High-Complexity WO3-Based Catalyst with Multi-Catalytic Species via 3D Printing
Abstract
:1. Introduction
2. Results and Discussion
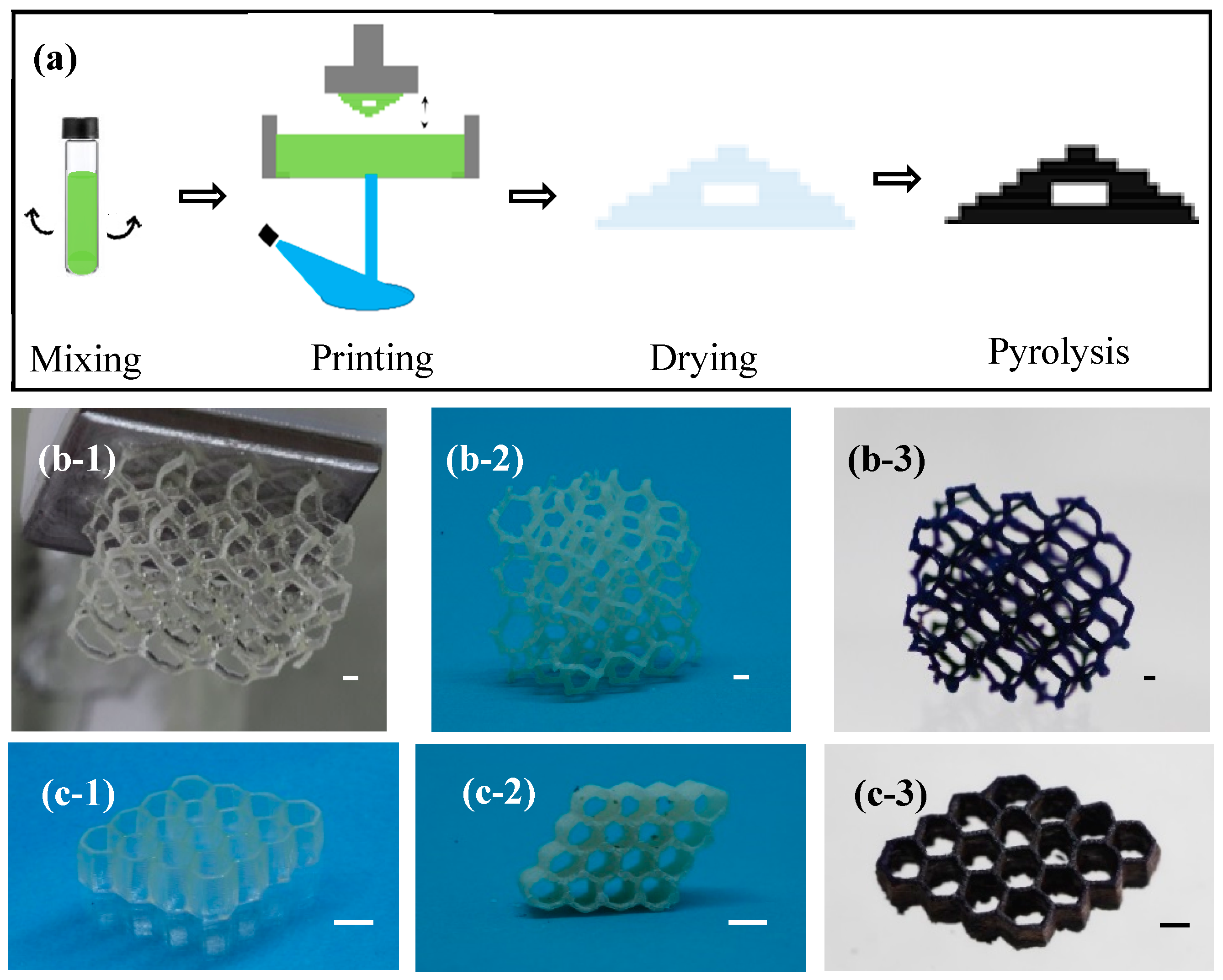
2.1. Preparation of 3D PtO2-WO3 Catalysts
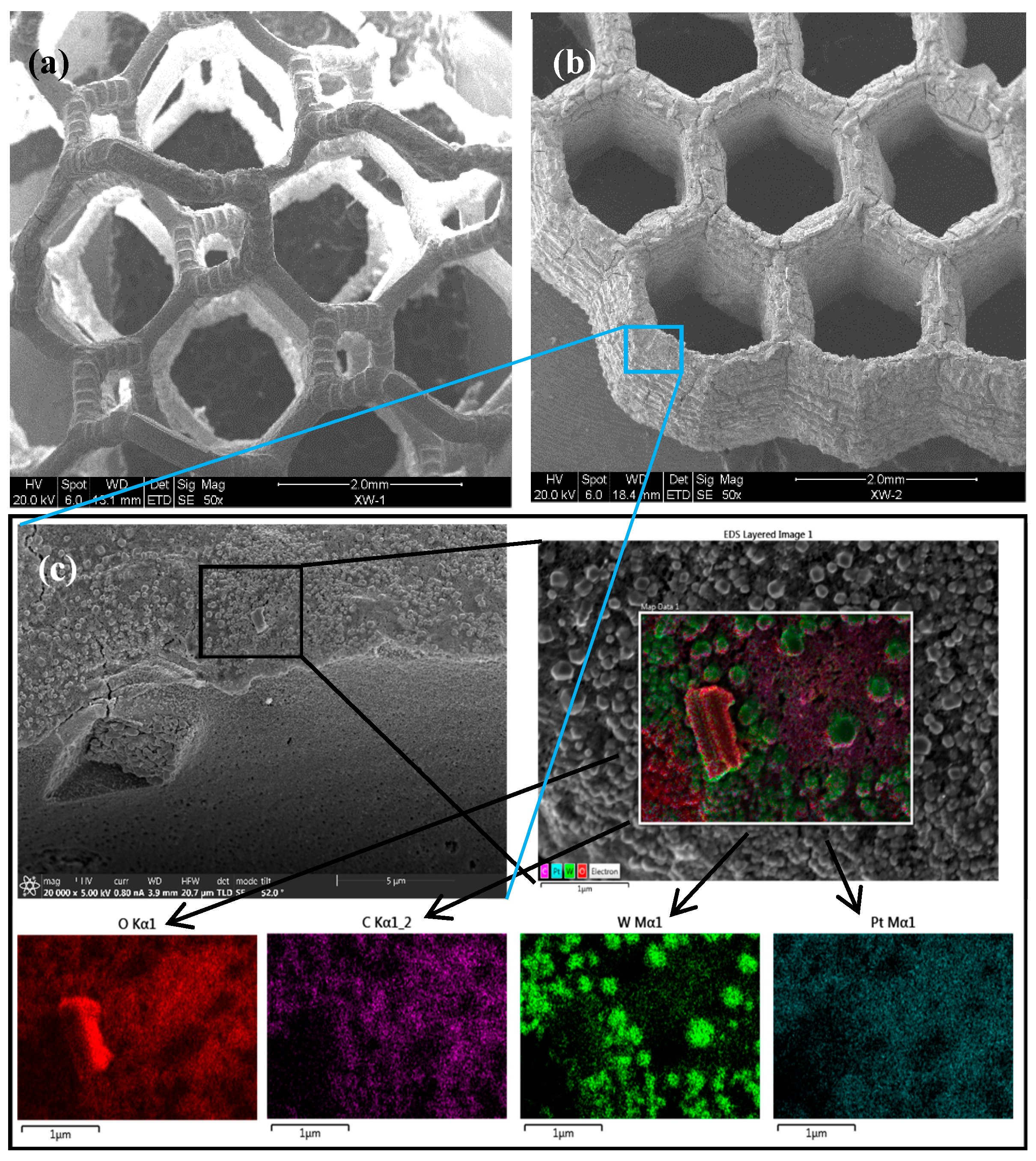
2.2. The Microstructure of the Printed PtO2-WO3 Catalyst
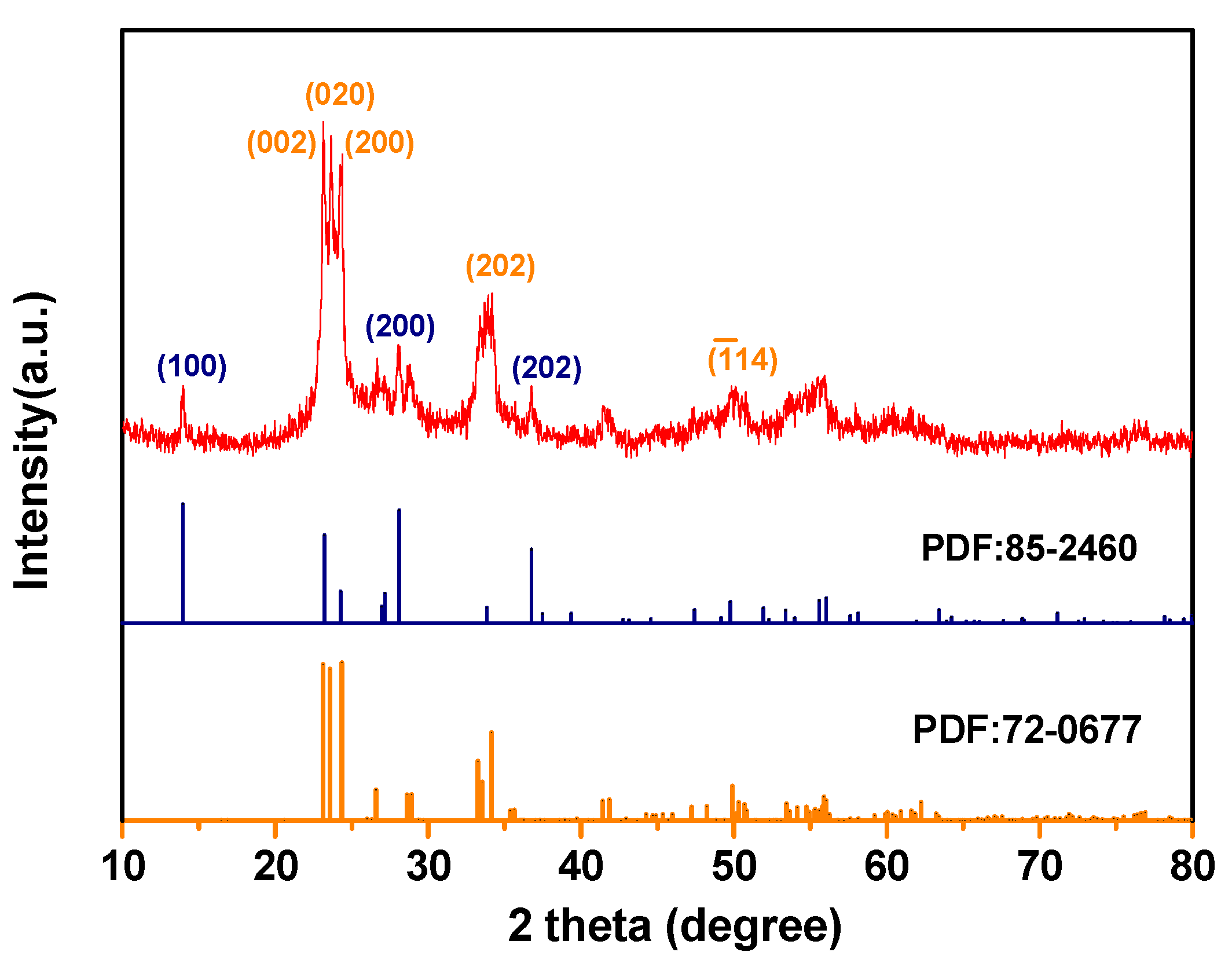
2.3. Composition of the Obtained PtO2-WO3 Catalyst
2.4. Properties of the Printed Catalyst
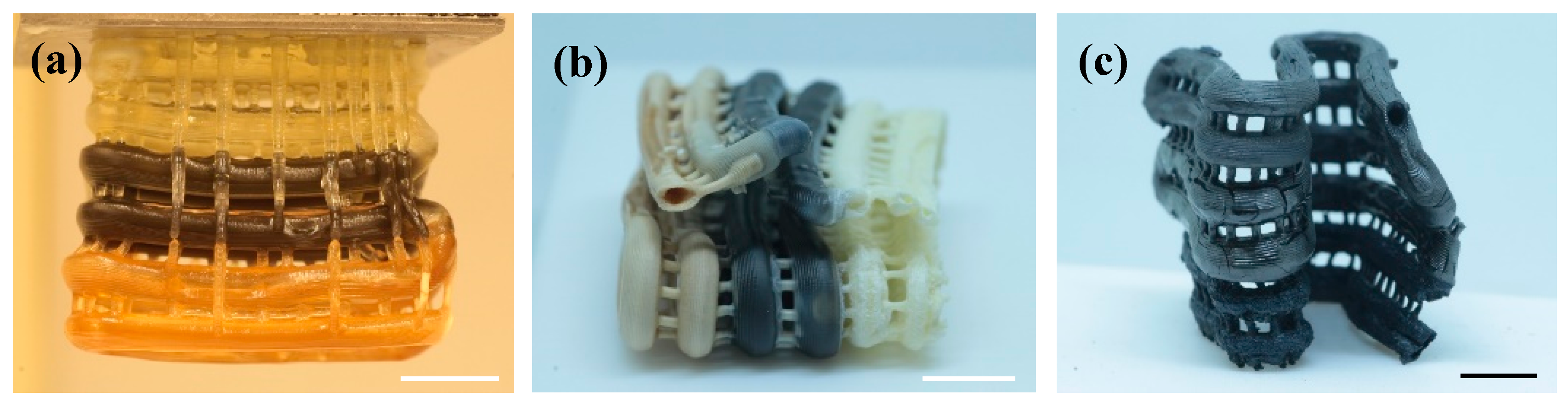
2.5. WO3-Based Catalyst with Multi-Catalytic Species
3. Materials and Methods
3.1. Materials
3.2. Ink Preparation
3.3. Ink Printing
3.4. Drying and Pyrolysis Process
3.5. Catalysis Reactions
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lewis, J.A. Direct ink writing of 3D functional materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Meteyer, S.; Xu, X.; Perry, N.; Zhao, Y.F. Energy and material flow analysis of binder-jetting additive manufacturing processes. Procedia Cirp 2014, 15, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Sachs, E.; Cima, M.; Williams, P.; Brancazio, D.; Cornie, J. Three dimensional printing: Rapid tooling and prototypes directly from a CAD model. CIRP Ann. 1990, 39, 201–204. [Google Scholar] [CrossRef]
- Anitha, R.; Arunachalam, S.; Radhakrishnan, P. Critical parameters influencing the quality of prototypes in fused deposition modelling. J. Mater. Process. Technol. 2001, 118, 385–388. [Google Scholar] [CrossRef]
- Han, W.; Jafari, M.A.; Danforth, S.C.; Safari, A. Tool path-based deposition planning in fused deposition process. J. Manuf. Sci. Eng. 2002, 124, 462–472. [Google Scholar] [CrossRef]
- Sood, A.K.; Ohdar, R.K.; Mahapatra, S.S. Parametric appraisal of mechanical property of fused deposition modelling processed parts. Mater. Des. 2010, 31, 287–295. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel materials for 3D printing by photopolymerization. Adv. Mater. 2018, 30, 1706344. [Google Scholar] [CrossRef]
- Cooperstein, I.; Shukrun, E.; Press, O.; Kamyshny, A.; Magdassi, S. Additive manufacturing of transparent silica glass from solutions. ACS Appl. Mater. Interfaces 2018, 10, 18879–18885. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, J.; Chua, C.K. Fundamentals, and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Akmal, J.S.; Salmi, M.; Makitie, A.; Bjorkstrand, R.; Partanen, J. Implementation of industrial additive manufacturing: Intelligent implants and drug delivery systems. J. Funct. Biomater. 2018, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Hashemi, M.; Moghaddam, N.S.; Elahinia, M. Improving corrosion resistance of additively manufactured nickel–titanium biomedical devices by micro-arc oxidation process. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- Lewis, J.A.; Ahn, B.Y. Device fabrication: Three-dimensional printed electronics. Nature 2015, 518, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Fantino, E.; Chiappone, A.; Roppolo, I.; Manfredi, D.G.; Bongiovanni, R.M.; Pirri, C.; Calignano, F. 3D printing of conductive complex structures with in situ generation of silver nanoparticles. Adv. Mater. 2016, 28, 3712–3717. [Google Scholar] [CrossRef]
- Ahn, B.Y.; Duoss, E.B.; Motala, M.J.; Guo, X.; Park, S.; Xiong, Y.; Yoon, Y.; Nuzzo, R.G.; Rogers, J.A.; Lewis, J.A. Omnidirectional printing of flexible, stretchable, and spanning silver microelectrodes. Science 2009, 323, 1590–1593. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3d printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Li, X.; Chen, Z.; Zhou, C.; Zhou, Q.; Chen, Y. Recent progress in biomimetic additive manufacturing technology: From materials to functional structures. Adv. Mater. 2018, 30, 1706539. [Google Scholar] [CrossRef]
- Attaran, M. The rise of 3D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Dilberoglu, U.M.; Gharehpapagh, B.; Yaman, U.; Dolen, M. The role of additive manufacturing in the era of industry 4.0. Procedia Manuf. 2017, 11, 545–554. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Dehghanghadikolaei, A.; Fotovvati, B.; Vosooghnia, A.; Emamian, S.S.; Gisario, A. The Potential of Additive Manufacturing in the Smart Factory Industrial 4.0: A Review. Appl. Sci. 2019, 9, 3865. [Google Scholar] [CrossRef] [Green Version]
- Capel, A.J.; Rimington, R.P.; Lewis, M.P.; Christie, S.D. 3D printing for chemical, pharmaceutical and biological applications. Nat. Rev. Chem. 2018, 2, 422–436. [Google Scholar] [CrossRef]
- Symes, M.D.; Kitson, P.J.; Yan, J.; Richmond, C.J.; Cooper, G.J.; Bowman, R.W.; Vilbrandt, T.; Cronin, L. Integrated 3D-printed reactionware for chemical synthesis and analysis. Nat. Chem. 2012, 4, 349–354. [Google Scholar] [CrossRef]
- Kitson, P.J.; Glatzel, S.; Chen, W.; Lin, C.G.; Song, Y.F.; Cronin, L. 3D printing of versatile reaction ware for chemical synthesis. Nat. Protoc. 2016, 11, 920–936. [Google Scholar] [CrossRef] [PubMed]
- Kitson, P.J.; Marshall, R.J.; Long, D.; Forgan, R.S.; Cronin, L. 3D printed high throughput hydrothermal reactionware for discovery, optimization, and scale-up. Angew. Chem. Int. Ed. 2014, 53, 12723–12728. [Google Scholar] [CrossRef]
- Kitson, P.J.; Glatzel, S.; Cronin, L. The digital code driven autonomous synthesis of ibuprofen automated in a 3D-printer-based robot. Beilstein J. Org. Chem. 2016, 12, 2776–2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragone, V.; Sans, V.; Rosnes, M.H.; Kitson, P.J.; Cronin, L. 3D-printed devices for continuous-flow organic chemistry. Beilstein J. Org. Chem. 2013, 9, 951–959. [Google Scholar] [CrossRef]
- Kitson, P.J.; Symes, M.D.; Dragone, V.; Cronin, L. Combining 3D printing and liquid handling to produce user-friendly reaction ware for chemical synthesis and purification. Chem. Sci. 2013, 4, 3099–3103. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Liu, C.J. Three-dimensional printing for catalytic applications: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1701134. [Google Scholar] [CrossRef]
- Parra-Cabrera, C.; Achille, C.; Kuhn, S.; Ameloot, R. 3D printing in chemical engineering and catalytic technology: Structured catalysts, mixers and reactors. Chem. Soc. Rev. 2018, 47, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. Heterogeneous catalysis: Understanding for designing, and designing for applications. Angew. Chem. Int. Ed. 2016, 55, 6112–6113. [Google Scholar] [CrossRef] [PubMed]
- Tubío, C.R.; Azuaje, J.; Escalante, L.; Coelho, A.; Guitián, F.; Sotelo, E.; Gil, A. 3D printing of a heterogeneous copper-based catalyst. J. Catal. 2016, 334, 110–115. [Google Scholar] [CrossRef]
- Zhu, C.; Qi, Z.; Beck, V.A.; Luneau, M.; Lattimer, J.; Chen, W.; Friend, C.M. Toward digitally controlled catalyst architectures: Hierarchical nanoporous gold via 3D printing. Sci. Adv. 2018, 4, eaas9459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Marta, A.S.; Tubío, C.R.; Carbajales, C.; Fernández, C.; Escalante, L.; Sotelo, E.; Coelho, A. Three-dimensional printing in catalysis: Combining 3D heterogeneous copper and palladium catalysts for multicatalytic multicomponent reactions. ACS Catal. 2017, 8, 392–404. [Google Scholar] [CrossRef]
- Rosental, T.; Magdassi, S. A new approach to 3D printing dense ceramics by ceramic precursor binders. Adv. Eng. Mater. 2019, 21, 1900604. [Google Scholar] [CrossRef]
- Houbertz, R.; Fröhlich, L.; Popall, M.; Streppel, U.; Dannberg, P.; Bräuer, A.; Serbin, J.; Chichkov, B. Inorganic–Organic Hybrid Polymers for Information Technology: From Planar Technology to 3D Nanostructures. Adv. Eng. Mater. 2003, 5, 551–555. [Google Scholar] [CrossRef]
- Cooperstein, I.; Indukuri, S.R.K.C.; Bouketov, A.; Levy, U.; Magdassi, S. 3D printing of micrometer-sized transparent ceramics with on-demand optical-gain properties. Adv. Mater. 2020, 32, 2001675. [Google Scholar] [CrossRef]
- Sun, P.; Shao, G.Q.; Duan, X.L.; Shi, X.; Zhou, F. Low temperature synthesis of tungsten carbide by embedment-direct reduction carburization process. Solid State Phenom. 2007, 121–123, 171–174. [Google Scholar] [CrossRef]
- Hunyadi, D.; Sajó, I.; Szilágyi, I.M. Structure and thermal decomposition of ammonium metatungstate. J. Therm. Anal. Calorim. 2014, 116, 329–337. [Google Scholar] [CrossRef] [Green Version]
- ThermoFisher Scientific. Available online: https://www.fishersci.com/store/msds?partNumber=AC195360010&productDescription=POTASSIUM+TETRACHLOROPLA+1GR&vendorId=VN00032119&countryCode=US&language=en (accessed on 9 June 2020).
- Wang, X.; Wang, R.; Peng, C.; Li, T.; Liu, B. Polyacrylamide gel method: Synthesis and property of BeO nanopowders. J. Sol-Gel Sci. Technol. 2011, 57, 115–127. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Peng, C.; Li, T.; Liu, B. Growth of BeO nanograins synthesized by polyacrylamide gel route. J. Mater. Sci. Technol. 2011, 27, 147–152. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, C.; Wang, R.; Wang, X.; Liu, B. Influence of calcining process on optical properties of Al-doped-ZnO powders. J. Inorg. Mater. 2013, 28, 171–176. [Google Scholar] [CrossRef]
- Sin, A.; Odier, P. Gelation by acrylamide, a quasi-universal medium for the synthesis of fine oxide powders for electroceramic applications. Adv. Mater. 2000, 12, 649–652. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L.; Li, W.; Li, Q. Preparation of nanoscale α-Al2O3 powder by the polyacrylamide gel method. Nanostruct. Mater. 1999, 11, 1263–1267. [Google Scholar] [CrossRef]
- Tahmasebpour, M.; Babaluo, A.A.; Aghjeh, M.K.R. Synthesis of zirconia nanopowders from various zirconium salts via polyacrylamide gel method. J. Eur. Ceram. Soc. 2008, 28, 773–778. [Google Scholar] [CrossRef]
- Douy, A.; Odier, P. The polyacrylamide gel: A novel route to ceramic and glassy oxide powders. Mater. Res. Bull. 1989, 24, 1119–1126. [Google Scholar] [CrossRef]
- Liu, N.; Yuan, Y.; Majewski, P.; Aldinger, F. Synthesis of La0.85Sr0.15Ga0.85Mg0.15O2.85 materials for SOFC applications by acrylamide polymerization. Mater. Res. Bull. 2006, 41, 461–468. [Google Scholar] [CrossRef]
- Rajagopal, S.; Nataraj, D.; Mangalaraj, D.; Djaoued, Y.; Robichaud, J.; Khyzhun, O.Y. Controlled growth of WO3 nanostructures with three different morphologies and their structural, optical, and photodecomposition studies. Nanoscale Res. Lett. 2009, 4, 1335–1342. [Google Scholar] [CrossRef] [Green Version]
- Anukorn, P.; Oranuch, Y.; Titipun, T.; Thongtem, S. Hydrothermal synthesis of hexagonal WO3 nanowires with high aspect ratio and their electrochemical properties for lithium-ion batteries. Russ. J. Phys. Chem. A 2017, 91, 2441–2447. [Google Scholar]
- Hidayat, D.; Purwanto, A.; Wang, W.; Okuyama, K. Preparation of size-controlled tungsten oxide nanoparticles and evaluation of their adsorption performance. Mater. Res. Bull. 2010, 45, 165–173. [Google Scholar] [CrossRef]
- Nogueira, H.I.S.; Cavaleiro, A.M.V.; Rocha, J.; Trindade, T.; Jesus, J.D.P.D. Synthesis and characterization of tungsten trioxide powders prepared from tungstic acids. Mater. Res. Bull. 2004, 39, 683–693. [Google Scholar] [CrossRef] [Green Version]
| Entry | Alkyne | Catalyst | Conversion (%) 2 | Selectivity of Alkene (%) 2 |
|---|---|---|---|---|
| 1 |  | PtO2-WO3 | 100 | 82 |
| 2 | 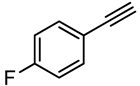 | PtO2-WO3 | 100 | 72 |
| 3 |  | PtO2-WO3 | 100 | 70 |
| 4 | 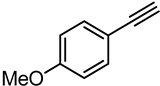 | PtO2-WO3 | 100 | 82 |
| 5 3 |  | PtO2-WO3 | 72 | 83 |
| 6 | 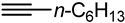 | PtO2-WO3 | 75 | 67 |
| 7 | 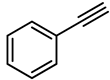 | Pt/C (10%) | 100 | 0 |
| 8 | 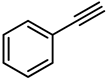 | PtO2 | 100 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Guo, W.; Abu-Reziq, R.; Magdassi, S. High-Complexity WO3-Based Catalyst with Multi-Catalytic Species via 3D Printing. Catalysts 2020, 10, 840. https://doi.org/10.3390/catal10080840
Wang X, Guo W, Abu-Reziq R, Magdassi S. High-Complexity WO3-Based Catalyst with Multi-Catalytic Species via 3D Printing. Catalysts. 2020; 10(8):840. https://doi.org/10.3390/catal10080840
Chicago/Turabian StyleWang, Xiaofeng, Wei Guo, Raed Abu-Reziq, and Shlomo Magdassi. 2020. "High-Complexity WO3-Based Catalyst with Multi-Catalytic Species via 3D Printing" Catalysts 10, no. 8: 840. https://doi.org/10.3390/catal10080840
APA StyleWang, X., Guo, W., Abu-Reziq, R., & Magdassi, S. (2020). High-Complexity WO3-Based Catalyst with Multi-Catalytic Species via 3D Printing. Catalysts, 10(8), 840. https://doi.org/10.3390/catal10080840







