Carbon Dioxide Reforming of Methane over Ni Supported SiO2: Influence of the Preparation Method on the Resulting Structural Properties and Catalytic Activity
Abstract
1. Introduction
2. Results and Discussion
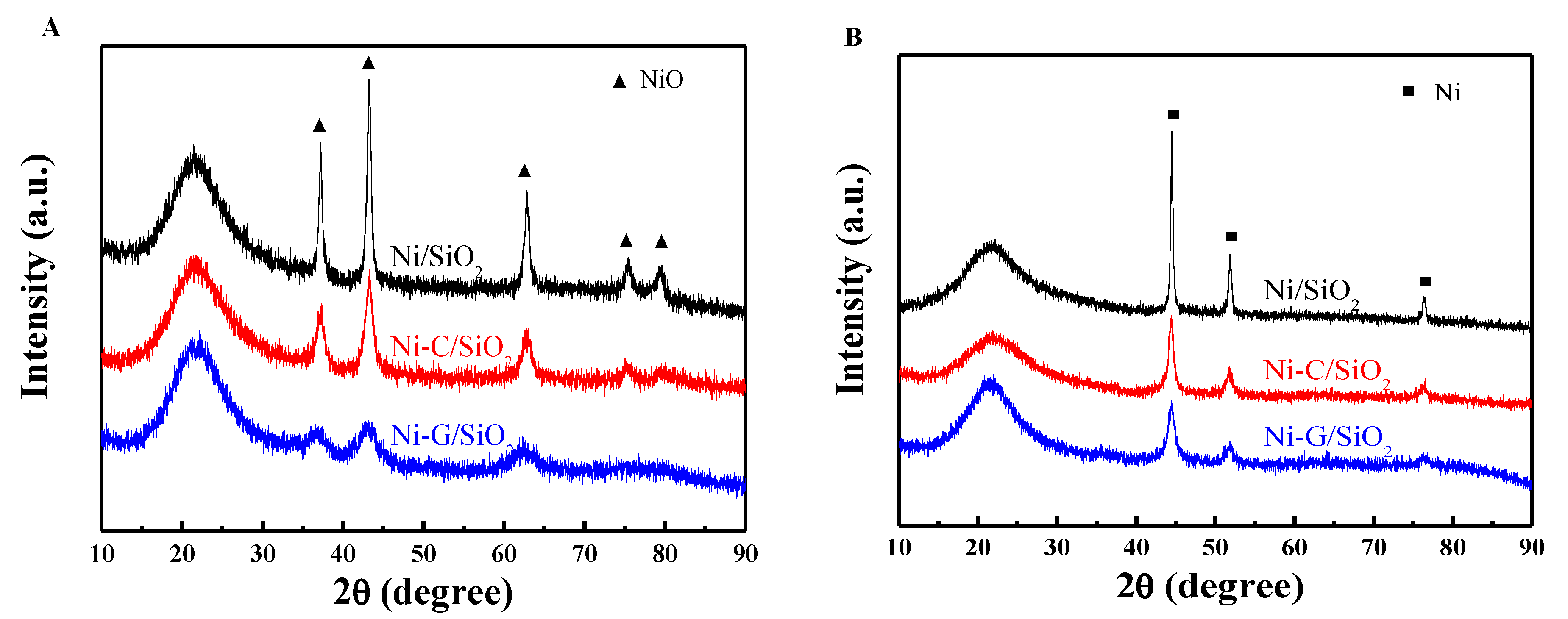
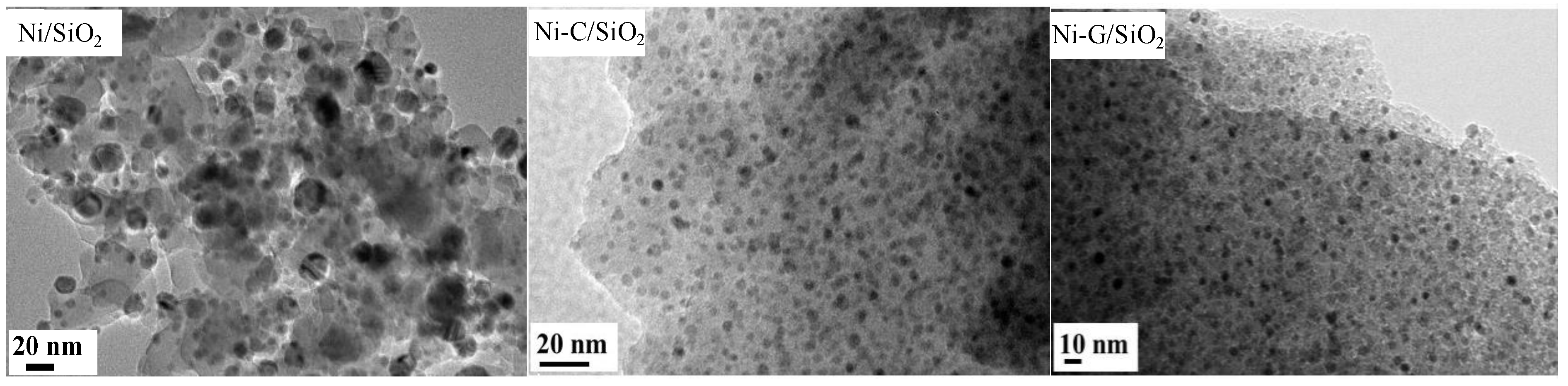
2.1. Structural Properties of Catalyst
2.2. Textural Properties of the Catalysts
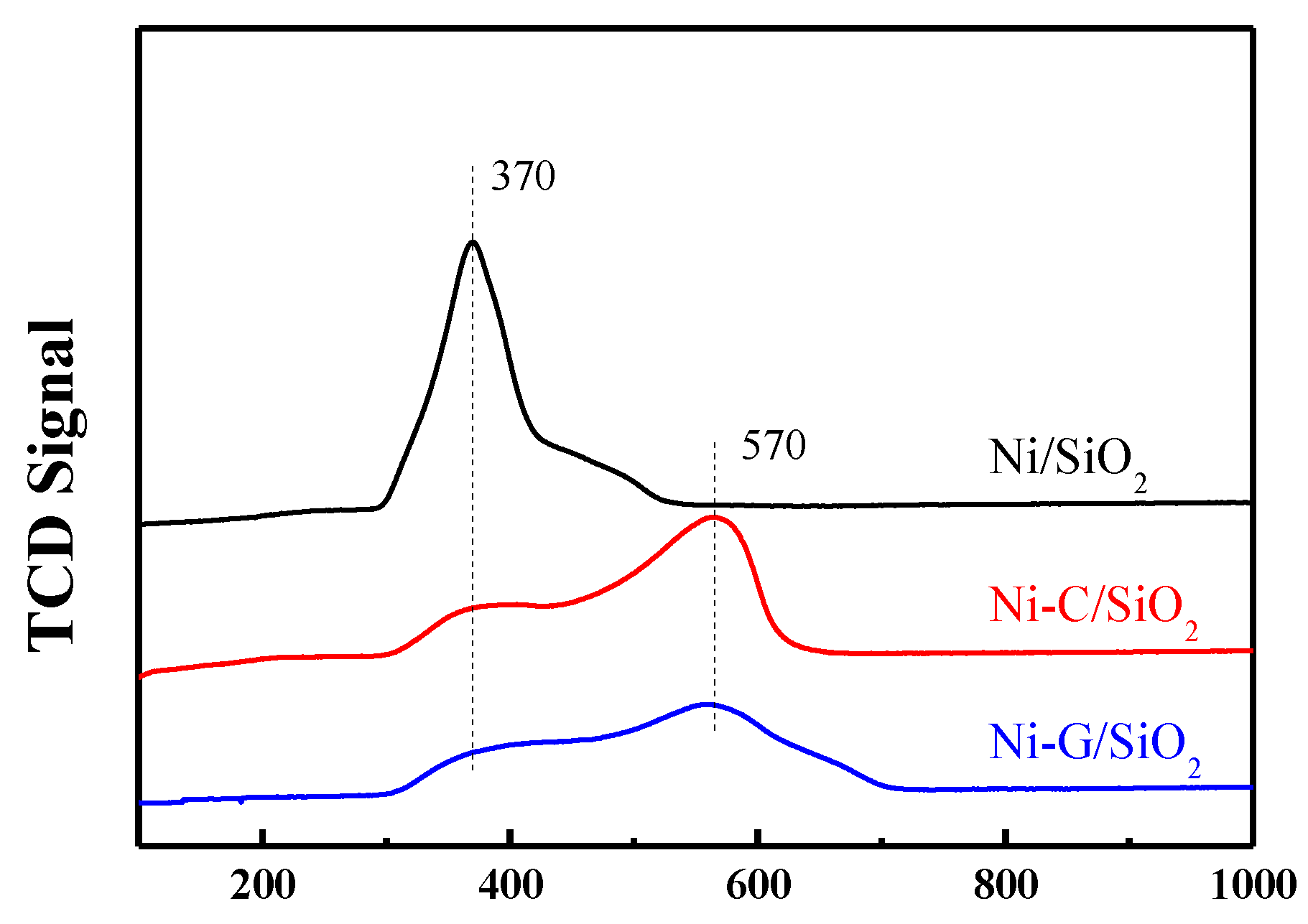
2.3. Reduction Behavior of the Catalysts
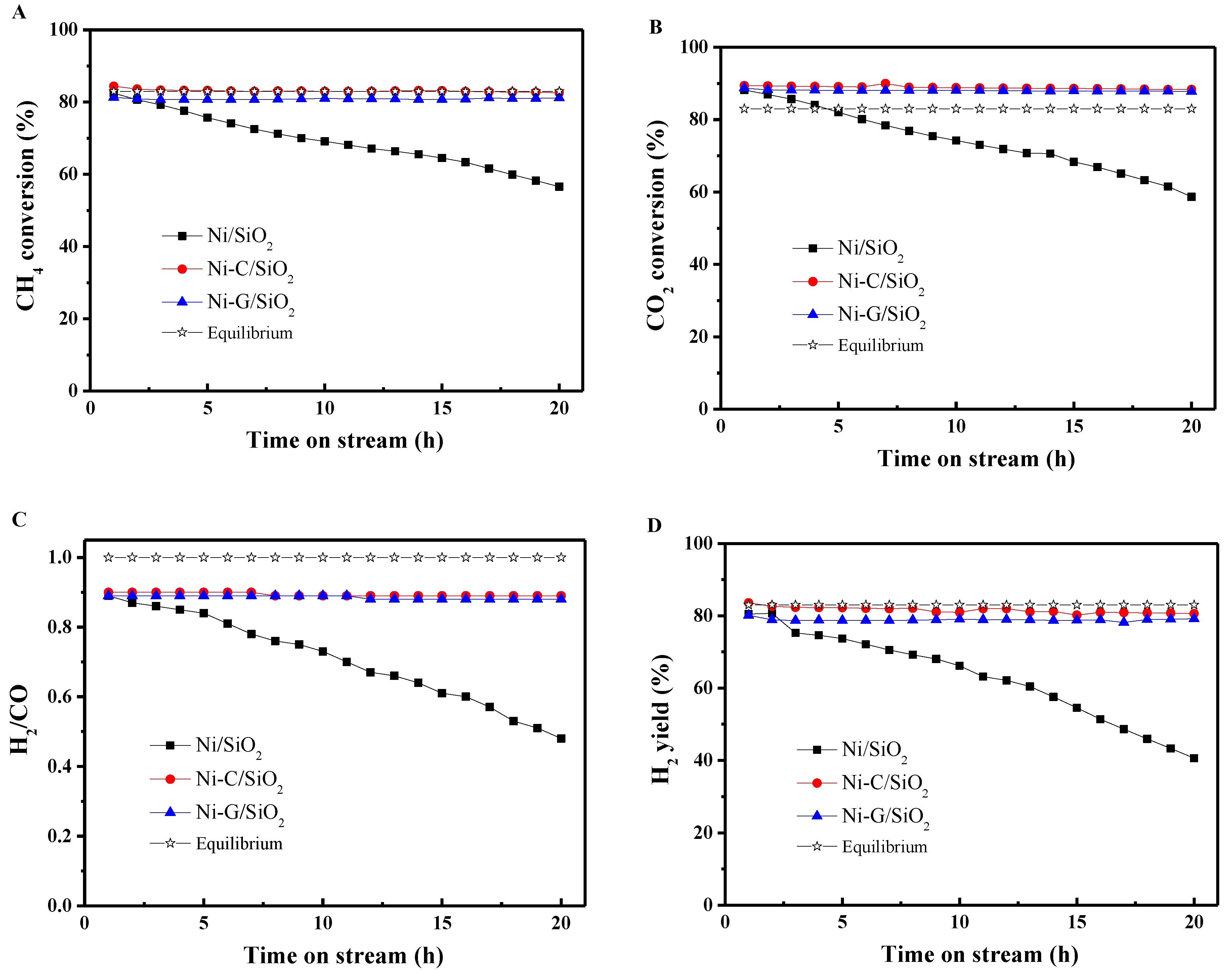
2.4. Catalytic Performance
2.5. Characterization of Used Catalysts
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Characterization Techniques Used on the Catalysts
3.3. Activity Evaluation of the Catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silva, C.G.; Passos, F.B.; Silva, V.T. Influence of the support on the activity of a supported nickel-promoted molybdenum carbide catalyst for dry reforming of methane. J. Catal. 2019, 375, 507–518. [Google Scholar] [CrossRef]
- Wang, F.; Han, B.; Zhang, L.; Xu, L.; Yu, H.; Shi, W. CO2 reforming with methane over small-sized Ni@SiO2 catalysts with unique features of sintering-free and low carbon. Appl. Catal. B Environ. 2018, 235, 26–35. [Google Scholar] [CrossRef]
- Xie, T.; Shi, L.; Zhang, J.; Zhang, D. Immobilizing Ni nanoparticles to mesoporous silica with size and location control via a polyol-assisted route for coking- and sintering-resistant dry reforming of methane. Chem. Commun. 2014, 50, 7250–7253. [Google Scholar] [CrossRef]
- Yang, W.; Liu, H.; Li, Y.; Wu, H.; He, D. CO2 reforming of methane to syngas over highly-stable Ni/SBA-15 catalysts prepared by P123-assisted method. Int. J. Hydrog. Energy 2016, 41, 1513–1523. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, H.; Zeng, L.; Zhao, Z.-J.; Gong, J. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B Environ. 2017, 202, 683–694. [Google Scholar] [CrossRef]
- Kumar, N.; Roy, A.; Wang, Z.; Abbate, E.M.; Haynes, D.; Shekhawat, D.; Spivey, J.J. Bi-reforming of methane on Ni-based pyrochlore catalyst. Appl. Catal. A Gen. 2016, 517, 211–216. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Kawi, S.; Kathiraser, Y.; Ni, J.; Oemar, U.; Li, Z.; Saw, E.T. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane. ChemSusChem 2015, 8, 3556–3575. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef]
- Liu, C.-J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- García, V.; Fernández, J.J.; Ruíz, W.; Mondragón, F.; Moreno, A. Effect of MgO addition on the basicity of Ni/ZrO2 and on its catalytic activity in carbon dioxide reforming of methane. Catal. Commun. 2009, 11, 240–246. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Pupulin, E.; Menegazzo, F.; Ghedini, E.; Michele, A.D.; Mattarelli, M.; Cruciani, G.; Signoretto, M. Nickel based catalysts for methane dry reforming: Effect of supports on catalytic activity and stability. Int. J. Hydrog. Energy 2019, 44, 28065–28076. [Google Scholar] [CrossRef]
- Xu, Y.; Du, X.; Li, J.; Wang, P.; Zhu, J.; Ge, F.; Zhou, J.; Song, M.; Zhu, W. A comparison of Al2O3 and SiO2 supported Ni-based catalysts in their performance for the dry reforming of methane. J. Fuel Chem. Technol. 2019, 47, 199–208. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Tzounis, L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Investigating the correlation between deactivation and the carbon deposited on the surface of Ni/Al2O3 and Ni/La2O3-Al2O3 catalysts during the biogas reforming reaction. Appl. Surf. Sci. 2019, 474, 42–56. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Liu, J.; Xu, Y.; Lv, Y. Production of syngas via CO2 methane reforming process: Effect of cerium and calcium promoters on the performance of Ni-MSC catalysts. Int. J. Hydrog. Energy 2020, 45, 640–649. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P. Effect of a second metal (Y, K, Ca, Mn or Cu) addition on the carbon dioxide reforming of methane over nanostructured palladium catalysts. Appl. Catal. B Environ. 2012, 115, 190–200. [Google Scholar] [CrossRef]
- Taherian, Z.; Gharahshiran, V.S.; Khataee, A.; Meshkani, F.; Orooji, Y. Comparative study of modified Ni catalysts over mesoporous CaO-Al2O3 support for CO2/methane reforming. Catal. Commun. 2020, 145, 106100. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Pino, L.; Italiano, C.; Vita, A.; Laganà, M.; Recupero, V. Ce0.70La0.20Ni0.10O2-δ catalyst for methane dry reforming: Influence of reduction temperature on the catalytic activity and stability. Appl. Catal. B Environ. 2017, 218, 779–792. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in depth investigation of deactivation through carbon formation during the biogas dry reforming reaction for Ni supported on modified with CeO2 and La2O3 zirconia catalysts. Int. J. Hydrog. Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Álvarez, A.; Bobadilla, M.L.F.; Garcilaso, V.; Centeno, M.A.; Odriozola, J.A. CO2 reforming of methane over Ni-Ru supported catalysts: On the nature of active sites by operando DRIFTS study. J. CO2 Util. 2018, 24, 509–515. [Google Scholar] [CrossRef]
- Turap, Y.; Wang, I.; Fu, T.; Wu, Y.; Wang, Y.; Wang, W. Co-Ni alloy supported on CeO2 as a bimetallic catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2020, 45, 6538–6548. [Google Scholar] [CrossRef]
- Andraos, S.; Abbas-Ghaleb, R.; Chlala, D.; Vita, A.; Italiano, C.; Laganà, M.; Pino, L.; Nakhl, M.; Specchia, S. Production of hydrogen by methane dry reforming over ruthenium-nickel based catalysts deposited on Al2O3, MgAl2O4, and YSZ. Int. J. Hydrog. Energy 2019, 44, 25706–25716. [Google Scholar] [CrossRef]
- Li, Z.; Mo, L.; Kathiraser, Y.; Kawi, S. Yolk-satellite-shell structured Ni-Yolk@Ni@SiO2 nanocomposite: Superb catalyst toward methane CO2 reforming reaction. ACS Catal. 2014, 4, 1526–1536. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F. Coke-resistant Ni@SiO2 catalyst for dry reforming of methane. Appl. Catal. B Environ. 2015, 176, 513–521. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Zhu, J.; Han, B.; Fan, W.; Zhao, L.; Cai, W.; Li, Z.; Xu, L.; Yu, H.; et al. CO2 reforming with methane reaction over Ni@SiO2 catalysts coupled by size effect and metal-support interaction. Fuel 2019, 256, 115954. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Huang, L.; Yu, X.; Qian, W.; Chu, W. Facile route for synthesizing ordered mesoporous Ni-Ce-Al oxide materials and their catalytic performance for methane dry reforming to hydrogen and syngas. ACS Catal. 2013, 3, 1638–1651. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, B.; Wang, Z.; Kawi, S. High carbon resistant Ni@Ni phyllosilicate@SiO2 core shell hollow sphere catalysts for low temperature CH4 dry reforming. J. CO2 Util. 2018, 27, 238–246. [Google Scholar] [CrossRef]
- Wang, C.; Jie, X.; Qiu, Y.; Zhao, Y.; Al-Megren, H.A.; Alshihri, S.; Edwards, P.P.; Xiao, T. The importance of inner cavity space within Ni@SiO2 nano-capsule catalysts for excellent coking resistance in the high-space-velocity dry reforming of methane. Appl. Catal. B Environ. 2019, 259, 118019. [Google Scholar] [CrossRef]
- Niu, J.; Ran, J.; Chen, D. Understanding the mechanism of CO2 reforming of methane to syngas on Ni@Pt surface compared with Ni(111) and Pt(111). Appl. Surf. Scie. 2020, 513, 145840. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Zhao, B.; Wang, Z.; Wang, Y.; Liu, C.-J. Methanation over Ni/SiO2: Effect of the catalyst preparation methodologies. Int. J. Hydrog. Energy 2013, 38, 2283–2291. [Google Scholar] [CrossRef]
- Yan, X.; Hu, T.; Liu, P.; Li, S.; Zhao, B.; Zhang, Q.; Jiao, W.; Chen, S.; Wang, P.; Lu, J.; et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Catal. B Environ. 2019, 246, 221–231. [Google Scholar] [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and heterogeneous catalysis: A review on selected catalytic processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef]
- Zhan, H.; Shi, X.; Huang, X.; Zhao, N. Highly coke-resistant ordered mesoporous Ni/SiC with large surface areas in CO2 reforming of CH4. J. Fuel Chem. Technol. 2019, 47, 942–948. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Cao, K.; Yang, W.; Gao, H.; Wang, Y.; Li, H. Highly dispersed nickel loaded on mesoporous silica: One-spot synthesis strategy and high performance as catalysts for methane reforming with carbon dioxide. Appl. Catal. B Environ. 2012, 125, 324–330. [Google Scholar] [CrossRef]
- Tomiyama, S.; Takahashi, R.; Sato, S.; Sodesawa, T.; Yoshida, S. Preparation of Ni/SiO2 catalyst with high thermal stability for CO2-reforming of CH4. Appl. Catal. A Gen. 2003, 241, 349–361. [Google Scholar] [CrossRef]
- Ren, H.-P.; Hao, Q.-Q.; Wang, W.; Song, Y.-H.; Cheng, J.; Liu, Z.-W.; Liu, Z.-T.; Lu, J.; Hao, Z. High-performance Ni-SiO2 for pressurized carbon dioxide reforming of methane. Int. J. Hydrog. Energy 2014, 39, 11592–11605. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Marceau, E.; Beauniera, P.; Chea, M.; Train, C. Stabilization of hexagonal close-packed metallic nickel for alumina-supported systems prepared from Ni(II) glycinate. J. Solid State Chem. 2007, 180, 22–30. [Google Scholar] [CrossRef]
- Ren, H.-P.; Hao, Q.-Q.; Ding, S.-Y.; Zhao, Y.-Z.; Zhu, M.; Tian, S.-P.; Ma, Q.; Song, W.-Q.; Miao, Z.; Liu, Z.-T. A high-performance Ni/SiO2 prepared by the complexed-impregnation method with citric acid for carbon dioxide reforming of methane. Ind. Eng. Chem. Res. 2018, 57, 16257–16263. [Google Scholar] [CrossRef]
- Zhang, Q.; Long, K.; Wang, J.; Zhang, T.; Song, Z.; Lin, Q. A novel promoting effect of chelating ligand on the dispersion of Ni species over Ni/SBA-15 catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2017, 42, 14103–14114. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S. Methane reforming with CO2 using nickel catalysts supported on yttria-doped SBA-15 mesoporous materials via sol-gel process. Int. J. Hydrog. Energy 2013, 38, 14250–14260. [Google Scholar] [CrossRef]
- Jiang, N.; Xu, X.-W.; Song, H.-L.; Song, H.; Zhang, F.-Y. Effect of Citric Acid on the Hydrodesulfurization Performance of Unsupported Nickel Phosphide. Ind. Eng. Chem. Res. 2016, 55, 555–559. [Google Scholar] [CrossRef]
- Castillo-Villalón, P.; Ramirez, J.; Vargas-Luciano, J.A. Analysis of the role of citric acid in the preparation of highly active HDS catalysts. J. Catal. 2014, 320, 127–136. [Google Scholar] [CrossRef]
- Bian, Z.; Xin, Z.; Meng, X.; Tao, M.; Lv, Y.; Gu, J. Effect of citric acid on the synthesis of CO methanation catalysts with high activity and excellent stability. Ind. Eng. Chem. Res. 2017, 56, 2383–2392. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 supported nickel-based bimetallic catalysts with superior stability during carbon dioxide reforming of methane: Effect of strong metal-support interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Samojeden, B.; Kamienowska, M.; Colorado, A.I.; Galvez, M.E.; Kolebuk, I.; Motak, M.; Costa, P.D. Novel nickel- and magnesium-modified cenospheres as catalysts for dry reforming of methane at moderate temperatures. Catalysts 2019, 9, 1066. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, W.; Li, S.; Wu, G.; Ma, X.; Wang, X.; Gong, J. Sintering-resistant Ni-based reforming catalysts obtained via the nanoconfinement effect. Chem. Commun. 2013, 49, 9383–9385. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, T.; Wang, J.; Sun, M.; Wang, H.; Sun, H.; Ning, P. Facile template-free synthesis of Ni-SiO2 catalyst with excellent sintering and coking-resistance for dry reforming of methane. Catal. Commun. 2019, 131, 105782. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Shi, W. Syngas production from CO2 reforming with methane over core-shell Ni@SiO2 catalysts. J. CO2 Util. 2016, 16, 318–327. [Google Scholar] [CrossRef]
- Świrk, K.; Zhang, H.; Li, S.; Chen, Y.; Rønning, M.; Motak, M.; Grzybek, T.; Costa, P.D. Carbon-resistant NiO-Y2O3-nanostructured catalysts derived from double layered hydroxides for dry reforming of methane. Catal. Today 2020. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Tzounis, L.; Sebastian, V.; Stolojan, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. The Relationship between Reaction Temperature and Carbon Deposition on Nickel Catalysts Based on Al2O3, ZrO2 or SiO2 Supports during the Biogas Dry Reforming Reaction. Catalysts 2019, 9, 676. [Google Scholar] [CrossRef]


| Samples | SBET (m2·g−1) a | Vp (cm3·g−1) b | Dp (nm) c | dNiO (nm) d | dNi (nm) e | dNi (nm) f | Dispersion (D%) g |
|---|---|---|---|---|---|---|---|
| SiO2 | 271.3 | 0.72 | 11.2 | -- | -- | -- | -- |
| Ni/SiO2 | 236.9 | 0.60 | 8.9 | 16.54 | 25.34 | 32.75 | 3.83 |
| Ni-C/SiO2 | 248.1 | 0.57 | 9.1 | 11.58 | 12.13 | 12.96 | 8.00 |
| Ni-G/SiO2 | 256.6 | 0.56 | 8.8 | 10.01 | 11.23 | 12.04 | 8.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.-P.; Tian, S.-P.; Ding, S.-Y.; Huang, G.-Q.; Zhu, M.; Ma, Q.; Song, W.-Q.; Zhao, Y.-Z.; Miao, Z.; Wang, W. Carbon Dioxide Reforming of Methane over Ni Supported SiO2: Influence of the Preparation Method on the Resulting Structural Properties and Catalytic Activity. Catalysts 2020, 10, 795. https://doi.org/10.3390/catal10070795
Ren H-P, Tian S-P, Ding S-Y, Huang G-Q, Zhu M, Ma Q, Song W-Q, Zhao Y-Z, Miao Z, Wang W. Carbon Dioxide Reforming of Methane over Ni Supported SiO2: Influence of the Preparation Method on the Resulting Structural Properties and Catalytic Activity. Catalysts. 2020; 10(7):795. https://doi.org/10.3390/catal10070795
Chicago/Turabian StyleRen, Hua-Ping, Shao-Peng Tian, Si-Yi Ding, Gui-Qiu Huang, Min Zhu, Qiang Ma, Wen-Qi Song, Yu-Zhen Zhao, Zongcheng Miao, and Wei Wang. 2020. "Carbon Dioxide Reforming of Methane over Ni Supported SiO2: Influence of the Preparation Method on the Resulting Structural Properties and Catalytic Activity" Catalysts 10, no. 7: 795. https://doi.org/10.3390/catal10070795
APA StyleRen, H.-P., Tian, S.-P., Ding, S.-Y., Huang, G.-Q., Zhu, M., Ma, Q., Song, W.-Q., Zhao, Y.-Z., Miao, Z., & Wang, W. (2020). Carbon Dioxide Reforming of Methane over Ni Supported SiO2: Influence of the Preparation Method on the Resulting Structural Properties and Catalytic Activity. Catalysts, 10(7), 795. https://doi.org/10.3390/catal10070795




