and genotoxic potential of the type I photoinitiators BAPO and TPO on human oral keratinocytes and V79 fibroblasts. Dent. Mater. 2018, 34, 1783–1796. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Krifka, S.; Hiller, K.A.; Bolay, C.; Waha, C.; Van Meerbeek, B.; Schmalz, G.; Schweikl, H. Evaluation of cell responses toward adhesives with different photoinitiating systems. Dent. Mater. 2015, 31, 916–927. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Nzulu, F.; Telitel, S.; Stoffelbach, F.; Graff, B.; Morlet-Savary, F.; Lalevée, J.; Fensterbank, L.; Goddard, J.P.; Ollivier, C. A dinuclear gold(i) complex as a novel photoredox catalyst for light-induced atom transfer radical polymerization. Polym. Chem. 2015, 6, 4605–4611. [Google Scholar] [CrossRef]
- Romańczyk, P.P.; Kurek, S.S. The Reduction Potential of Diphenyliodonium Polymerisation Photoinitiator Is Not −0.2 V vs. SCE. A Computational Study. Electrochim. Acta 2017, 255, 482–485. [Google Scholar] [CrossRef]
- Strehmel, B.; Ernst, S.; Reiner, K.; Keil, D.; Lindauer, H.; Baumann, H. Application of nir-photopolymers in the graphic industry: From physical chemistry to lithographic applications. Z. Phys. Chem. 2014, 228, 129–153. [Google Scholar] [CrossRef]
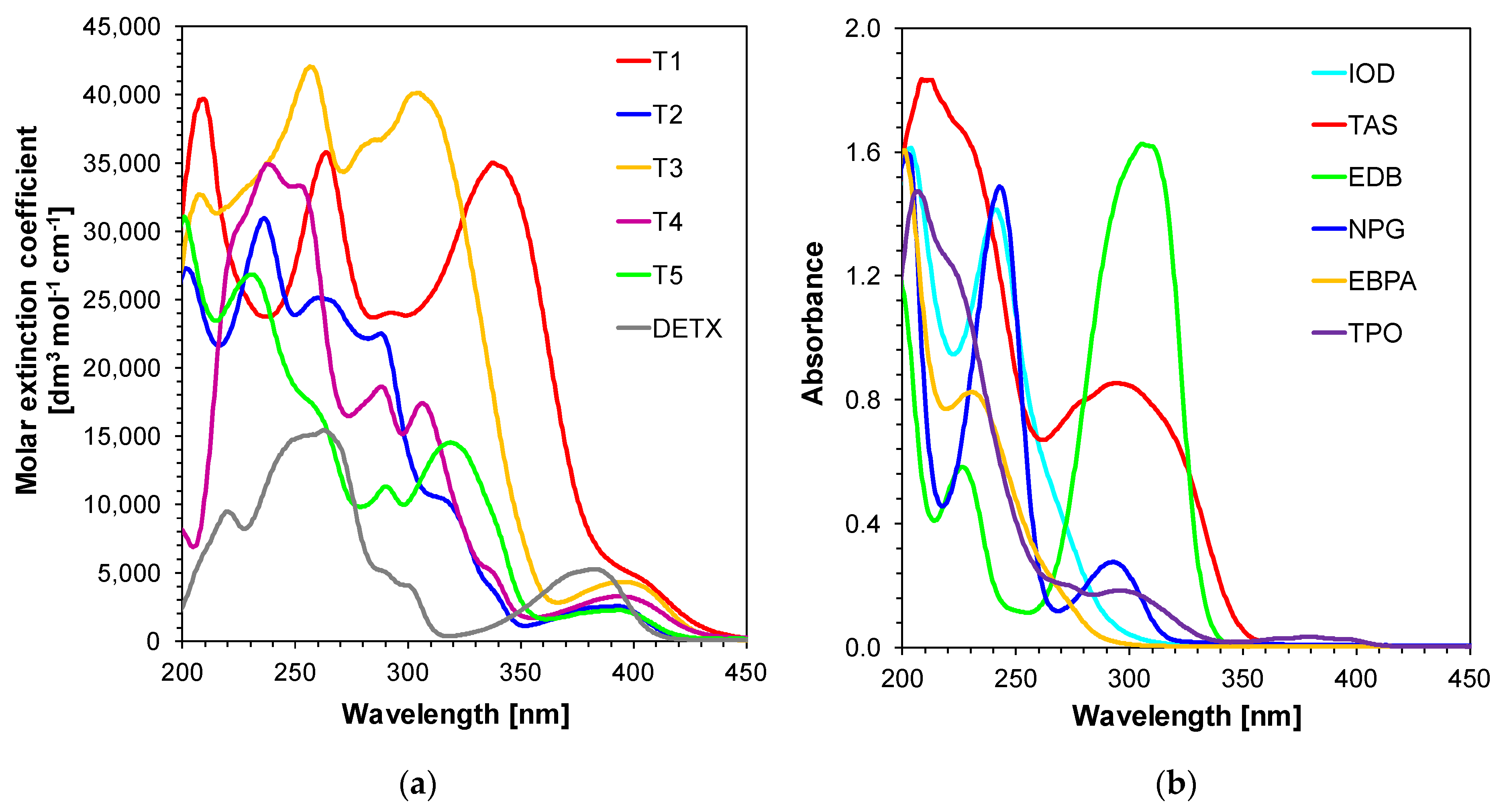
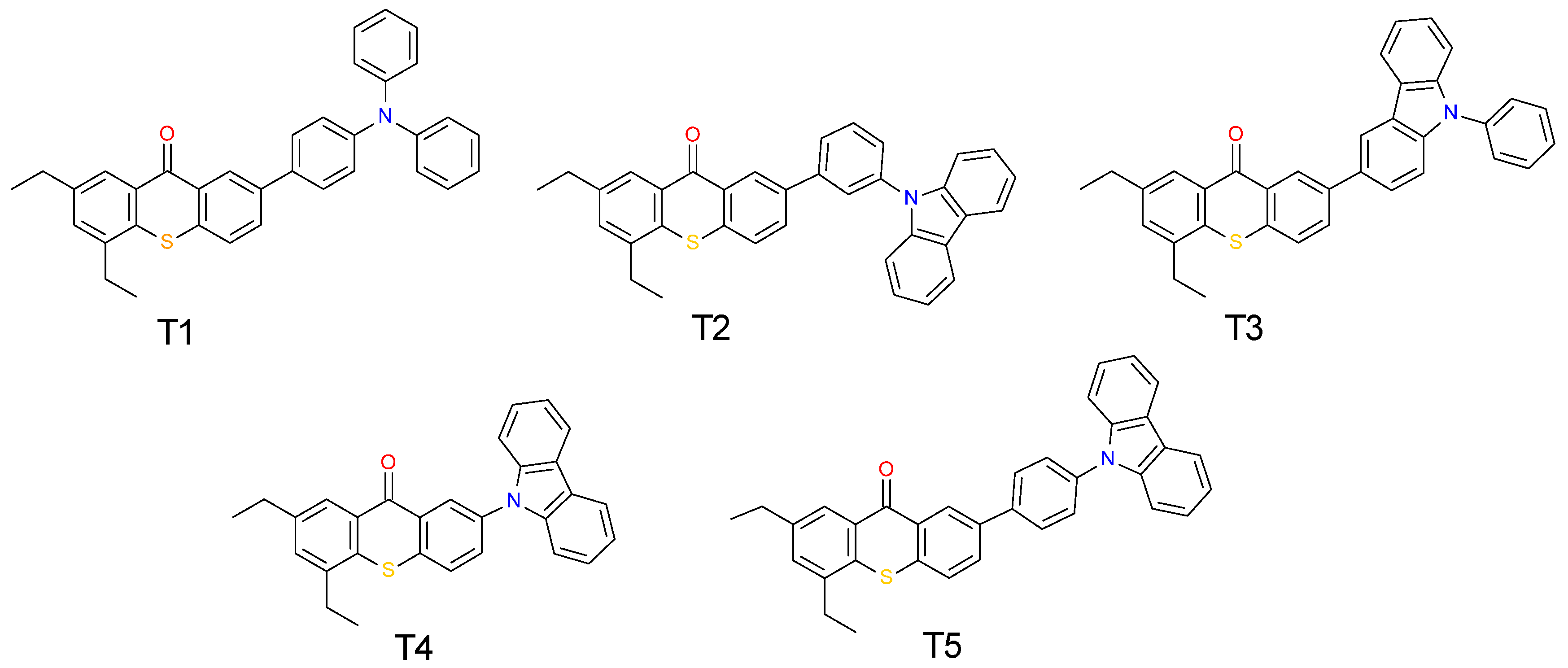
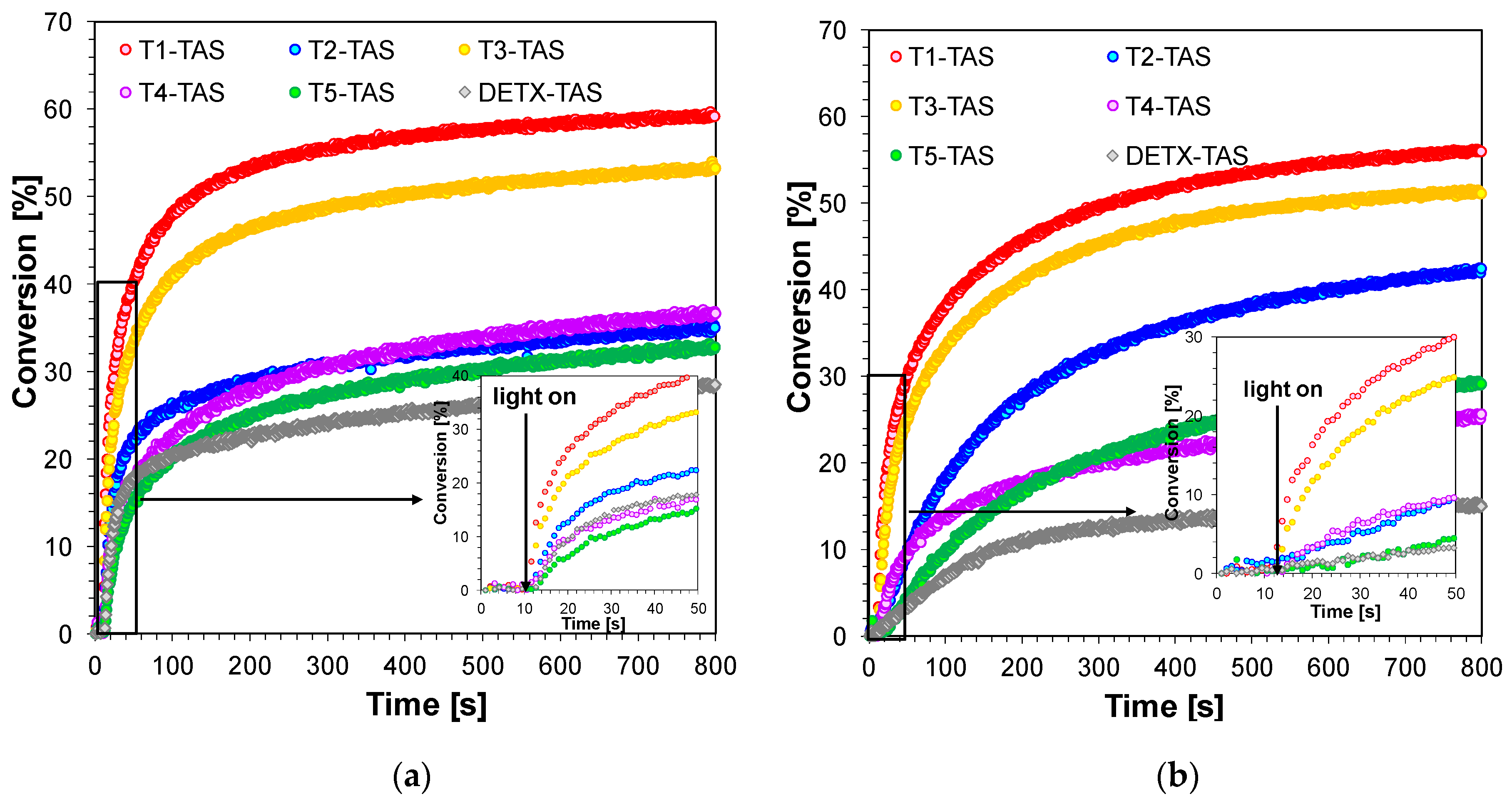
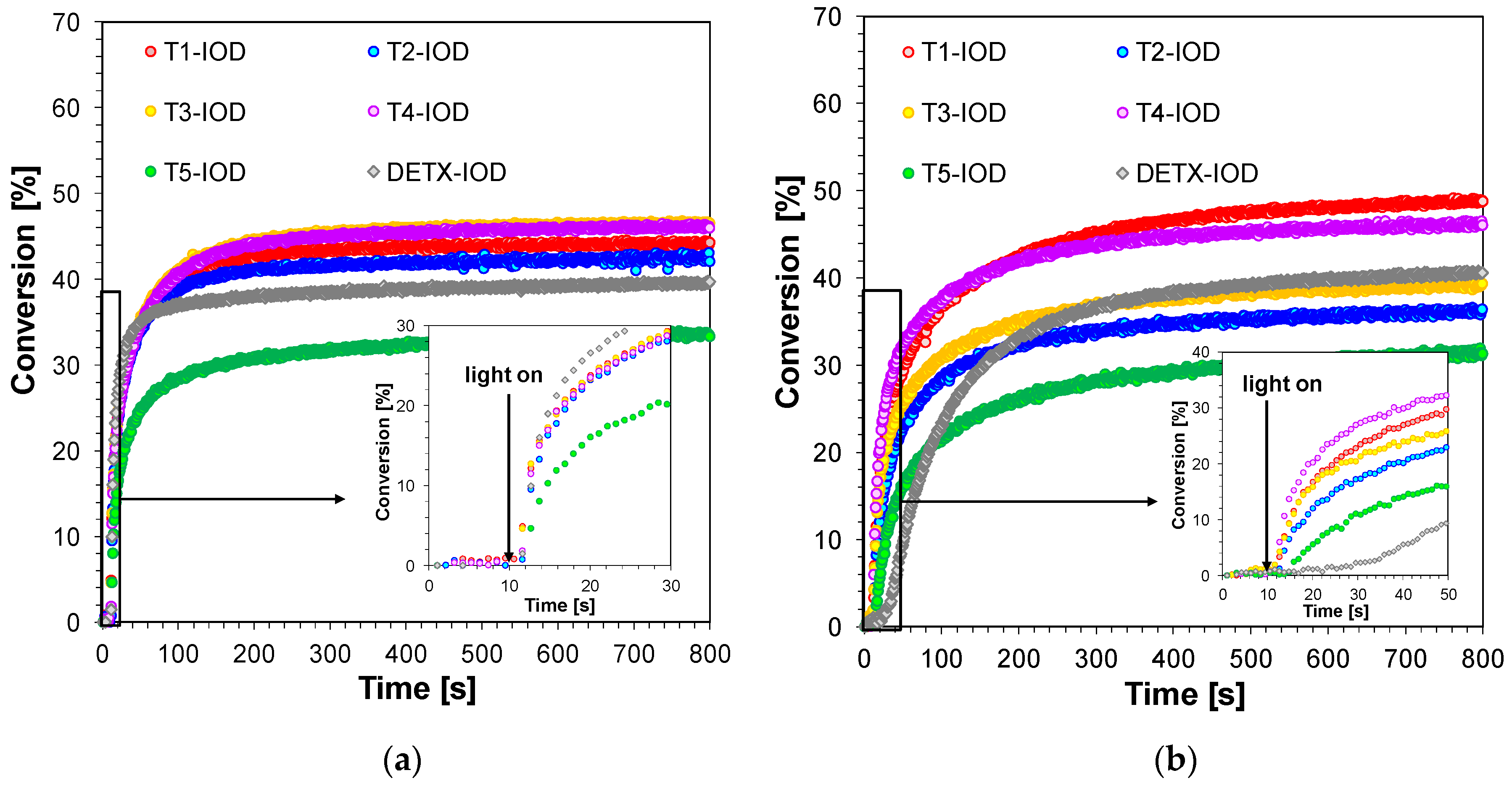
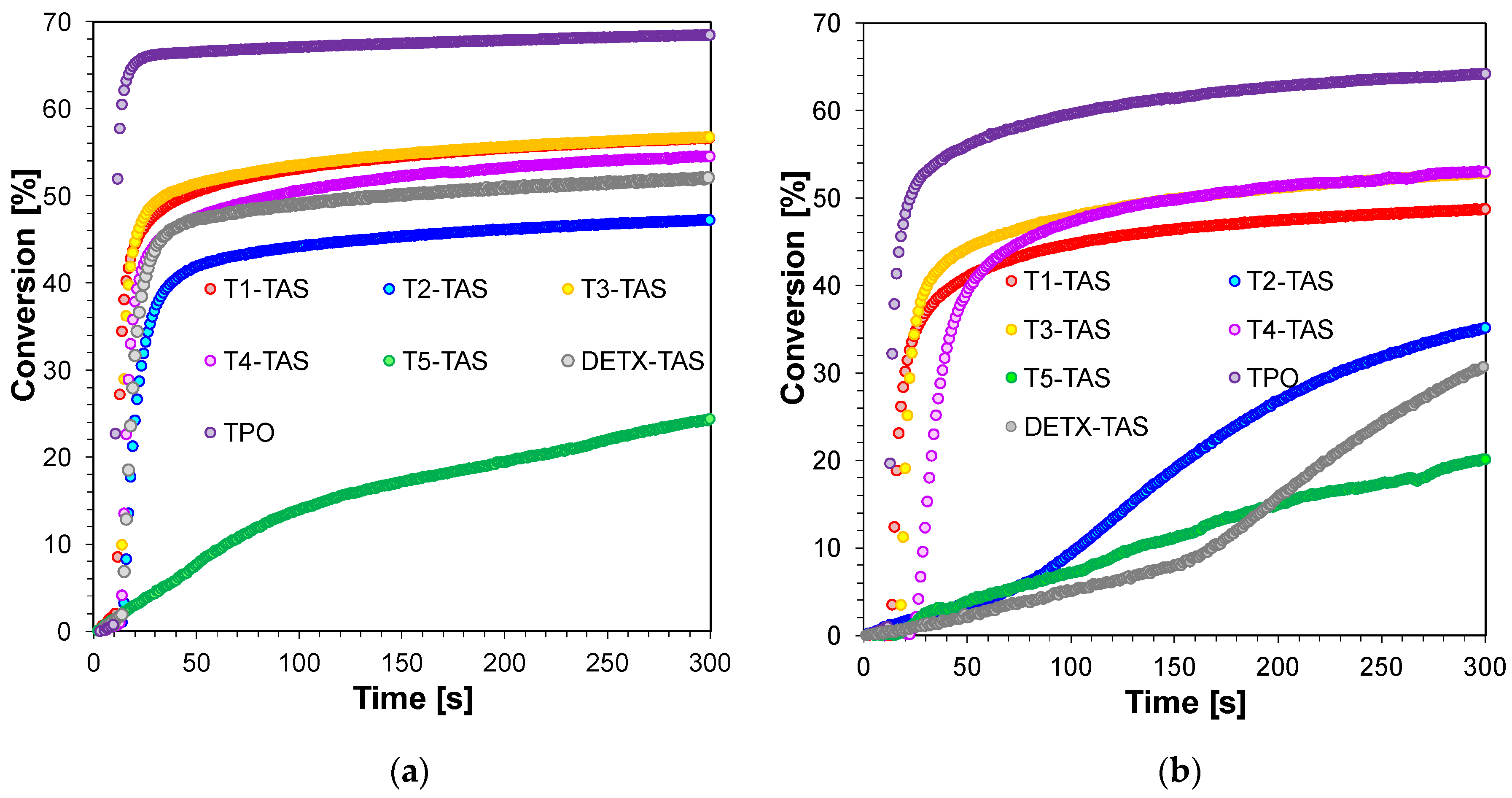
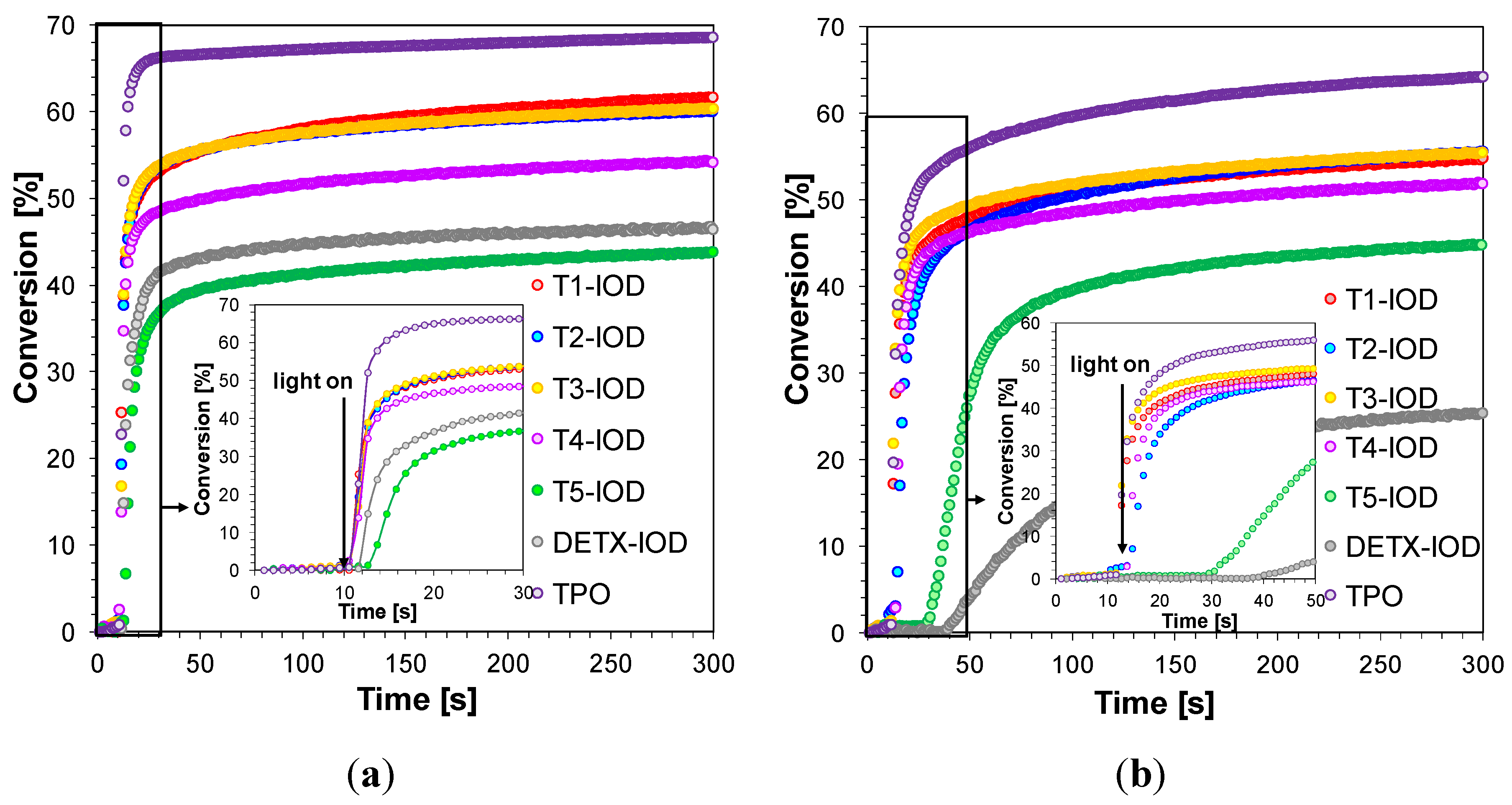




| Compound | Concentration [mol·dm−3] | λmax-abs [nm] | εmax | ε405 nm | ε420 nm |
|---|---|---|---|---|---|
| [dm3·mol−1·cm−1] | |||||
| T1 | 4.03 × 10−5 | 338 | 34,923 | 4405 | 2204 |
| T2 | 2.24 × 10−5 | 393 | 2553 | 1615 | 268 |
| T3 | 4.40 × 10−5 | 396 | 4329 | 3942 | 1598 |
| T4 | 3.60 × 10−5 | 393 | 33,312 | 2892 | 1334 |
| T5 | 2.75 × 10−5 | 393 | 2261 | 1644 | 432 |
| DETX | 9.70 × 10−5 | 382 | 5263 | 1116 | 134 |
| IOD | 6.54 × 10−5 | 241 | - | - | - |
| TAS | 3.42 × 10−2 * | 294 | - | - | - |
| EDB | 6.83 × 10−5 | 306 | - | - | - |
| NPG | 1.53 × 10−4 | 293 | - | - | - |
| EBPA | 1.53 × 10−4 | 231 | - | - | - |
| TPO | 6.60 × 10−5 | 298 380 | - | - | - |
| Sensitizer | Photoinitiating System with TAS (2%) | Photoinitiating System with IOD (1%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 405 nm, I0 = 17.24 mW·cm−2 | 420 nm, I0 = 14.87 mW·cm−2 | 405 nm, I0 = 17.24 mW·cm−2 | 420 nm, I0 = 14.87 mW·cm−2 | |||||
| tind [s] | FC [%] | tind [s] | FC [%] | tind [s] | FC [%] | tind [s] | FC [%] | |
| T1 | 1 | 59 | 1 | 56 | 1 | 44 | 1 | 48 |
| T2 | 1 | 34 | 6 | 42 | 1 | 42 | 2 | 36 |
| T3 | 1 | 53 | 2 | 51 | 1 | 46 | 1 | 39 |
| T4 | 1 | 36 | 2 | 25 | 1 | 46 | 1 | 46 |
| T5 | 1 | 33 | 14 | 29 | 2 | 33 | 4 | 31 |
| DETX | 1 | 28 | 18 | 15 | 1 | 39 | 17 | 40 |
| Sensitizer | Photoinitiating System with TAS (2%) | Photoinitiating System with IOD (1%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 405 nm, I0 = 17.24 mW·cm−2 | 420 nm, I0 = 14.87 mW·cm−2 | 405 nm, I0 = 17.24 mW·cm−2 | 420 nm, I0 = 14.87 mW·cm−2 | |||||
| tind [s] | FC [%] | tind [s] | FC [%] | tind [s] | FC [%] | tind [s] | FC [%] | |
| T1 | 0 | 56 | 1 | 48 | 0 | 61 | 1 | 54 |
| T2 | 3 | 47 | 30 | 35 | 0 | 60 | 3 | 55 |
| T3 | 3 | 56 | 6 | 53 | 0 | 60 | 1 | 55 |
| T4 | 2 | 54 | 14 | 53 | 0 | 54 | 3 | 51 |
| T5 | 3 | 24 | 10 | 20 | 2 | 44 | 19 | 44 |
| DETX | 2 | 52 | 15 | 30 | 1 | 46 | 28 | 25 |
| TPO * | 0 | 68 | 1 | 64 | 0 | 68 | 1 | 64 |
| Concentration of Photoinitiator T1 | tind [s] | FC [%] |
|---|---|---|
| 5.3 × 10−3 mol·dm−3 (0.2%) | 9 | 49 |
| 10.6 × 10−3 mol·dm−3 (0.4%) | 3 | 57 |
| 15.9 × 10−3 mol·dm−3 (0.6%) | 3 | 60 |
| Photo- Sensitizer | Conversion of the Acrylate Monomer TMPTA [%] | |||||
|---|---|---|---|---|---|---|
| One-Component Photoinitiatng System | Two-Component Photoinitiating System | |||||
| with NPG | with NPG | |||||
| @405 nm I0 = 17.24 mW·cm−2 | @420 nm, I0 = 14.87 mW·cm−2 | @405 nm I0 = 17.24 mW·cm−2 | @420 nm, I0 = 14.87 mW·cm−2 | @405 nm I0 = 17.24 mW·cm−2 | @420 nm I0 = 14.87 mW·cm−2 | |
| T1 | 49 | 44 | 53 | 50 | 57 | 53 |
| T2 | 25 | 10 | 50 | 47 | 51 | 56 |
| T3 | 43 | 39 | 47 | 47 | 56 | 52 |
| T4 | 51 | 47 | 48 | 48 | 53 | 53 |
| T5 | - | - | 30 | 27 | 42 | 40 |
| DETX | - | - | 45 | 42 | 50 | 51 |
| Photoinitiating System | Concentration [%] | tind [s] | Final Conversion [%] |
|---|---|---|---|
| T1/EDB/IOD | 0.2%/1.5%/1.0% | 7 | 49 |
| T1/EDB | 0.2%/1.5% | 21 | 44 |
| T1/IOD | 0.2%/1.0% | 14 | 39 |
| T1/NPG | 0.2%/1.17% | 0 | 47 |
| T1/NPG/IOD | 0.2%/1.17%/1.0% | 2 | 57 |
| T1/EDB/TAS | 0.2%/1.5%/2.0% | 10 | 48 |
| T1/TAS | 0.2%/2.0% | 41 | 29 |
| T1/TAS/NPG | 0.2%/2.0%/1.17% | 1 | 55 |
| T1/EBPA | 0.2%/1.5% | 16 | 49 |
| T1/EBPA/EDB | 0.2%/1.5%/1.5% | 9 | 44 |
| T1/EBPA/NPG | 0.2%/1.5%/1.17% | 3 | 53 |
| LOT | EOX [mV] vs. Ag/AgCl | ES1 [eV] | ET1 [eV] | ΔGetS1 vs. IOD [eV] | ΔGetT1 vs. IOD [eV] | ΔGetS1 vs. TAS [eV] | ΔGetT1 vs. TAS [eV] |
|---|---|---|---|---|---|---|---|
| T1 | 1063 | 2.76 | 2.52 | −1.06 | −0.82 | −0.88 | −0.64 |
| T2 | 1319 | 3.03 | 2.61 | −1.07 | −0.65 | −0.90 | −0.48 |
| T3 | 1182 | 2.91 | 2.56 | −1.09 | −0.74 | −0.91 | −0.56 |
| T4 | 1347 | 2.90 | 2.50 | −0.91 | −0.51 | −0.74 | −0.34 |
| T5 | 1353 | 3.00 | 2.59 | −1.01 | −0.60 | −0.83 | −0.42 |
| DETX | 1675 | 3.00 | 2.64 | −0.69 | −0.33 | −0.51 | −0.10 |
| LOT | Ered [mV] vs. Ag/AgCl | ES1 [eV] | ET1 [eV] | ΔGetS1 vs. EDB [eV] | ΔGetT1 vs. EDB [eV] | ΔGetS1 vs. NPG [eV] | ΔGetT1 vs. NPG [eV] |
|---|---|---|---|---|---|---|---|
| T1 | −1602 | 2.76 | 2.52 | −0.07 | 0.17 | −0.03 | 0.21 |
| T2 | −1591 | 3.03 | 2.61 | −0.35 | 0.07 | −0.31 | 0.11 |
| T3 | −1625 | 2.91 | 2.56 | −0.20 | 0.15 | −0.16 | 0.20 |
| T4 | −1528 | 2.90 | 2.50 | −0.28 | 0.12 | −0.24 | 0.16 |
| T5 | - | 3.00 | 2.59 | - | - | - | - |
| DETX | −1770 | 3.00 | 2.64 | −0.14 | 0.22 | −0.10 | 0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hola, E.; Pilch, M.; Ortyl, J. Thioxanthone Derivatives as a New Class of Organic Photocatalysts for Photopolymerisation Processes and the 3D Printing of Photocurable Resins under Visible Light. Catalysts 2020, 10, 903. https://doi.org/10.3390/catal10080903
Hola E, Pilch M, Ortyl J. Thioxanthone Derivatives as a New Class of Organic Photocatalysts for Photopolymerisation Processes and the 3D Printing of Photocurable Resins under Visible Light. Catalysts. 2020; 10(8):903. https://doi.org/10.3390/catal10080903
Chicago/Turabian StyleHola, Emilia, Maciej Pilch, and Joanna Ortyl. 2020. "Thioxanthone Derivatives as a New Class of Organic Photocatalysts for Photopolymerisation Processes and the 3D Printing of Photocurable Resins under Visible Light" Catalysts 10, no. 8: 903. https://doi.org/10.3390/catal10080903
APA StyleHola, E., Pilch, M., & Ortyl, J. (2020). Thioxanthone Derivatives as a New Class of Organic Photocatalysts for Photopolymerisation Processes and the 3D Printing of Photocurable Resins under Visible Light. Catalysts, 10(8), 903. https://doi.org/10.3390/catal10080903