Catalytic Degradation of Nerve Agents
Abstract
1. Introduction
2. Definition of NAs
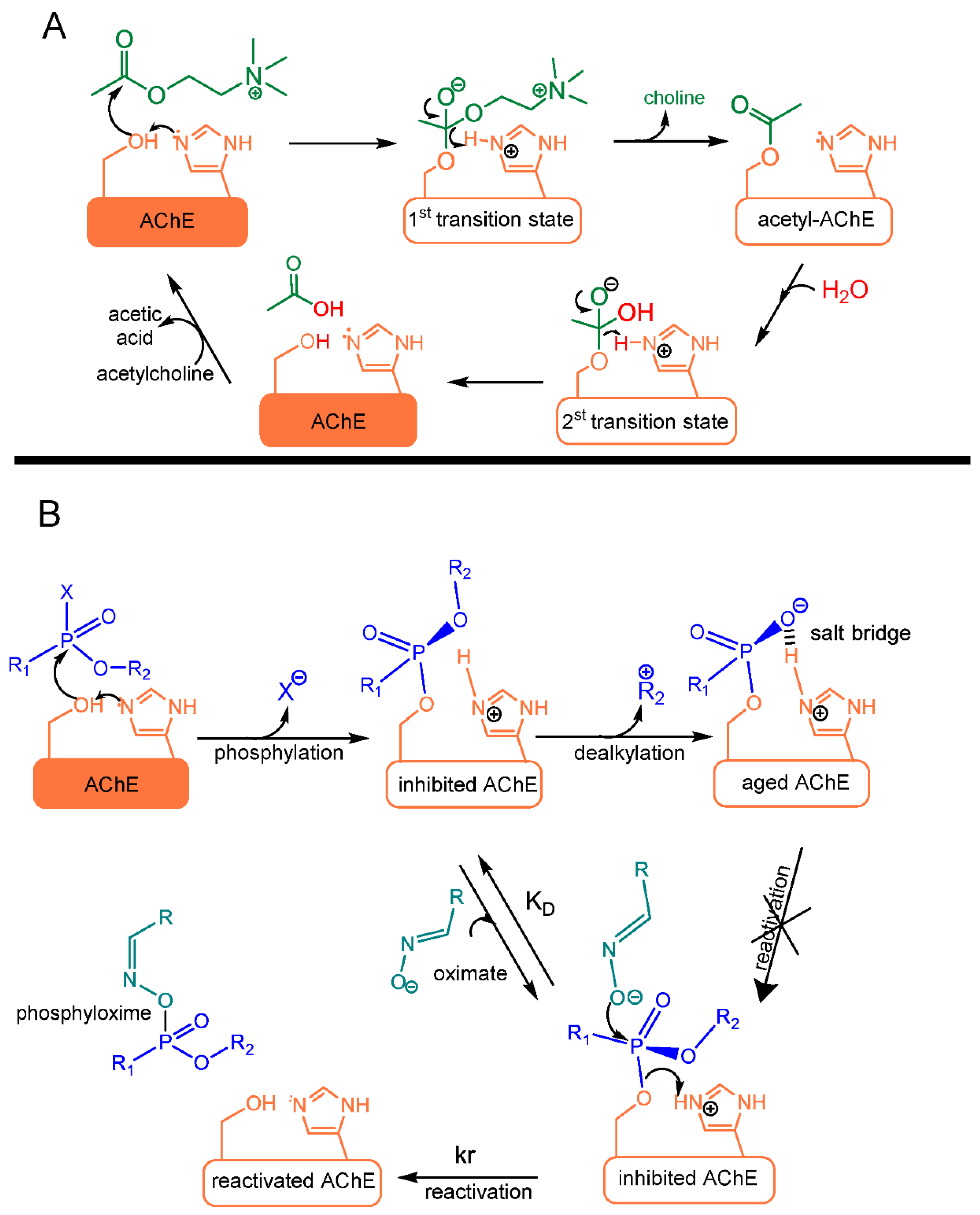
3. Toxicity of NAs
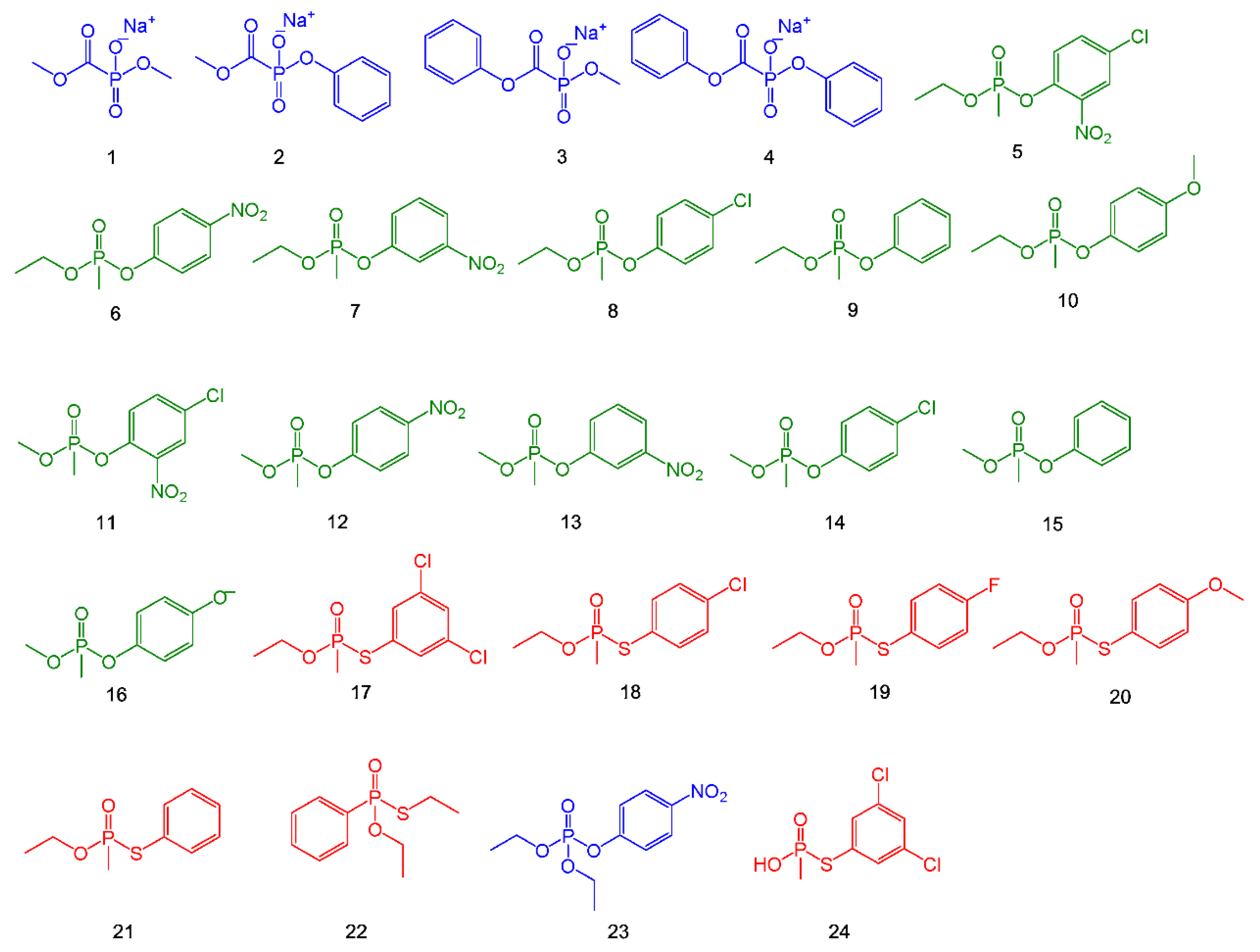
4. Detection of Nerve Agents
5. Chemical Hydrolysis
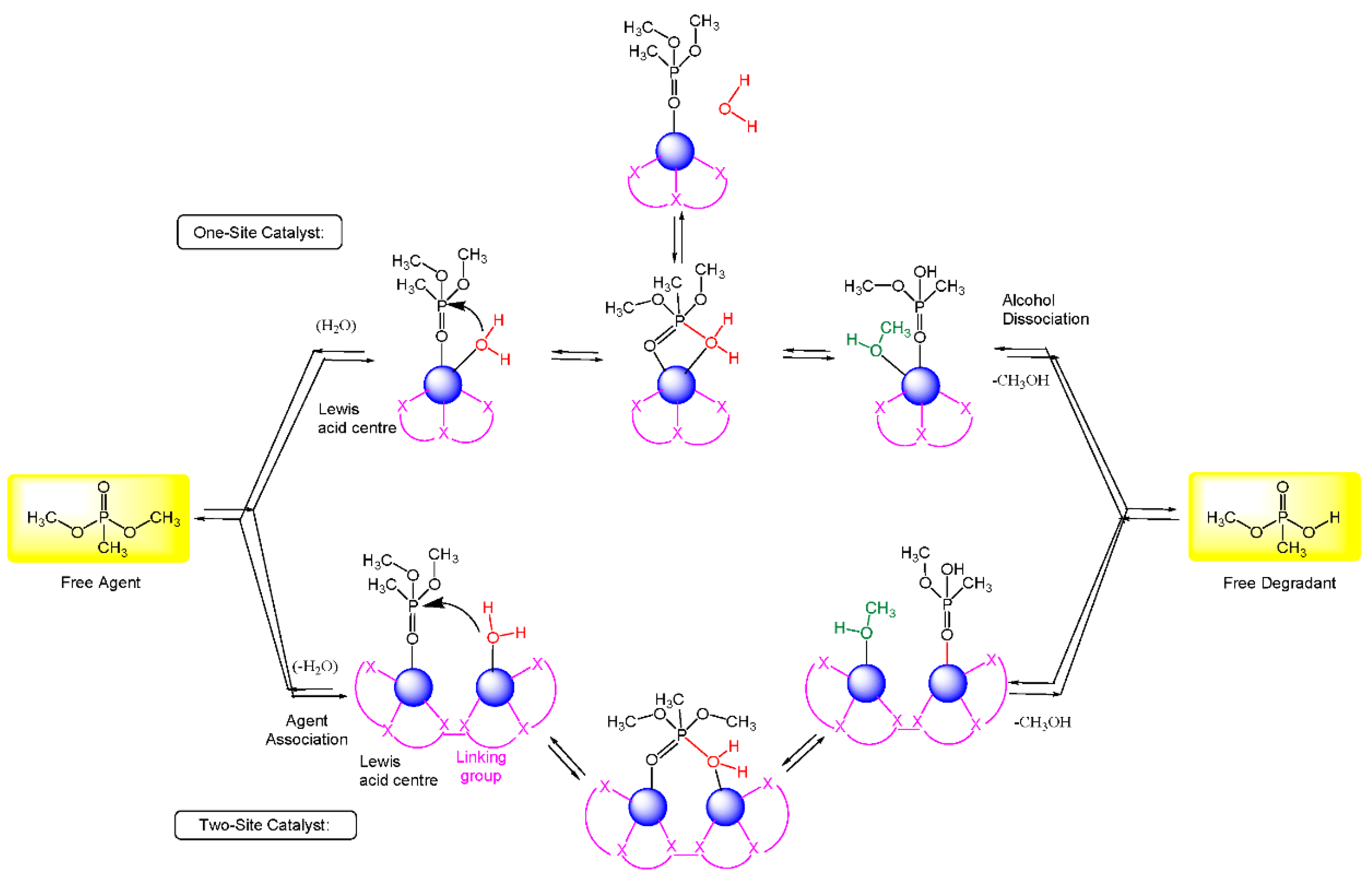
5.1. Metal-Catalyzed Hydrolysis
5.2. Polymeric/Heterogeneous Hydrolysis
5.3. Enzymatic Hydrolysis
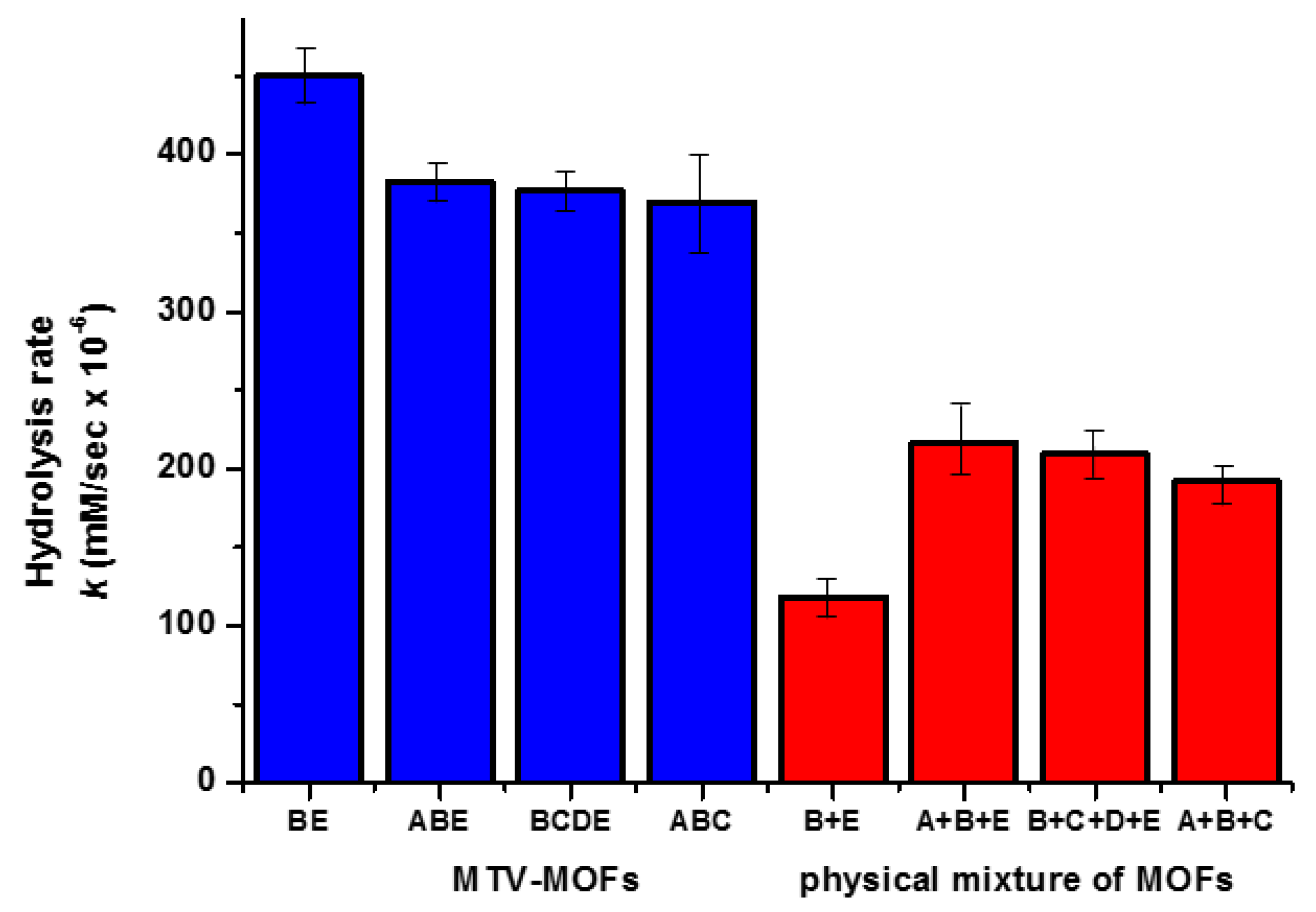
5.4. Hydrolysis by MOFs
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stone, R.U.K. Attack puts nerve agent in the spotlight. Science 2018, 359, 1314–1315. [Google Scholar] [CrossRef] [PubMed]
- Stone, R. How to defeat a nerve agent. Science 2018, 359, 23. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Fox, S.C. Acute and Long-Term Impact of Chemical Weapons: Lessons from the Iran-Iraq War. Forensic Sci. Rev. 2014, 26, 97–114. [Google Scholar] [PubMed]
- Seto, Y.; Sekiguchi, H.; Maruko, H.; Yamashiro, S.; Sano, Y.; Takayama, Y.; Sekioka, R.; Yamaguchi, S.; Kishi, S.; Satoh, T.; et al. Sensitive and Comprehensive Detection of Chemical Warfare Agents in Air by Atmospheric Pressure Chemical Ionization Ion Trap Tandem Mass Spectrometry with Counterflow Introduction. Anal. Chem. 2014, 86, 4316–4326. [Google Scholar] [CrossRef]
- Bizzigotti, G.O.; Castelly, H.; Hafez, A.M.; Smith, W.H.B.; Whitmire, M.T. Parameters for Evaluation of the Fate, Transport, and Environmental Impacts of Chemical Agents in Marine Environments. Chem. Rev. 2009, 109, 236–256. [Google Scholar] [CrossRef]
- Valdez, C.A.; Leif, R.N.; Hok, S.; Hart, B.R. Analysis of chemical warfare agents by gas chromatography-mass spectrometry: Methods for their direct detection and derivatization approaches for the analysis of their degradation products. Rev. Anal. Chem. 2018, 37, 20170007. [Google Scholar] [CrossRef]
- Pacsial-Ong, E.J.; Aguilar, Z.P. Chemical warfare agent detection: A review of current trends and future perspective. Front. Biosci. 2013, 5, 516–543. [Google Scholar] [CrossRef]
- Picard, B.; Chataigner, I.; Maddaluno, J.; Legros, J. Introduction to chemical warfare agents, relevant simulants and modern neutralisation methods. Org. Biomol. Chem. 2019, 17, 6528–6537. [Google Scholar] [CrossRef]
- Liu, Y.; Howarth, A.J.; Vermeulen, N.A.; Moon, S.-Y.; Hupp, J.T.; Farha, O.K. Catalytic degradation of chemical warfare agents and their simulants by metal-organic frameworks. Coord. Chem. Rev. 2017, 346, 101–111. [Google Scholar] [CrossRef]
- Vellingiri, K.; Philip, L.; Kim, K. –H. Metal-organic frameworks as media for the catalytic degradation of chemical warfare agents. Coord. Chem. Rev. 2017, 353, 159–179. [Google Scholar] [CrossRef]
- Islamoglu, T.; Chen, Z.; Wasson, M.C.; Buru, C.T.; Kirlikovali, K.O.; Afrin, U.; Mian, M.R.; Farha, O.K. Metal-Organic Frameworks against Toxic Chemicals. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kirlikovali, K.O.; Chen, Z.; Islamoglu, T.; Hupp, J.T.; Farha, O.K. Zirconium-Based Metal—Organic Frameworks for the Catalytic Hydrolysis of Organophosphorus Nerve Agents. ACS Appl. Mater. Interfaces 2020, 12, 14702–14720. [Google Scholar] [CrossRef] [PubMed]
- Manco, G.; Porzio, E.; Suzumoto, Y. Enzymatic detoxification: A sustainable means of degrading toxic organophosphate pesticides and chemical warfare nerve agents. J. Chem. Technol. Biotechnol. 2018, 93, 2064–2082. [Google Scholar] [CrossRef]
- Singh, B.; Prasad, G.K.; Pandey, K.S.; Danikhel, R.K.; Vijayaraghavan, R. Decontamination of chemical warfare age. Def. Sci. J. 2010, 60, 428–441. [Google Scholar] [CrossRef]
- Wagner, G.W. Decontamination of Chemical Warfare Agents Using Household Chemica. Ind. Eng. Chem. Res. 2011, 50, 12285–12287. [Google Scholar] [CrossRef]
- Costanzi, S.; Machado, J.-H.; Mitchell, M. Nerve Agents: What They Are, How They Work, How to Counter Them. ACS Chem. Neurosci. 2018, 9, 873–885. [Google Scholar] [CrossRef]
- Vale, J.A.; Marrs, T.C.; Maynard, R.L. Novichok: A murderous nerve agent attack in the UK. Clin. Toxicol. 2018, 56, 1096–1097. [Google Scholar] [CrossRef]
- Kloske, M.; Witkiewicz, Z. Novichoks—The A group of organophosphorus chemical warfare agents. Chemosphere 2019, 221, 672–682. [Google Scholar] [CrossRef]
- Subramanyam, C.; Ramana, K.V.; Rasheed, S.; Adam, S.; Raju, C. Naga. Synthesis and Biological Activity of Novel Diphenyl N-Substituted Carbamimidoylphosphoramidate Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 1228–1235. [Google Scholar] [CrossRef]
- Kuca, K.; Jun, D.; Musilek, K.; Bajgar, J. Reactivators of tabun-inhibited acetylcholinesterase: Structure-biological activity relationship. Front. Drug Des. Discov. 2007, 3, 381–394. [Google Scholar]
- Abou-Donia, M.B.; Siracuse, B.; Gupta, N.; Sobel Sokol, A. Sarin (GB, O-isopropyl methylphosphonofluoridate) neurotoxicity: Critical review. Crit. Rev. Toxicol. 2016, 46, 845–875. [Google Scholar] [CrossRef] [PubMed]
- van Helden, H.P.M.; Joosen, M.J.A.; Philippens, I.H.C. Non-enzymatic pretreatment of nerve agent (soman) poisoning: A brief state-of-the-art review. Toxicol. Lett. 2011, 206, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Krejcova, G.; Kuca, K.; Sevelova, L. Cyclosarin. An organophosphate nerve agent. Def. Sci. J. 2005, 55, 105–115. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Webster, R.L.; Micich, E.; McDowall, L.J.; McGill, N.W.; Williams, J.; Zanatta, S.D. The identification of chemical attribution signatures of stored VX nerve agents using NMR, GC-MS, and LC-HRM. Talanta 2020, 211, 120753. [Google Scholar] [CrossRef]
- Ghanem, E.; Li, Y.; Xu, C.; Raushel, F.M. Characterization of a Phosphodiesterase Capable of Hydrolyzing EA 2192, the Most Toxic Degradation Product of the Nerve Agent VX. Biochemistry 2007, 46, 9032–9040. [Google Scholar] [CrossRef]
- Witkiewicz, Z.; Neffe, S.; Sliwka, E.; Quagliano, J. Analysis of the precursors, simulants and degradation products of chemical warfare agents. Crit. Rev. Anal. Chem. 2018, 48, 337–371. [Google Scholar] [CrossRef]
- Agrawal, M.; Gallis, D.F.S.; Greathouse, J.A.; Sholl, D. How Useful Are Common Simulants of Chemical Warfare Agents at Predicting Adsorption Behavior? J. Phys. Chem. C 2018, 122, 26061–26069. [Google Scholar] [CrossRef]
- Tu, A.T. Aum Shinrikyo’s Chemical and Biological Weapons: More Than Sarin. Forensic Sci. Rev. 2014, 26, 115–120. [Google Scholar]
- Somani, S.M.; Solana, R.P.; Dube, S.N. Toxicodynamics of nerve agents. In Chemical Warfare Agents; Somani, S.M., Ed.; Academic Press: Cambridge, MA, USA, 1992; pp. 67–123. [Google Scholar]
- Spradling, K.D.; Dillman, J.F. The molecular toxicology of chemical warfare nerve agents. In Advances in Molecular Toxicology; James, C.F., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; pp. 111–144. [Google Scholar]
- Mercey, G.; Verdelet, T.; Renou, J.; Kliachina, M.; Baati, R.; Nachon, F.; Jean, L.; Renard, P.-Y. Acc. Chem. Res. 2012, 45, 756–766. [CrossRef]
- Ekström, F.; Hörnberg, A.; Artursson, E.; Hammarström, L.G.; Schneider, G.; Pang, Y.P. Structure of HI-6 *sarin-acetylcholinesterase determined by X-ray crystallography and molecular dynamics simulation: Reactivator mechanism and design. PLoS ONE 2009, 4, e5957. [Google Scholar] [CrossRef]
- Rupa, I.; Brian, I.; Alex, L. Developments in alternative treatments for organophosphate poisoning. Toxicol. Lett. 2015, 233, 200–206. [Google Scholar]
- Sidell, F.R. Soman and Sarin: Clinical manifestations and treatment of accidental poisoning by organophosphates. Clin Toxicol. 1974, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sidell, F.R.; Groff, W.A. The reactivatibility of cholinesterase inhibited by VX and Sarin in man. Toxicol. Appl. Pharmacol. 1974, 27, 241–252. [Google Scholar] [CrossRef]
- Sidell, F.R.; Borak, J. Chemical warfare agents: II. Nerve agents. Ann. Emerg. Med. 1992, 21, 865–871. [Google Scholar] [CrossRef]
- Noort, D.; Benschop, H.P.; Black, R.M. Biomonitoring of Exposure to Chemical Warfare Agents: A Review. Toxicol. Appl. Pharm. 2002, 184, 116–126. [Google Scholar] [CrossRef]
- Ghanei, M.; Fathi, H.; Mohammad, M.M.; Aslani, J.; Nematizadeh, F. Long-Term Respiratory Disorders of Claimers with Subclinical Exposure to Chemical Warfare Agents. Inhal. Toxicol. 2004, 16, 491–495. [Google Scholar] [CrossRef]
- Wiener, S.W.; Hoffman, R.S. Nerve Agents: A Comprehensive Review. J. Intensive Care Med. 2004, 19, 22–37. [Google Scholar] [CrossRef]
- Baygildiev, T.; Vokuev, M.; Ogorodnikov, R.; Braun, A.; Rybalchenko, I.; Rodin, I. Simultaneous determination of organophosphorus nerve agent markers in urine by IC-MS/MS using anion-exchange solid-phase extraction. J. Chrom. B 2019, 1132, 121815. [Google Scholar] [CrossRef]
- B’Hymer, C. A brief overview of HPLC-MS analysis of alkyl methylphosphonic acid degradation products of nerve agents. J. Chrom. Sci. 2019, 57, 606–617. [Google Scholar] [CrossRef]
- Kim, H.; Cho, Y.; Lee, B.S.; Choi, I.S. In-situ derivatization and headspace solid-phase microextraction for gas chromatography-mass spectrometry analysis of alkyl methylphosphonic acids following solid-phase extraction using thin film. J. Chrom. A 2019, 1599, 17–24. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Update 1 of: Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2015, 115, PR1–PR76. [Google Scholar] [CrossRef]
- Chen, L.; Wu, D.; Yoon, J. Recent Advances in the Development of Chromophore-Based Chemosensors for Nerve Agents and Phosgene. ACS Sens. 2018, 3, 27–43. [Google Scholar] [CrossRef]
- Dey, N.; Jha, S.; Bhattacharya, S. Visual detection of a nerve agent simulant using chemically modified paper strips and dye-assembled inorganic nanocomposite. Analyst 2018, 143, 528–535. [Google Scholar] [CrossRef]
- Sambrook, M.R.; Notman, S. Supramolecular chemistry and chemical warfare agents: From fundamentals of recognition to catalysis and sensing. Chem. Soc. Rev. 2013, 42, 9251–9267. [Google Scholar] [CrossRef] [PubMed]
- Border, S.E.; Pavlovic, R.Z.; Zhiquan, L.; Badjic, J.D. Removal of Nerve Agent Simulants from Water Using Light-Responsive Molecular Baskets. J. Am. Chem. Soc. 2017, 139, 18496–18499. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ruan, Y.; Brown, J.D.; Hadad, C.M.; Badjic, J.D. Recognition Characteristics of an Adaptive Vesicular Assembly of Amphiphilic Baskets for Selective Detection and Mitigation of Toxic Nerve Agents. J. Am. Chem. Soc. 2014, 136, 17337–17342. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Dalkilic, E.; Peterson, P.W.; Pandit, A.; Dastan, A.; Brown, J.D.; Polen, S.M.; Hadad, C.M.; Badjic, J.D. Trapping of organophosphorus chemical nerve agents in water with amino acid functionalized baskets. Chem. Eur. J. 2014, 20, 4251–4256. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ruan, Y.; Brown, J.D.; Gallucci, J.; Maslak, V.; Hadad, C.M.; Badjic, J.D. Assembly of Amphiphilic Baskets into Stimuli-Responsive Vesicles. Developing a Strategy for the Detection of Organophosphorus Chemical Nerve Agents. J. Am. Chem. Soc. 2013, 135, 14964–14967. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Lee, M.; Seo, H.O.; Song, S.G.; Kim, K.; Park, C.H.; Kim, I.L.; Kim, Y.D.; Song, C. Structural Effect of Thioureas on the Detection of Chemical Warfare Agent Simulants. ACS Sens. 2017, 2, 1146–1151. [Google Scholar] [CrossRef]
- Hiscock, J.R.; Piana, F.; Sambrook, M.R.; Wells, N.J.; Clark, A.J.; Vincent, J.C.; Busschaert, N.; Brown, R.C.D.; Gale, P.A. Detection of nerve agent via perturbation of supramolecular gel formation. Chem. Commun. 2013, 49, 9119–9121. [Google Scholar] [CrossRef]
- Esipenko, N.A.; Koutnik, P.; Minami, T.; Mosca, L.; Lynch, V.M.; Zyryanov, G.V.; Anzenbacher, P., Jr. First supramolecular sensors for phosphonate anions. Chem. Sci. 2013, 4, 3617–3623. [Google Scholar] [CrossRef]
- Hiscock, J.R.; Sambrook, M.R.; Wells, N.J.; Gale, P.A. Detection and remediation of organophosphorus compounds by oximate containing organogels. Chem. Sci. 2015, 6, 5680–5684. [Google Scholar] [CrossRef]
- Ishihara, S.; Azzarelli, J.M.; Krikorian, M.; Swager, T.M. Ultratrace Detection of Toxic Chemicals: Triggered Disassembly of Supramolecular Nanotube Wrappers. J. Am. Chem. Soc. 2016, 138, 8221–8227. [Google Scholar] [CrossRef]
- Hiscock, J.R.; Wells, N.J.; Ede, J.A.; Gale, P.A.; Sambrook, M.R. Biasing hydrogen bond donating host systems towards chemical warfare agent recognition. Org. Biomol. Chem. 2016, 14, 9560–9567. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.K.; Ha, S.; Woo, T.G.; Kim, Y.D.; Song, C.; Kim, S.K. Binding thiourea derivatives with dimethyl methylphosphonate for sensing nerve agents. RSC Adv. 2019, 9, 10693–10701. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, C.S.; Park, S.J.; Noh, S.; Kim, S.; Kong, H.J.; Bae, J.; Lee, C.-S.; Yoon, H. Carboxylic Acid-Functionalized Conducting-Polymer Nanotubes as Highly Sensitive Nerve-Agent Chemiresistors. Sci. Rep. 2016, 6, 33724. [Google Scholar] [CrossRef]
- Fennell, J.F., Jr.; Hamaguchi, H.; Yoon, B.; Swager, T.M. Chemiresistor Devices for Chemical Warfare Agent Detection Based on Polymer Wrapped Single-Walled Carbon Nanotubes. Sensors 2017, 17, 982. [Google Scholar] [CrossRef]
- Pappalardo, A.; Amato, M.E.; Ballistreri, F.P.; La Paglia Fragola, V.; Tomaselli, G.A.; Toscano, R.M.; Sfrazzetto, G.T. Binding of reactive organophosphate by oximes via hydrogen bond. J. Chem. Sci. 2013, 125, 869–873. [Google Scholar] [CrossRef]
- Sfrazzetto, G.T.; Millesi, S.; Pappalardo, A.; Tomaselli, G.A.; Ballistreri, F.P.; Toscano, R.M.; Fragalà, I.; Gulino, A. Nerve Gas Simulant Sensing by a Uranyl–Salen Monolayer Covalently Anchored on Quartz Substrates. Chem. Eur. J. 2017, 23, 1576–1583. [Google Scholar] [CrossRef]
- Puglisi, R.; Mineo, P.G.; Pappalardo, A.; Gulino, A.; Sfrazzetto, G.T. Supramolecular Detection of a Nerve Agent Simulant by Fluorescent Zn–Salen Oligomer Receptors. Molecules 2019, 24, 2160. [Google Scholar] [CrossRef]
- Puglisi, R.; Pappalardo, A.; Gulino, A.; Sfrazzetto, G.T. Supramolecular recognition of a CWA simulant by metal–salen complexes: The first multi-topic approach. Chem. Comm. 2018, 54, 11156–11159. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, R.; Pappalardo, A.; Gulino, A.; Sfrazzetto, G.T. Multitopic Supramolecular Detection of Chemical Warfare Agents by Fluorescent Sensors. ACS Omega 2019, 4, 7550–7555. [Google Scholar] [CrossRef]
- Legnani, L.; Puglisi, R.; Pappalardo, A.; Chiacchio, M.A.; Sfrazzetto, G.T. Supramolecular recognition of phosphocholine by an enzyme-like cavitand receptor. Chem. Commun. 2020, 56, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.A.; Morales-Rojas, H.; Vijayaraghavan, S.; Tian, J.Z. Metal-Cation-Mediated Hydrolysis of Phosphonoformate Diesters: Chemoselectivity and Catalysis. J. Am. Chem. Soc. 2004, 126, 10923–10963. [Google Scholar] [CrossRef]
- Lewis, R.E.; Neverov, A.A.; Brown, R.S. Mechanistic studies of La3+ and Zn2+-catalyzed methanolysis of O-ethyl O-aryl methylphosphonate esters. An effective solvolytic method for the catalytic destruction of phosphonate CW simulants. Org. Biomol. Chem. 2005, 3, 4082–4088. [Google Scholar] [CrossRef]
- Melnychuk, S.A.; Neverov, A.A.; Brown, R.S. Catalytic decomposition of simulants for chemical warfare V agents: Highly efficient catalysis of the methanolysis of phosphonothioate esters. Angew. Chem., Int. Ed. 2006, 45, 1767–1770. [Google Scholar] [CrossRef]
- Kuo, L.Y.; Adint, T.T.; Akagi, A.E.; Zakharov, L. Degradation of a VX Analogue: First Organometallic Reagent To Promote Phosphonothioate Hydrolysis Through Selective P-S Bond Scission. Organometallics 2008, 27, 2560–2564. [Google Scholar] [CrossRef]
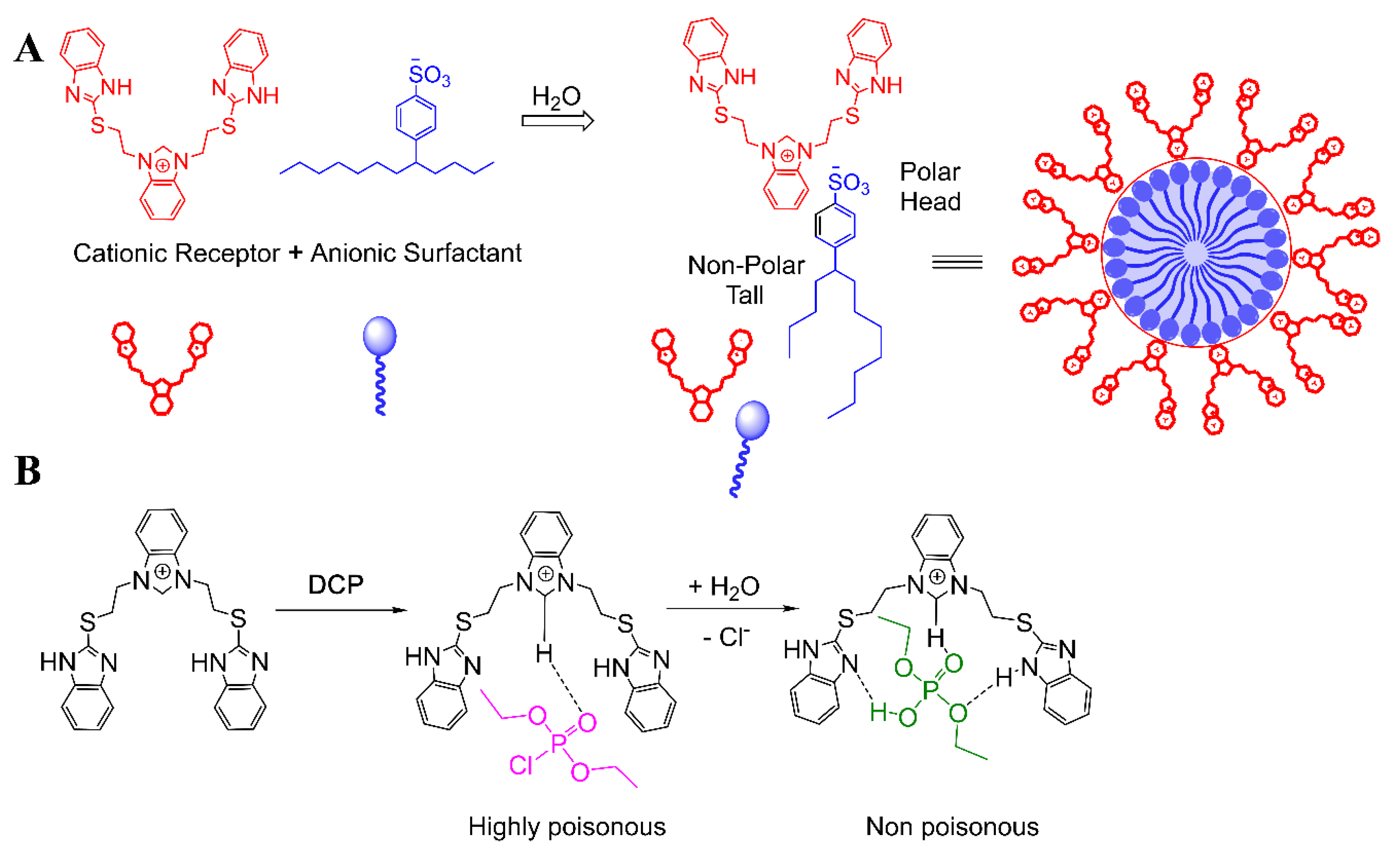
- Singh, A.; Raj, P.; Singh, N. Benzimidazolium-Based Self-Assembled Fluorescent Aggregates for Sensing and Catalytic Degradation of Diethylchlorophosphate. ACS Appl. Mater. Interfaces 2016, 8, 28641–28651. [Google Scholar] [CrossRef]
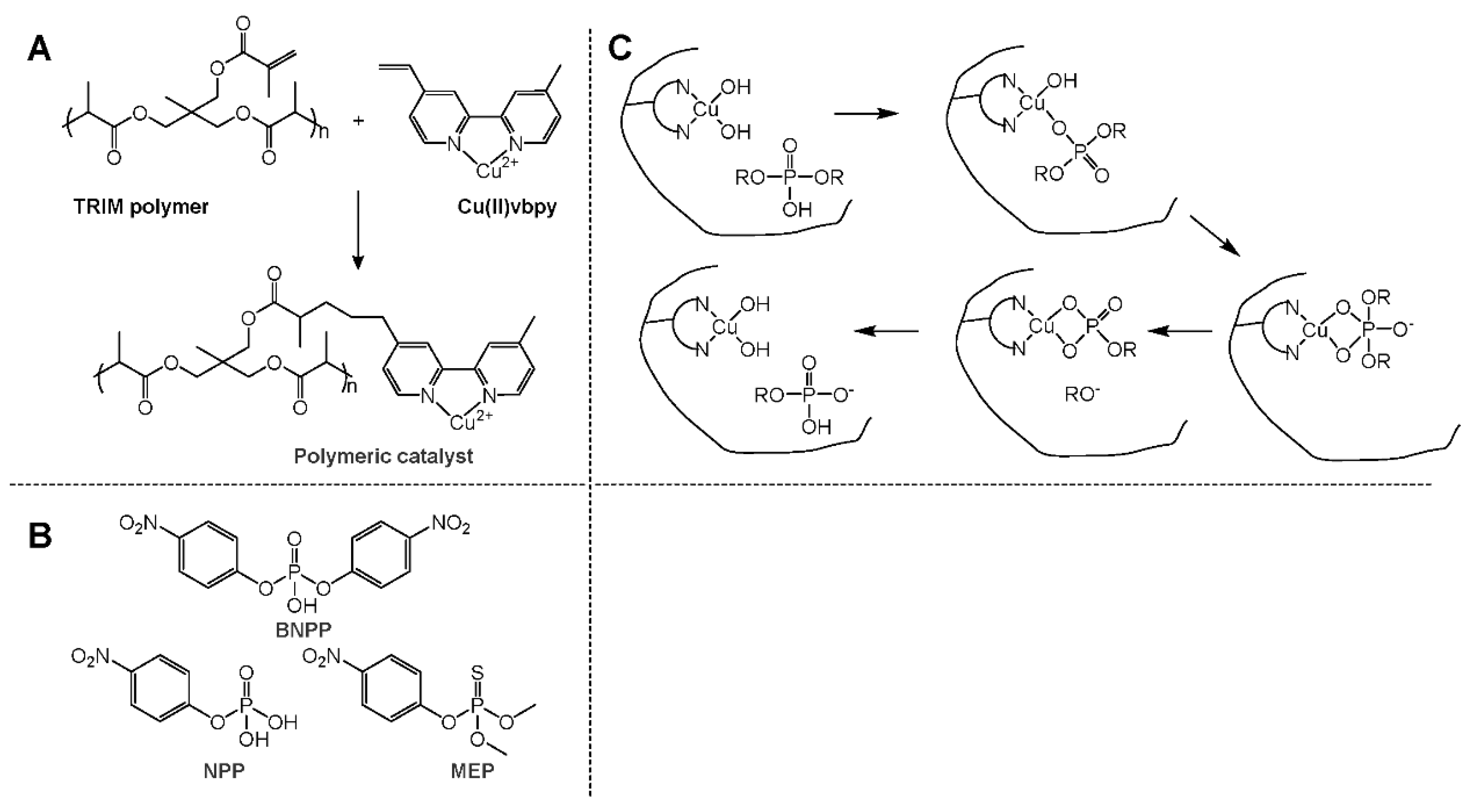
- Hartshorn, C.M.; Singh, A.; Chang, E.L. Metal-chelator polymers as organophosphate hydrolysis catalys. J. Mater. Chem. 2002, 12, 602–605. [Google Scholar] [CrossRef]
- Andrea, T.; Neverov, A.A.; Brown, R.S. Efficient Methanolytic Cleavage of Phosphate, Phosphonate, and Phosphonothioate Esters Promoted by Solid Supported Lanthanide Ions. Ind. Eng. Chem. Res. 2010, 49, 7027–7033. [Google Scholar] [CrossRef]
- Desloges, W.; Neverov, A.A.; Brown, R.S. Zn2+-Catalyzed Methanolysis of Phosphate Triesters: A Process for Catalytic Degradation of the Organophosphorus Pesticides Paraoxon and Fenitrothion. Inorg. Chem. 2004, 43, 6752–6761. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.M. Catalytic methods for the destruction of chemical warfare agents under ambient conditions. Chem. Soc. Rev. 2008, 37, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.J.; Erbeldinger, M.; DeFrank, J.J.; Kaar, J.; Drevon, G. Catalytic buffers enable positive-response inhibition-based sensing of nerve agents. Biotechnol. Bioeng. 2002, 77, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Suye, S.-I.; Kuroda, K.; Ueda, M.; Kitaguchi, T.; Tsuchiyama, K.; Fukuda, T.; Chen, W.; Mulchandani, A. Surface Display of Organophosphorus Hydrolase on Saccharomyces cerevisiae. Biotechnol. Prog. 2006, 22, 939–943. [Google Scholar] [CrossRef]
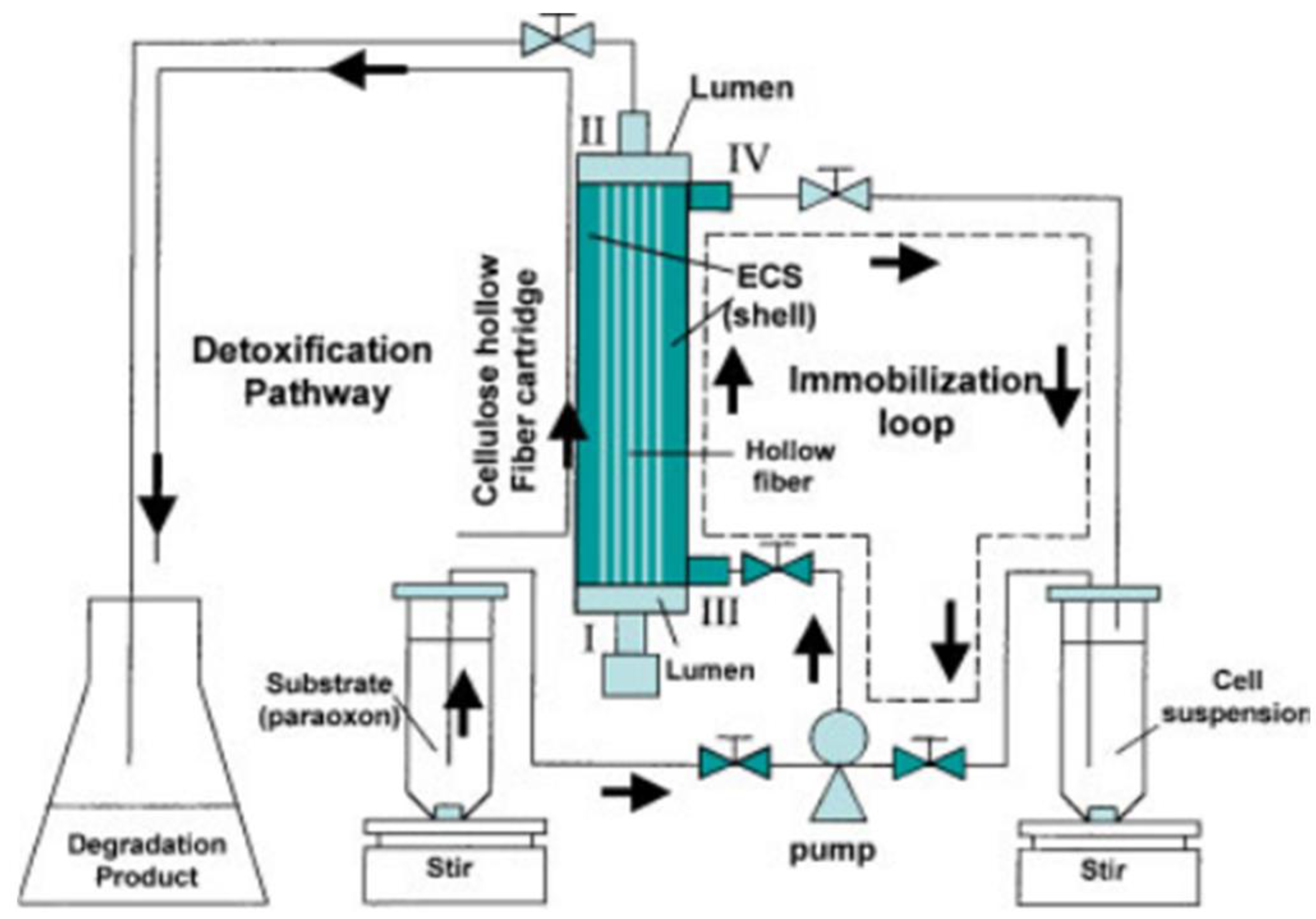
- Wang, A.A.; Chen, W.; Mulchandani, A. Detoxification of organophosphate nerve agents by immobilized dual functional biocatalysts in a cellulose hollow fiber bioreactor. Biotechnol. Bioeng. 2005, 91, 379–386. [Google Scholar] [CrossRef]
- Dumas, D.P.; Durst, H.D.; Landis, W.G.; Raushel, F.M.; Wild, J.R. Inactivation of organophosphorus nerve agents by the phosphotriesterase from Pseudomonas diminuta. Arch. Biochem. Biophys. 1990, 277, 155–159. [Google Scholar] [CrossRef]
- Kolakowski, J.E.; Defrank, J.J.; Harvey, S.P.; Szafraniec, L.L.; Beaudry, W.T.; Lai, K.; Wild, J.R. Enzymatic hydrolysis of the chemical warfare agent VX and its neurotoxic analogues by organophosphorus hydrolase. Biocatal. Biotransform 1997, 15, 297–312. [Google Scholar] [CrossRef]
- Mackness, M.I.; Arrol, S.; Durrington, P. Substrate specificity of human serum paraoxonase. Biochem. Soc Trans. 1991, 19, 304S. [Google Scholar] [CrossRef]
- Furlong, C.E.; Richter, R.J.; Chapline, C.; Crabb, J.W. Purification of rabbit and human serum paraoxonase. Biochemistry 1991, 30, 10133–10140. [Google Scholar] [CrossRef]
- Li, W.F.; Furlong, C.E.; Costa, L.G. Paraoxonase protects against chlorpyrifos toxicity in mice. Toxicol. Lett. 1995, 76, 219–226. [Google Scholar] [CrossRef]
- Hoskin, F.C. Diisopropylphosphorofluoridate and Tabun: Enzymatic hydrolysis and nerve function. Science 1971, 172, 1243–1245. [Google Scholar] [CrossRef] [PubMed]
- Merone, L.; Mandrich, L.; Porzio, E.; Rossi, M.; Muller, S.; Reiter, G.; Worek, F.; Manco, G. Improving the promiscuous nerve agent hydrolase activity of a thermostable archaeal lactonase. Bioresour. Technol. 2010, 101, 9204–9212. [Google Scholar] [CrossRef] [PubMed]
- Motkuri, R.K.; Thallapally, P.K.; Annapureddy, H.V.; Dang, L.X.; Krishna, R.; Nune, S.K.; Fernandez, C.A.; Liu, J.; McGrail, B.P. Separation of polar compounds using a flexible metal-organic framework. Chem. Commun. 2015, 51, 8421–8424. [Google Scholar] [CrossRef] [PubMed]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef]
- Motkuri, R.K.; Thallapally, P.K.; Nune, S.K.; Fernandez, C.A.; McGrail, B.P.; Atwood, J.L. Role of hydrocarbons in pore expansion and contraction of a flexible metalorganic framework. Chem. Commun. 2011, 47, 7077–7079. [Google Scholar] [CrossRef]
- Wilmer, C.E.; Farha, O.K.; Yildirim, T.; Eryazici, I.; Krungleviciute, V.; Sarjeant, A.A.; Snurr, R.Q.; Hupp, J.T. Gram-scale, High-yield Synthesis of a Robust Metal–Organic Framework for Storing Methane and Other Gases. Energ. Environ. Sci. 2013, 6, 1158–1163. [Google Scholar] [CrossRef]
- Motkuri, R.K.; Annapureddy, H.V.R.; Vijaykumar, M.; Schaef, H.T.; Martin, P.F.; McGrail, B.P.; Dang, L.X.; Krishna, R.; Thallapally, P.K. Fluorocarbon adsorption in hierarchical porous frameworks. Nat. Commun. 2014, 5, 4368. [Google Scholar] [CrossRef]
- An, J.; Farha, O.K.; Hupp, J.T.; Pohl, E.; Yeh, J.I.; Rosi, N.L. Metal-adeninate vertices for the construction of an exceptionally porous metal-organic framework. Nat. Commun. 2012, 3, 604. [Google Scholar] [CrossRef]
- Motkuri, R.K.; Thallapally, P.K.; McGrail, B.P.; Ghorishi, S.B. Dehydrated Prussian Blues for CO2 Storage and Separation Applications. CrystEngComm 2010, 12, 4003–4006. [Google Scholar] [CrossRef]
- Thallapally, P.K.; Motkuri, R.K.; Fernandez, C.A.; McGrail, B.P.; Behrooz, G.S. Prussian blue analogues for CO2 and SO2 capture and separation applications. Inorg. Chem. 2010, 49, 4909–4915. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Xamena, F.X.L.I. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, Y.; Zapata, F.; Lin, G.; Qian, G.; Lobkovsky, E.B. Luminescent Open Metal Sites within a Metal–Organic Framework for Sensing Small Molecules. Adv. Mater. 2007, 19, 1693–1696. [Google Scholar] [CrossRef]
- Chen, B.L.; Xiang, S.C.; Qian, G.D. Metal-Organic Frameworks with Functional Pores for Recognition of Small Molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- DeCoste, J.B.; Peterson, G.W. Metal-organic frameworks for air purification of toxic chemicals. Chem. Rev. 2014, 114, 5695–5727. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J.A. Toxic gas removal--metal-organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef]
- López-Maya, E.; Montoro, C.; Rodríguez-Albelo, L.M.; Cervantes, S.D.A.; Lozano-Pérez, A.A.; Cenis, J.L.; Barea, E.; Navarro, J.A.R. Textile/Metal-organic-framework composites as self-detoxifying filters for chemical-warfare agents. Angew. Chem. Int. Ed. 2015, 54, 6790–6794. [Google Scholar] [CrossRef]
- Bromberg, L.; Klichko, Y.; Chang, E.P.; Speakman, S.; Straut, C.M.; Wilusz, E.; Hatton, T.A. Alkylaminopyridine-modified aluminum aminoterephthalate metal-organic frameworks as components of reactive self-detoxifying materials. ACS Appl. Mater. Interfaces 2012, 4, 4595–4602. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Micropor. Mesopor. Mat. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Isley, W.C.; Ghosh, P.; Liao, P.L.; Bury, W.; Wagner, G.W.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W.; et al. Destruction of chemical warfare agents using metal–organic frameworks. Nat. Mater. 2015, 14, 512–516. [Google Scholar] [CrossRef]
- Greathouse, J.A.; Ockwig, N.W.; Criscenti, L.J.; Guilinger, T.R.; Pohl, P.; Allendorf, M.D. Computational screening of metal-organic frameworks for large-molecule chemical sensing. Phys. Chem. Chem. Phys. 2012, 12, 12621–12629. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.K.; Siberio-Perez, D.Y.; Kim, J.; Go, Y.B.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.B.T.; Hupp, J.T. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar]
- Motkuri, R.K.; Liu, J.; Fernandez, C.A.; Nune, S.K.; Thallapally, P.; McGrail, B.P. Metal-Organic Frameworks -Synthesis and Applications. In Chapter 3 in Industrial Catalysis and Separations: Innovations for Process Intensification; Apple Academic Press: Waretown, NJ, USA, 2014; pp. 61–103. [Google Scholar]
- Feng, D.; Wang, K.; Su, J.; Liu, T.-F.; Park, J.; Wei, Z.; Bosch, M.; Yakovenko, A.; Zou, X.; Zhou, H.-C. A highly stable zeotype mesoporous zirconium metal-organic framework with ultralarge pores. Angew. Chem. Int. Ed. 2015, 54, 149–154. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, J.; Ahn, W.S. Synthesis of metal-organic frameworks: A mini review. Korean, J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef]
- Conato, M.T.; Oleksiak, M.D.; McGrail, B.P.; Motkuri, R.K.; Rimer, J.D. Framework stabilization of Si-rich LTA zeolite prepared in organic-free media. Chem. Commun. 2015, 51, 269–272. [Google Scholar] [CrossRef]
- Katz, M.J.; Mondloch, J.E.; Totten, R.K.; Park, J.K.; Nguyen, S.B.T.; Farha, O.K.; Hupp, J.T. Simple and compelling biomimetic metal-organic framework catalyst for the degradation of nerve agent simulants. Angew. Chem. Int. Ed. 2014, 53, 497–501. [Google Scholar] [CrossRef]
- Peterson, G.W.; Wagner, G.W. Detoxification of chemical warfare agents by CuBTC. J. Porous Mater. 2014, 21, 121–126. [Google Scholar] [CrossRef]
- Nunes, P.; Gomes, A.C.; Pillinger, M.; Goncalves, I.S.; Abrantes, M. Promotion of phosphoester hydrolysis by the ZrIV-based metal-organic framework UiO-67. Micropor. Mesopor. Mat. 2015, 208, 21–29. [Google Scholar] [CrossRef]
- Peterson, G.W.; Moon, S.-Y.; Wagner, G.W.; Hall, M.G.; DeCoste, J.B.; Hupp, J.T.; Farha, O.K. Tailoring the Pore Size and Functionality of UiO-Type Metal-Organic Frameworks for Optimal Nerve Agent Destruction. Inorg. Chem. 2015, 54, 9684–9686. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ma, K.; Mahle, J.J.; Wang, H.; Syed, Z.H.; Atilgan, A.; Chen, Y.; Xin, J.H.; Islamoglu, T.; Peterson, G.W.; et al. Integration of Metal-Organic Frameworks on Protective Layers for Destruction of Nerve Agents under Relevant Conditions. J. Am. Chem. Soc. 2019, 141, 20016–20021. [Google Scholar] [CrossRef] [PubMed]
- Kalaj, M.; Palomba, J.M.; Bentz, K.C.; Cohen, S.M. Multiple functional groups in UiO-66 improve chemical warfare agent simulant degradation. Chem. Commun. 2019, 55, 5367–5370. [Google Scholar] [CrossRef] [PubMed]
- Ploskonka, A.B.; DeCoste, J.B. Insight into organophosphate chemical warfare agent simulant hydrolysis in metal-organic frameworks. J. Haz. Mat. 2019, 375, 191–197. [Google Scholar] [CrossRef]
- Zammataro, A.; Gangemi, C.M.A.; Pappalardo, A.; Toscano, R.M.; Puglisi, R.; Nicotra, G.; Fragalà, M.E.; Tuccitto, N.; Trusso Sfrazzetto, G. Covalently functionalized carbon nanoparticles with a chiral Mn-Salen: A new nanocatalyst for enantioselective epoxidation of alkenes. Chem. Commun. 2019, 55, 5255–5258. [Google Scholar] [CrossRef]
- Sfrazzetto, G.T.; Millesi, S.; Pappalardo, A.; Toscano, R.M.; Ballistreri, F.P.; Tomaselli, G.A.; Gulino, A. Olefin Epoxidation by a (salen) Mn(III) Catalyst Covalently Grafted on Glass Beads. Cat. Sci. Techn. 2015, 5, 673–679. [Google Scholar] [CrossRef]
- La Paglia Fragola, V.; Lupo, F.; Pappalardo, A.; Sfrazzetto, G.T.; Toscano, R.M.; Ballistreri, F.P.; Tomaselli, G.A.; Gulino, A. A surface-confined O=MnV(salen) oxene catalyst and high turnover values in asymmetric epoxidation of unfunctionalized olefins. J. Mater. Chem. 2012, 22, 20561–20565. [Google Scholar] [CrossRef]
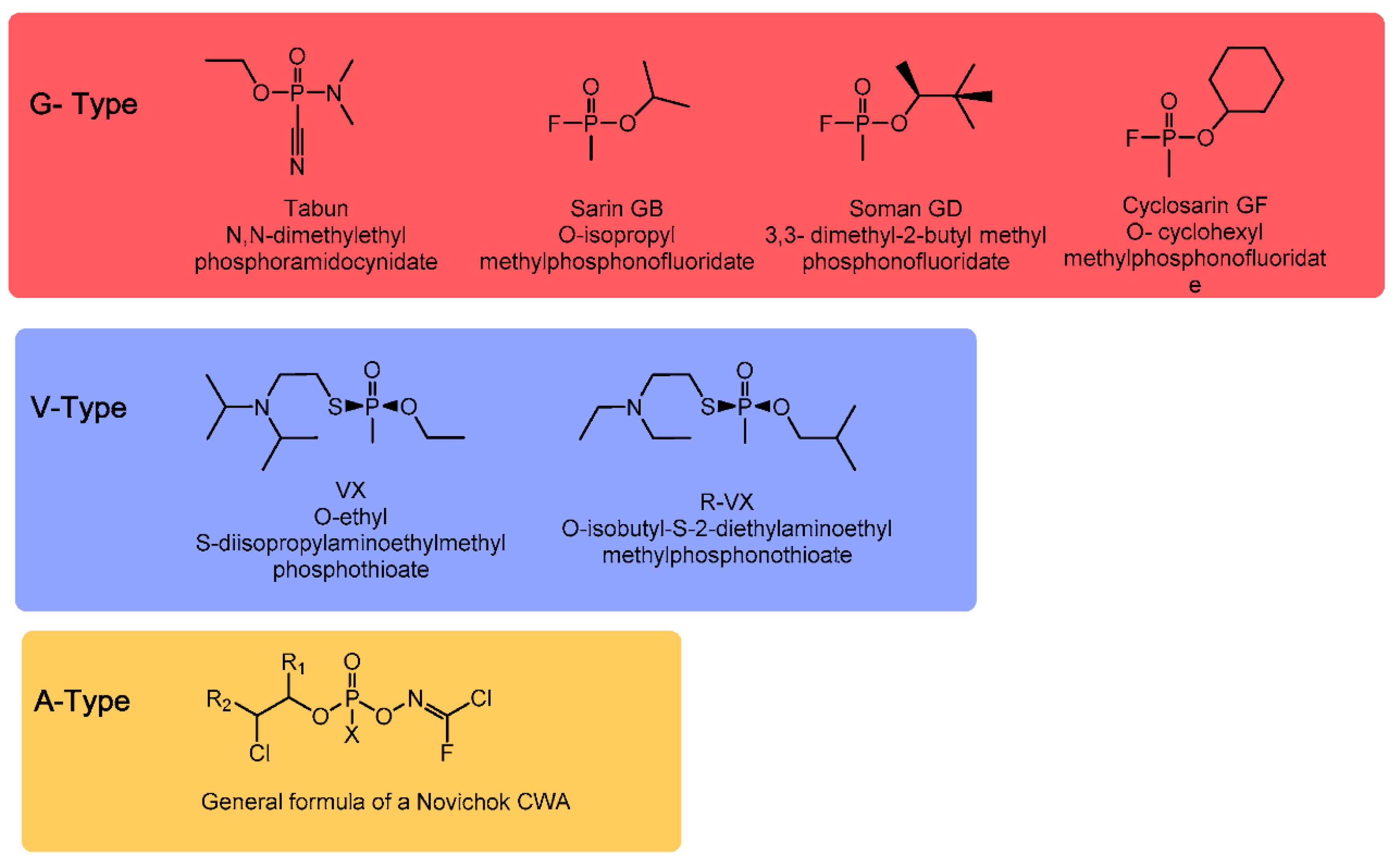


| Vapor Pressure | Volatility | Odor | Solubility | Persistency | |
|---|---|---|---|---|---|
| Tabun (GA) CAS#77-81-6 | 0.037 mm Hg at 20 °C | 576–610 mg/m3 at 25 °C | Fruity | 9.8 g/100 g at 25 °C | T1/2 = 24–36 h |
| Sarin (GB) CAS#107-44-8 | 2.1 mm Hg at 20 °C | 16,400–22,000 mg/m3 at 25 °C | Odorless | Miscible | 2–24 h at 5–25 °C |
| Soman CAS#96-64-0 | 0.40 mm Hg at 20 °C | 3060–3900 mg/m3 at 25 °C | Fruity; oil of camphor | 2.1 g/100 g at 20 °C | Relatively persistent |
| GF CAS#329-99-7 | 0.07 mm Hg at 25 °C | 59 ppm | Odorless | 3.7 g/100 g at 25 °C | Unknown |
| VX CAS#20820-80-8 | 0.0007 mm Hg at 20 °C | 3–30.0 (10.5) mg/m3 at 25 °C | Odorless | Miscibile at <9.4 °C “Slight” at 25 °C | 2–6 days |
| LD50 (Percutaneous) | LC50 | LCt50 | IDLH [39] | |
|---|---|---|---|---|
| Tabun (GA) CAS#77-81-6 | 1 gm/person | 2 ppm | 100–400 mg × min/m3 | 0.03 ppm |
| Sarin (GB) CAS#107-44-8 | 1.7 gm/person | 1.2 ppm | 50–100 mg × min/m3 | 0.03 ppm |
| Soman CAS#96-64-0 | 0.35 gm/person | 0.9 ppm | 25–70 mg × min/m3 | 0.008 |
| GF CAS#329-99-7 | 0.03 gm/person | Unknown | Unknown | Unknown |
| VX CAS#20820-80-8 | 0.01 gm/person | 0.3 ppm | 5–50 mg × min/m3 | 0.002 ppm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zammataro, A.; Santonocito, R.; Pappalardo, A.; Trusso Sfrazzetto, G. Catalytic Degradation of Nerve Agents. Catalysts 2020, 10, 881. https://doi.org/10.3390/catal10080881
Zammataro A, Santonocito R, Pappalardo A, Trusso Sfrazzetto G. Catalytic Degradation of Nerve Agents. Catalysts. 2020; 10(8):881. https://doi.org/10.3390/catal10080881
Chicago/Turabian StyleZammataro, Agatino, Rossella Santonocito, Andrea Pappalardo, and Giuseppe Trusso Sfrazzetto. 2020. "Catalytic Degradation of Nerve Agents" Catalysts 10, no. 8: 881. https://doi.org/10.3390/catal10080881
APA StyleZammataro, A., Santonocito, R., Pappalardo, A., & Trusso Sfrazzetto, G. (2020). Catalytic Degradation of Nerve Agents. Catalysts, 10(8), 881. https://doi.org/10.3390/catal10080881








