Effects of Sulfuric Acid Treatment on the Performance of Ga-Al2O3 for the Hydrolytic Decomposition of 1,1,1,2-Tetrafluoroethane (HFC-134a)
Abstract
1. Introduction
2. Results and Discussion
2.1. Improvement in Catalytic Performance in HFC-134a Decomposition
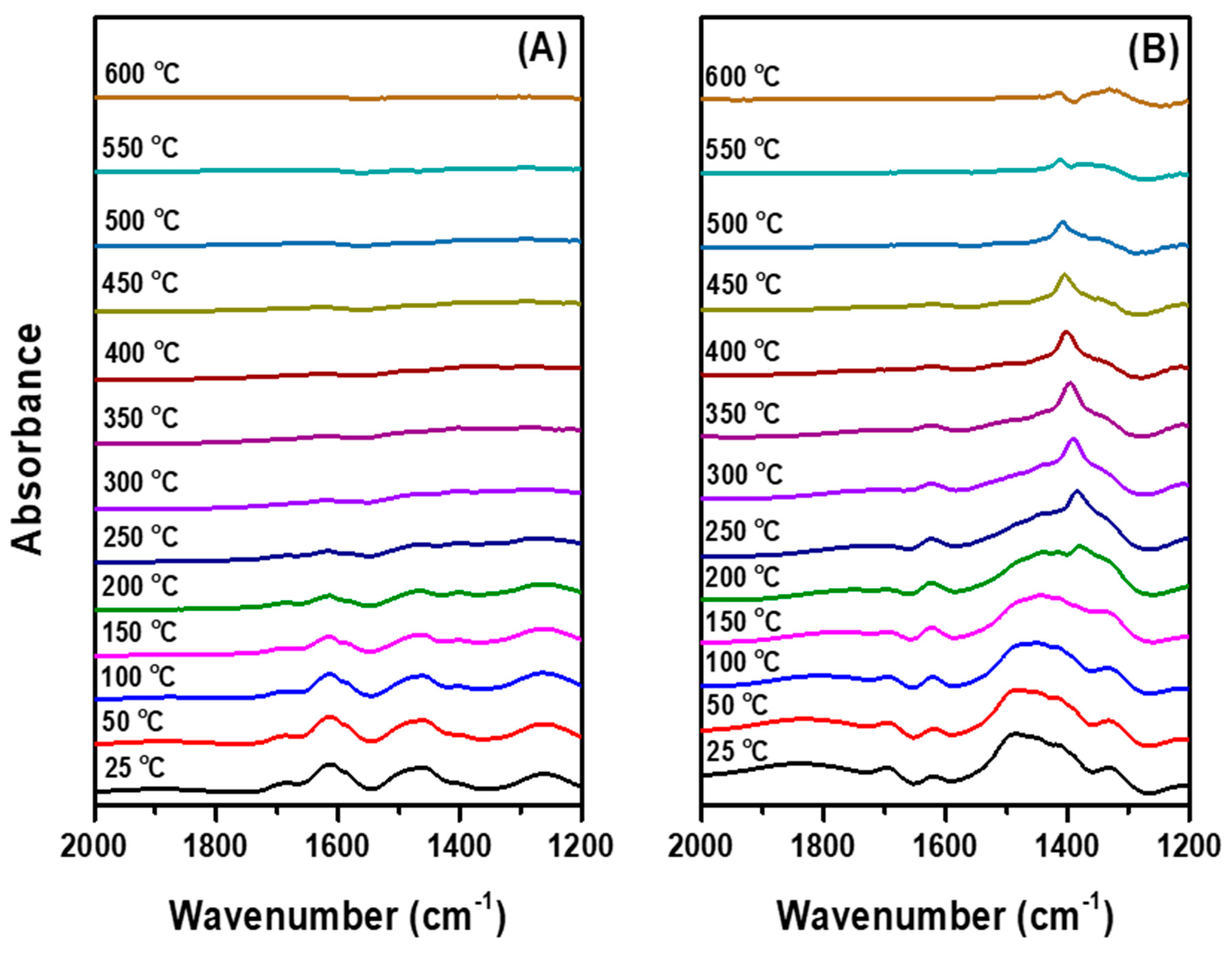
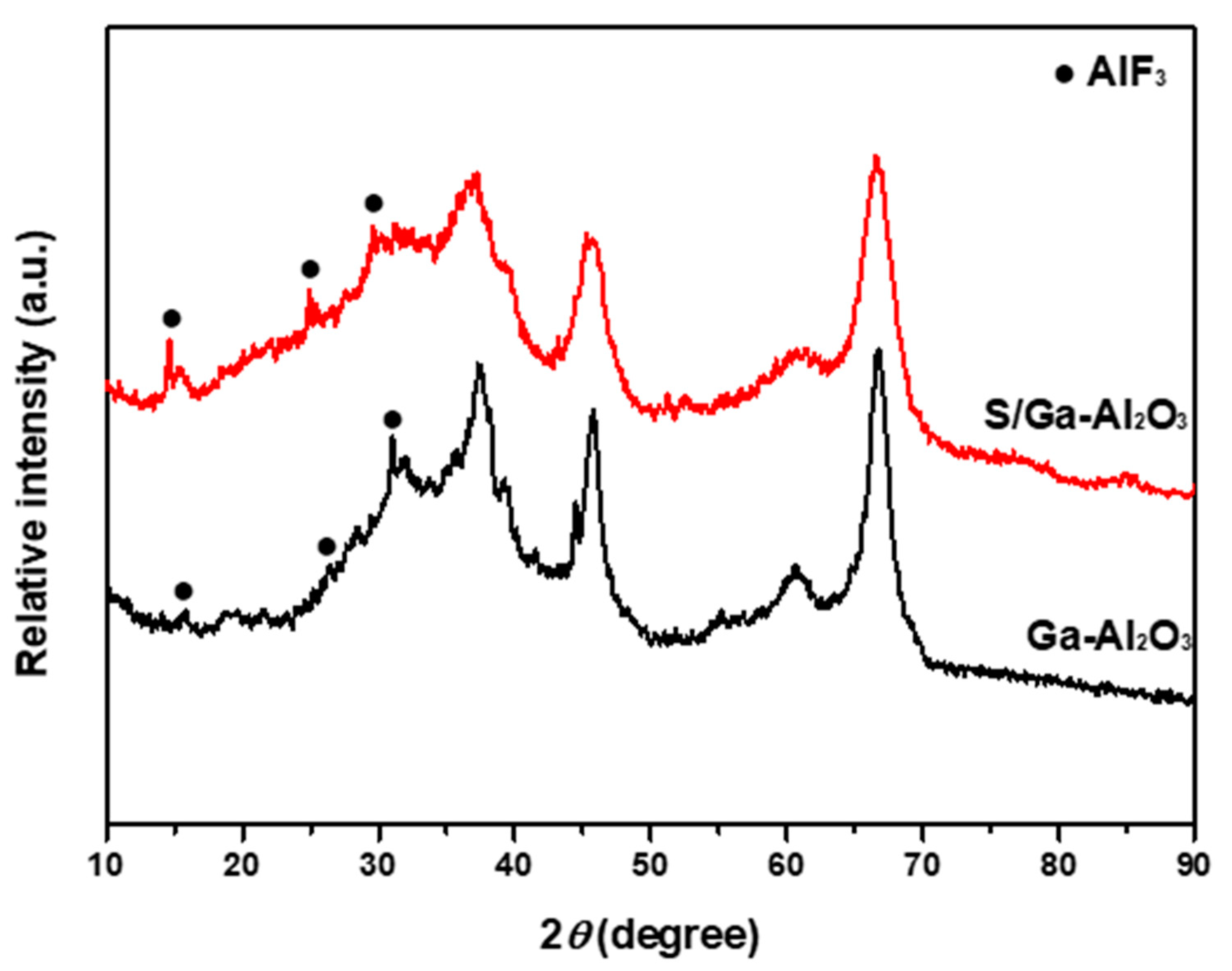
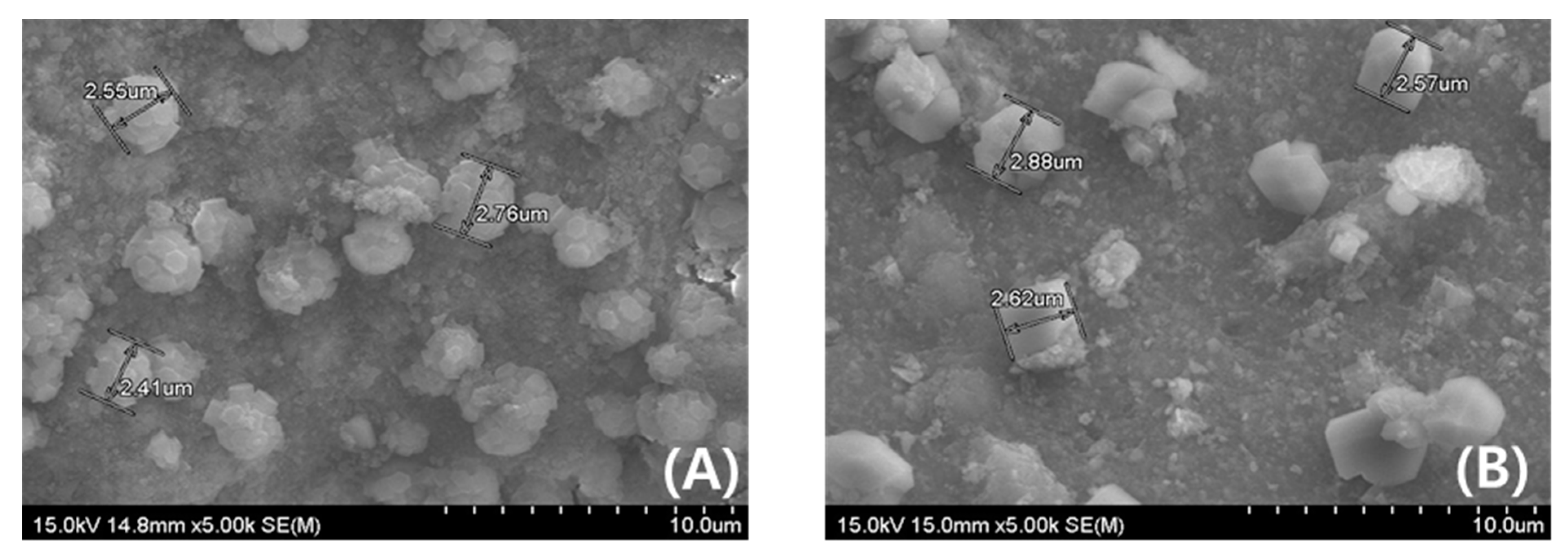
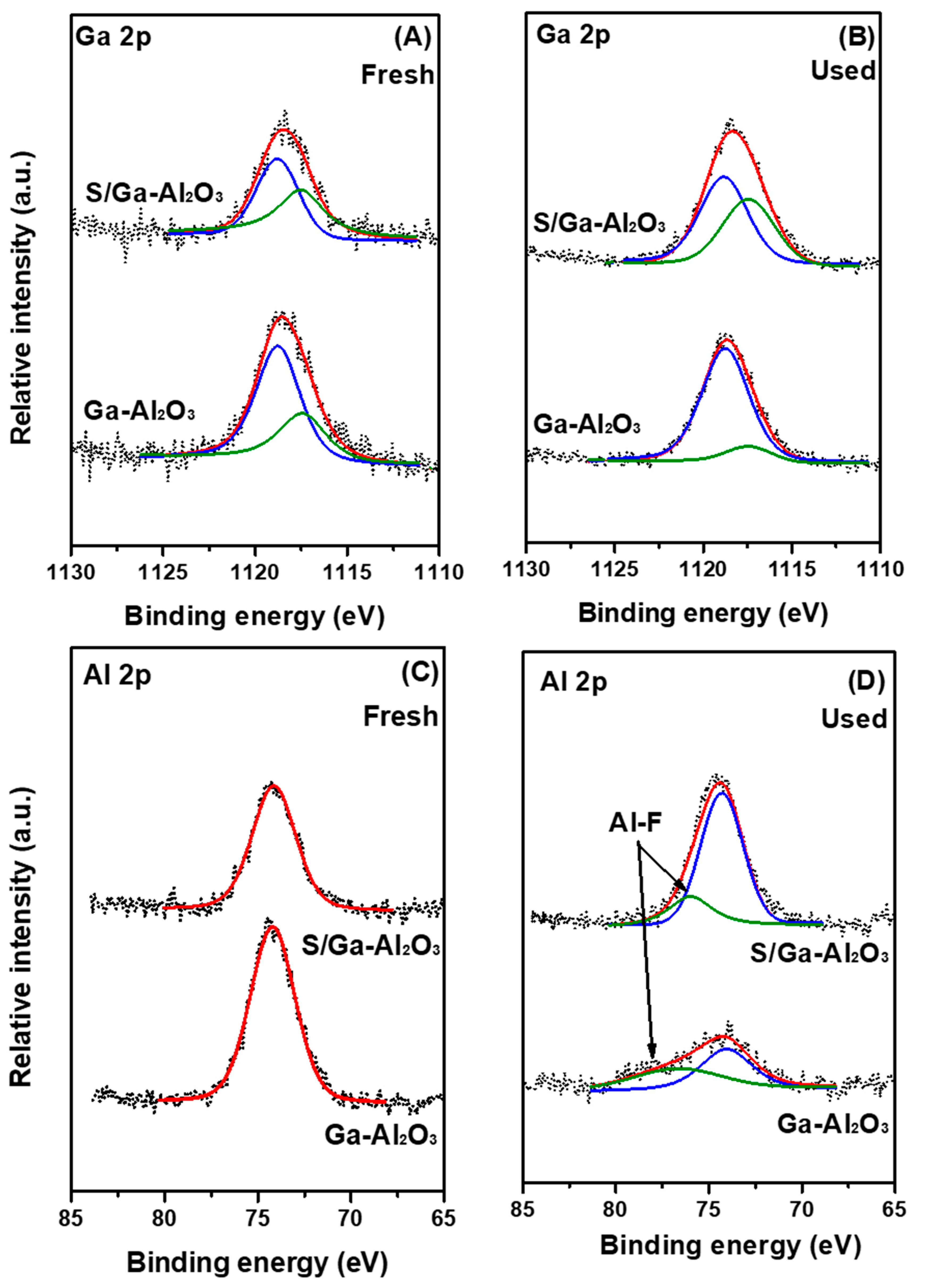
2.2. Observation of Change in Surface Properties by HF Poisoning
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Reaction
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravishankara, A.; Solomon, S.; Turnipseed, A.A.; Warren, R. Atmospheric lifetimes of long-lived halogenated species. Science 1993, 259, 194–199. [Google Scholar] [CrossRef]
- Franklin, J. The atmospheric degradation and impact of 1, 1, 1, 2-tetrafluoroethane (hydrofluorocarbon 134a). Chemosphere 1993, 27, 1565–1601. [Google Scholar] [CrossRef]
- Karmakar, S.; Greene, H.L. Oxidative destruction of chlorofluorocarbons (CFC11 and CFC12) by zeolite catalysts. J. Catal. 1992, 138, 364–376. [Google Scholar] [CrossRef]
- Choi, S.-S.; Park, D.-W.; Watanabe, T. Thermal plasma decomposition of fluorinated greenhouse gases. Nucl. Eng. Technol. 2012, 44, 21–32. [Google Scholar] [CrossRef]
- Mi, T.; Han, J.; He, X.; Qin, L. Investigation of HFC-134a decomposition by combustion and its kinetic characteristics in a laboratory scale reactor. Environ. Prot. Eng. 2015, 41, 143–150. [Google Scholar]
- Watanabe, T.; Tsuru, T. Water plasma generation under atmospheric pressure for HFC destruction. Thin Solid Films 2008, 516, 4391–4396. [Google Scholar] [CrossRef]
- Takita, Y.; Tanabe, T.; Ito, M.; Ogura, M.; Muraya, T.; Yasuda, S.; Nishiguchi, H.; Ishihara, T. Decomposition of CH2FCF3 (134a) over metal phosphate catalysts. Ind. Eng. Chem. Res. 2002, 41, 2585–2590. [Google Scholar] [CrossRef]
- Swamidoss, C.M.A.; Sheraz, M.; Anus, A.; Jeong, S.; Park, Y.-K.; Kim, Y.-M.; Kim, S. Effect of Mg/Al2O3 and calcination temperature on the catalytic decomposition of HFC-134a. Catalysts 2019, 9, 270. [Google Scholar] [CrossRef]
- Iizuka, A.; Ishizaki, H.; Mizukoshi, A.; Noguchi, M.; Yamasaki, A.; Yanagisawa, Y. Simultaneous decomposition and fixation of F-gases using waste concrete. Ind. Eng. Chem. Res. 2011, 50, 11808–11814. [Google Scholar] [CrossRef]
- Song, J.-Y.; Chung, S.-H.; Kim, M.-S.; Seo, M.-G.; Lee, Y.-H.; Lee, K.-Y.; Kim, J.-S. The catalytic decomposition of CF4 over Ce/Al2O3 modified by a cerium sulfate precursor. J. Mol. Catal. A Chem. 2013, 370, 50–55. [Google Scholar] [CrossRef]
- Takita, Y.; Moriyama, J.-I.; Nishiguchi, H.; Ishihara, T.; Hayano, F.; Nakajo, T. Decomposition of CCl2F2 over metal sulfate catalysts. Catal. Today 2004, 88, 103–109. [Google Scholar] [CrossRef]
- Takita, Y.; Moriyama, J.-I.; Yoshinaga, Y.; Nishiguchi, H.; Ishihara, T.; Yasuda, S.; Ueda, Y.; Kubo, M.; Miyamoto, A. Adsorption of water vapor on the AlPO4-based catalysts and reaction mechanism for CFCs decomposition. Appl. Catal. A Gen. 2004, 271, 55–60. [Google Scholar] [CrossRef]
- Takita, Y.; Ishihara, T. Catalytic decomposition of CFCs. Catal. Surv. Asia 1998, 2, 165–173. [Google Scholar] [CrossRef]
- Jia, W.; Wu, Q.; Lang, X.; Hu, C.; Zhao, G.; Li, J.; Zhu, Z. Influence of lewis acidity on catalytic activity of the porous alumina for dehydrofluorination of 1,1,1,2-tetrafluoroethane to trifluoroethylene. Catal. Lett. 2014, 145, 654–661. [Google Scholar] [CrossRef]
- Kashiwagi, D.; Takai, A.; Takubo, T.; Nagaoka, K.; Inoue, T.; Takita, Y. Metal phosphate catalysts effective for degradation of sulfur hexafluoride. Ind. Eng. Chem. Res. 2008, 48, 632–640. [Google Scholar] [CrossRef]
- Kashiwagi, D.; Takai, A.; Takubo, T.; Yamada, H.; Inoue, T.; Nagaoka, K.; Takita, Y. Catalytic activity of rare earth phosphates for SF6 decomposition and promotion effects of rare earths added into AlPO4. J. Colloid Interf. Sci. 2009, 332, 136–144. [Google Scholar] [CrossRef]
- Afonasenko, T.N.; Leont’eva, N.N.; Talzi, V.P.; Smirnova, N.S.; Savel’eva, G.G.; Shilova, A.V.; Tsyrul’nikov, P.G. Synthesis and properties of γ-Ga2O3–Al2O3 solid solutions. Russ. J. Phys. Chem. A 2017, 91, 1939–1945. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Ohnishi, R.; Ichikawa, M. Hydrolytic decomposition of CF4 over alumina-based binary metal oxide catalysts: High catalytic activity of gallia-alumina catalyst. Catal. Today 2004, 90, 283–290. [Google Scholar] [CrossRef]
- Mekhemer, G.A.H.; Khalaf, H.A.; Mansour, S.A.A.; Nohman, A.K.H. Sulfated alumina catalysts: Consequences of sulfate content and source. Monatsh. Chem. 2005, 136, 2007–2016. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. In situ DRIFTS and temperature-programmed technology study on NH3-SCR of NO over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B Environ. 2014, 156–157, 428–437. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Ohnishi, R.; Ichikawa, M. Hydrolysis of CF4 over alumina-based binary metal oxide catalysts. Appl. Catal. B Environ. 2003, 40, 81–91. [Google Scholar] [CrossRef]
- Han, T.U.; Yoo, B.-S.; Kim, Y.-M.; Hwang, B.; Sudibya, G.L.; Park, Y.-K.; Kim, S. Catalytic conversion of 1,1,1,2-tetrafluoroethane (HFC-134a). Korean J. Chem. Eng. 2018, 35, 1611–1619. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wang, Y.; Cen, W.; Wu, Z.; Wang, H.; Weng, X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl Catal. B Environ. 2014, 148–149, 582–588. [Google Scholar] [CrossRef]
- Takita, Y.; Wakamatsu, H.; Li, G.-L.; Moro-Oka, Y.; Nishiguchi, H.; Ishihara, T. Decomposition of chlorofluorocarbons over metal phosphate catalysis: II. Origin of the stability of AlPO4 and the location of Ce as a promoter. J. Mol. Catal. A Chem. 2000, 155, 111–119. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Zhang, J.; Huo, X.; Lai, W.; Lian, Y.; Fang, W. Co-aromatization of n-butane and methanol over PtSnK-Mo/ZSM-5 zeolite catalysts: The promotion effect of ball-milling. Catalysts 2018, 8, 307. [Google Scholar] [CrossRef]
- Chen, H.; Hu, J.; Li, G.D.; Gao, Q.; Wei, C.; Zou, X. Porous Ga-In bimetallic oxide nanofibers with controllable structures for ultrasensitive and selective detection of formaldehyde. ACS Appl. Mater. Interfaces 2017, 9, 4692–4700. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.; Wang, X.; Zhang, Q.; Xie, H.; Han, Y.; Tan, Y. A highly efficient Ga/ZSM-5 catalyst prepared by formic acid impregnation and in situ treatment for propane aromatization. Catal. Sci. Technol. 2015, 5, 4081–4090. [Google Scholar] [CrossRef]
- Limcharoen, A.; Pakpum, C.; Limsuwan, P. An X-ray photoelectroscopy investigation of re-deposition from fluorine-based plasma etch on magnetic recording slider head substrate. Procedia Eng. 2012, 32, 1043–1049. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, B.J.; Kang, S.K.; Kong, B.H.; Cho, H.K.; Yeom, G.Y.; Heo, S.; Hwang, H. Characteristics of Al2O3 gate dielectrics partially fluorinated by a low energy fluorine beam. Appl. Phys. Lett. 2008, 93, 191506. [Google Scholar] [CrossRef]
- Zhang, T.; Park, J.Y.; Huang, W.; Somorjai, G.A. Influence of reaction with XeF2 on surface adhesion of Al and Al2O3 surfaces. Appl. Phys. Lett. 2008, 93, 141905. [Google Scholar] [CrossRef]



| Sample | Ga (wt.%) * | Al (wt.%) * | S (wt.%) ** |
|---|---|---|---|
| Ga-Al2O3 | 14.7 | 52.8 | |
| S/Ga-Al2O3 | 15.3 | 53.5 | 1.51 |
| Samples | BET Surface Area [m2 g−1] | Pore Volume [m3 g−1] | Amount of Acid Site [mmol g−1] | |||
|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Total | |||
| Ga-Al2O3 | 227.5 | 0.35 | 0.072 | 0.154 | 0.342 | 0.568 |
| S/Ga-Al2O3 | 187.4 | 0.29 | 0.117 | 0.207 | 0.312 | 0.646 |
| Elements (Weight %) | Ga-Al2O3 | S/Ga-Al2O3 |
|---|---|---|
| Ga | 13.9 | 13.8 |
| Al | 30.6 | 34.4 |
| O | 30.3 | 37.2 |
| F | 25.2 | 13.8 |
| S | 0.8 |
| Samples | Ga0/(Ga0 + Ga3+) | Binding Energy (eV) | |
|---|---|---|---|
| Ga0 | Ga3+ | ||
| Ga-Al2O3 | 0.30 | 1117.4 | 1118.7 |
| S/Ga-Al2O3 | 0.44 | 1117.5 | 1118.8 |
| Ga-Al2O3 used | 0.11 | 1117.5 | 1118.7 |
| S/Ga-Al2O3 used | 0.41 | 1117.4 | 1118.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Kim, Y.; Youn, J.-R.; Choi, I.-H.; Hwang, K.-R.; Kim, S.G.; Park, Y.-K.; Moon, S.-H.; Lee, K.B.; Jeon, S.G. Effects of Sulfuric Acid Treatment on the Performance of Ga-Al2O3 for the Hydrolytic Decomposition of 1,1,1,2-Tetrafluoroethane (HFC-134a). Catalysts 2020, 10, 766. https://doi.org/10.3390/catal10070766
Kim M-J, Kim Y, Youn J-R, Choi I-H, Hwang K-R, Kim SG, Park Y-K, Moon S-H, Lee KB, Jeon SG. Effects of Sulfuric Acid Treatment on the Performance of Ga-Al2O3 for the Hydrolytic Decomposition of 1,1,1,2-Tetrafluoroethane (HFC-134a). Catalysts. 2020; 10(7):766. https://doi.org/10.3390/catal10070766
Chicago/Turabian StyleKim, Min-Jae, Yeonjin Kim, Jae-Rang Youn, Il-Ho Choi, Kyung-Ran Hwang, Seung Gon Kim, Young-Kwon Park, Seung-Hyun Moon, Ki Bong Lee, and Sang Goo Jeon. 2020. "Effects of Sulfuric Acid Treatment on the Performance of Ga-Al2O3 for the Hydrolytic Decomposition of 1,1,1,2-Tetrafluoroethane (HFC-134a)" Catalysts 10, no. 7: 766. https://doi.org/10.3390/catal10070766
APA StyleKim, M.-J., Kim, Y., Youn, J.-R., Choi, I.-H., Hwang, K.-R., Kim, S. G., Park, Y.-K., Moon, S.-H., Lee, K. B., & Jeon, S. G. (2020). Effects of Sulfuric Acid Treatment on the Performance of Ga-Al2O3 for the Hydrolytic Decomposition of 1,1,1,2-Tetrafluoroethane (HFC-134a). Catalysts, 10(7), 766. https://doi.org/10.3390/catal10070766







