Low-Noble-Metal-Loading Hybrid Catalytic System for Oxygen Reduction Utilizing Reduced-Graphene-Oxide-Supported Platinum Aligned with Carbon-Nanotube-Supported Iridium
Abstract
1. Introduction
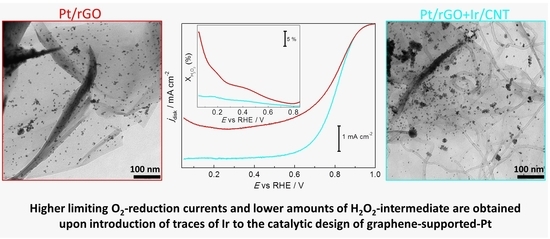
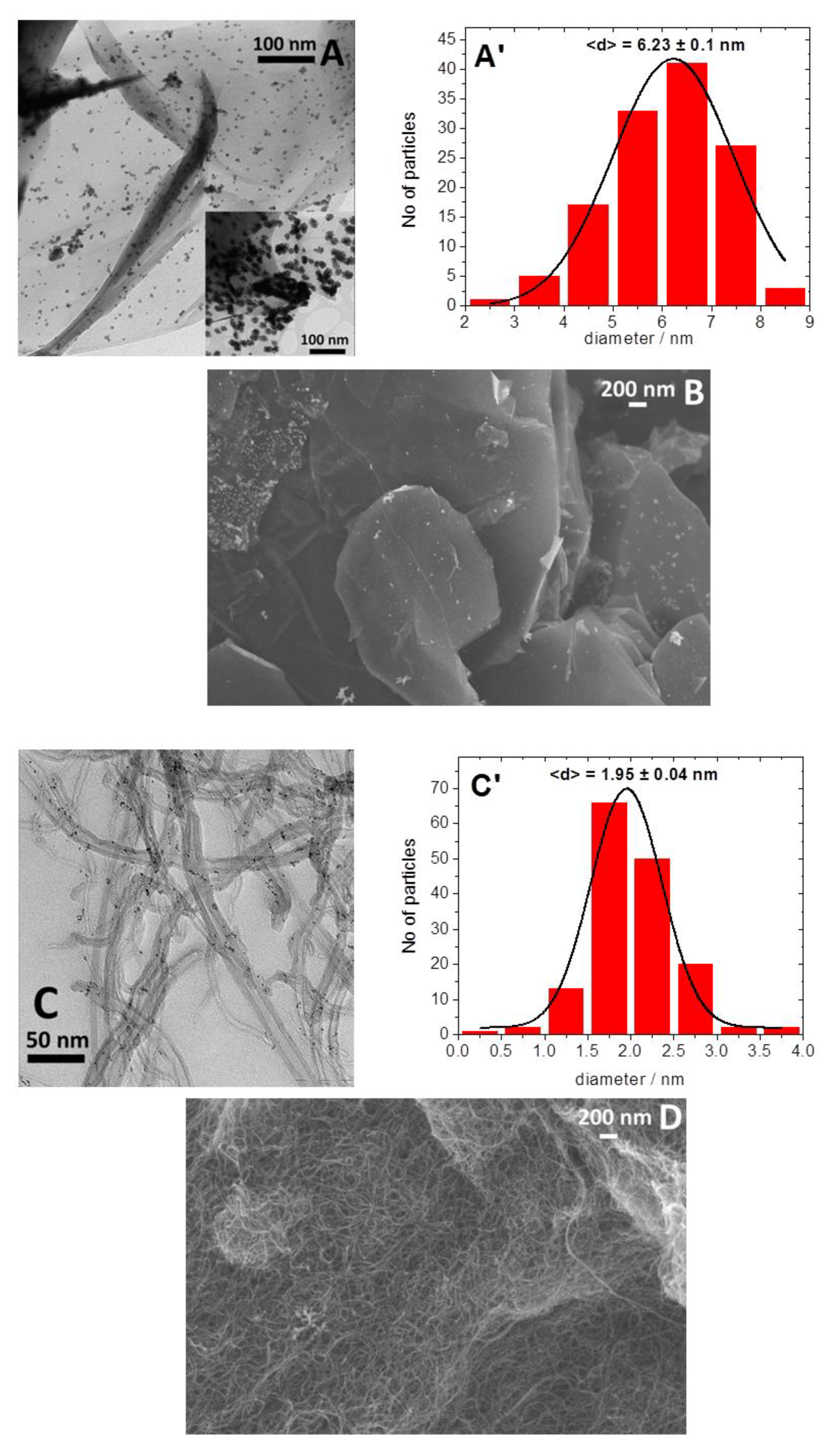
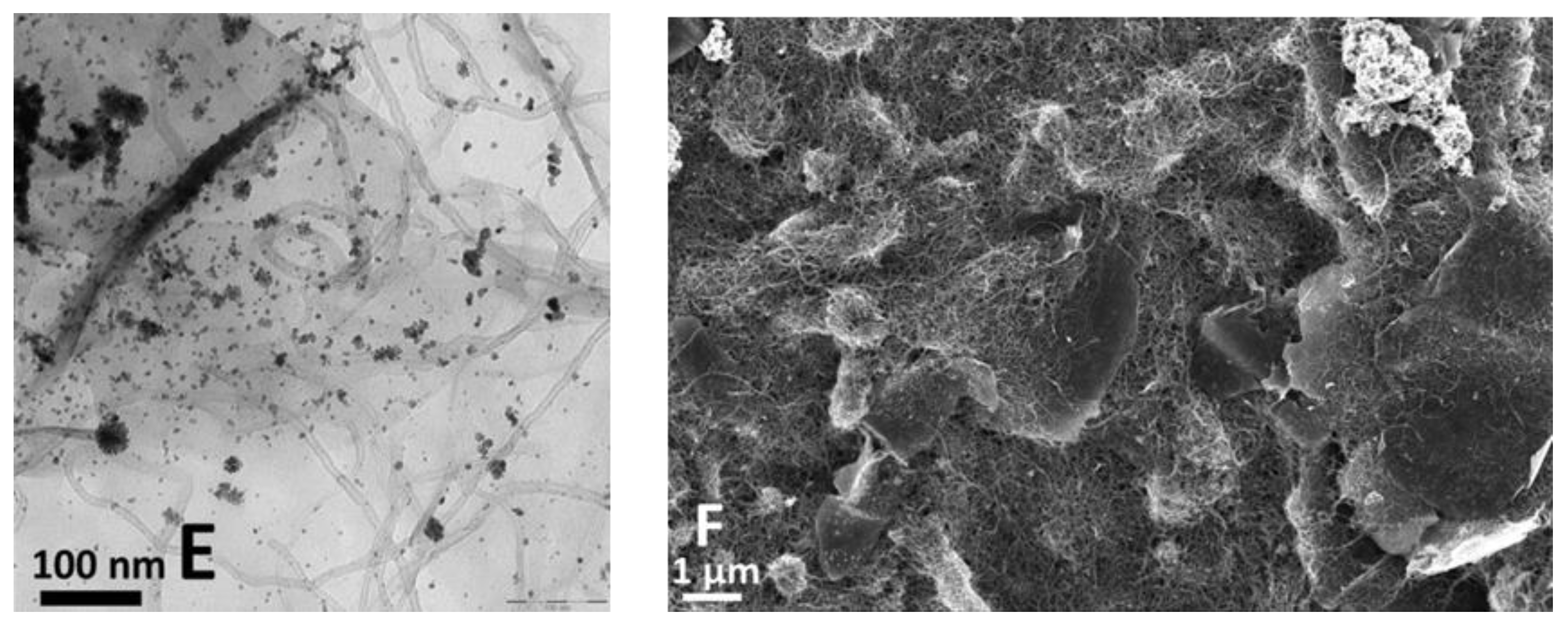
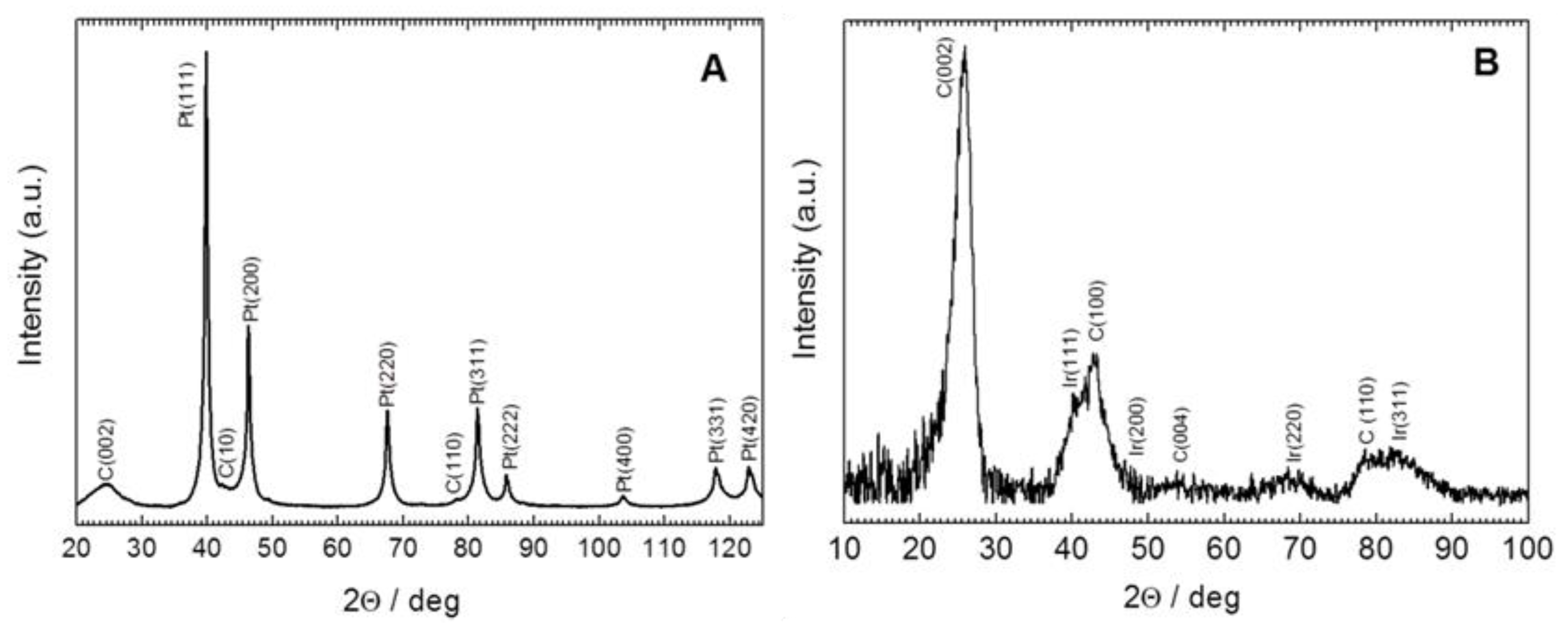
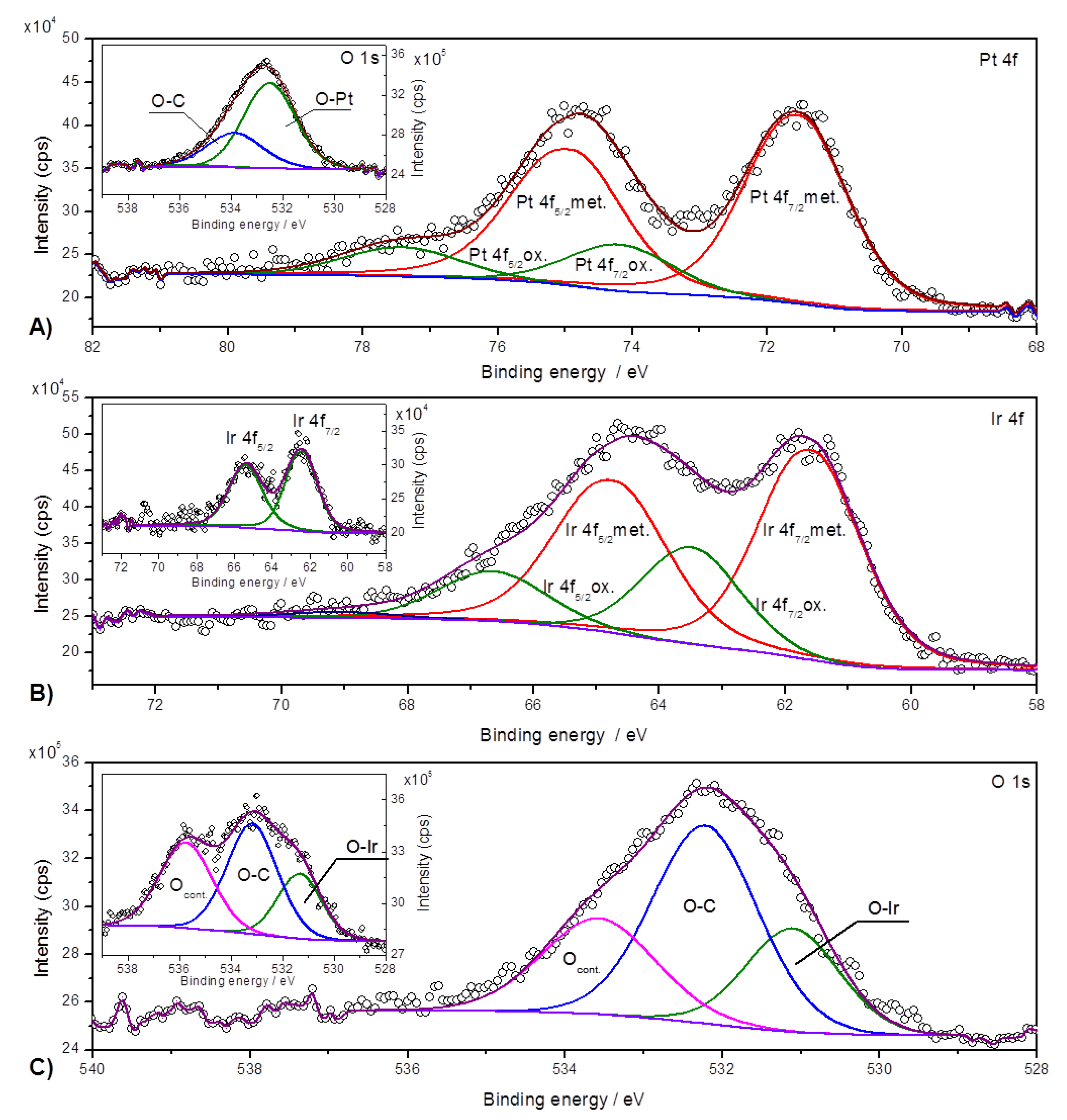
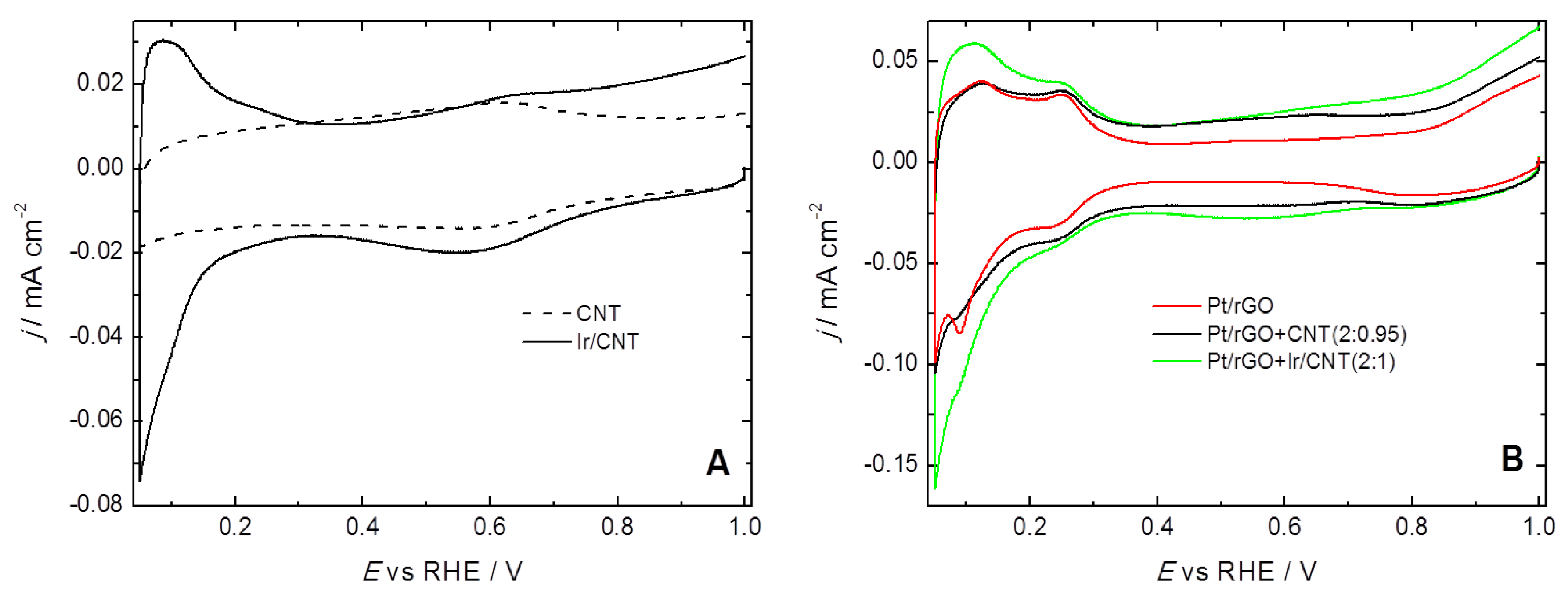
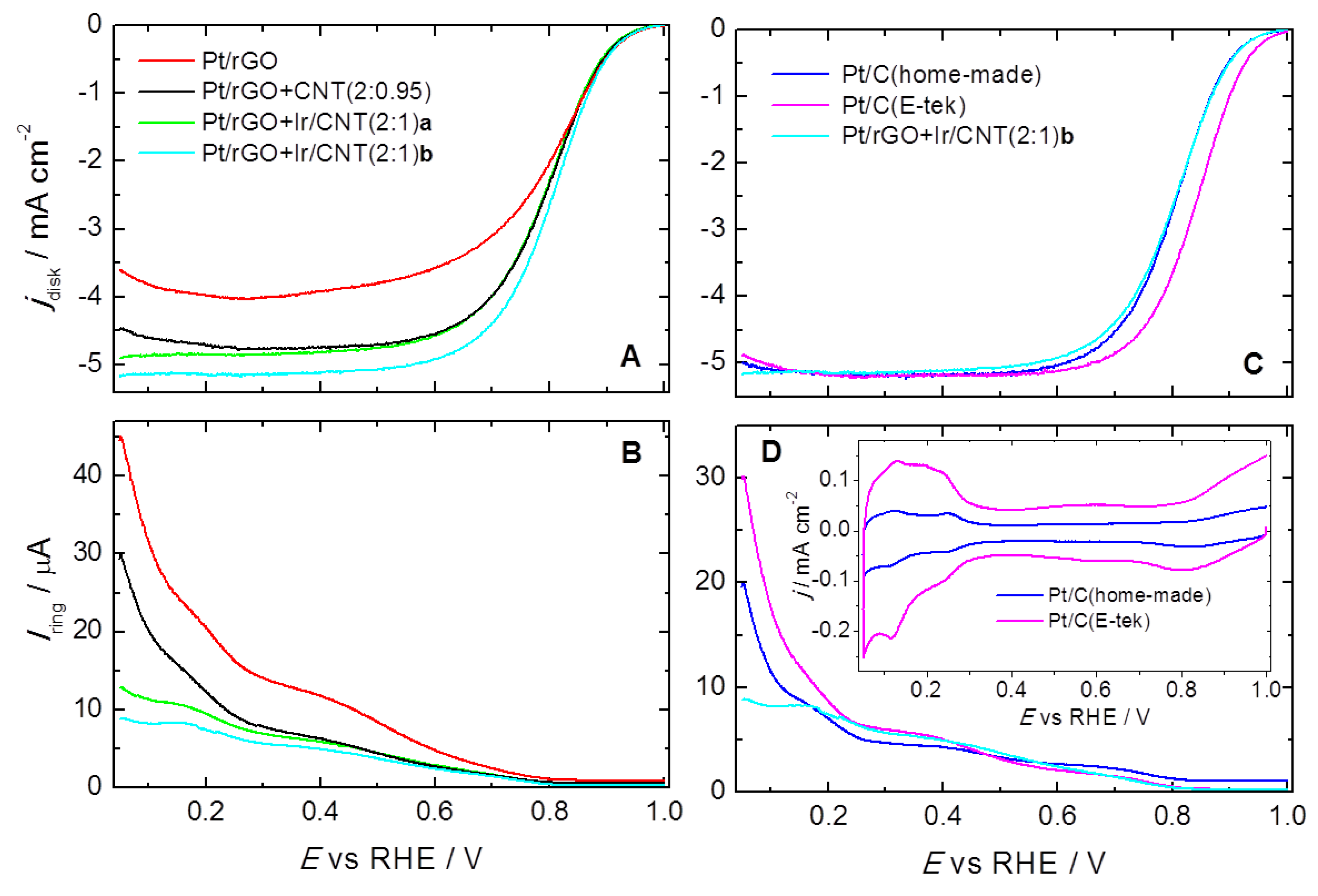
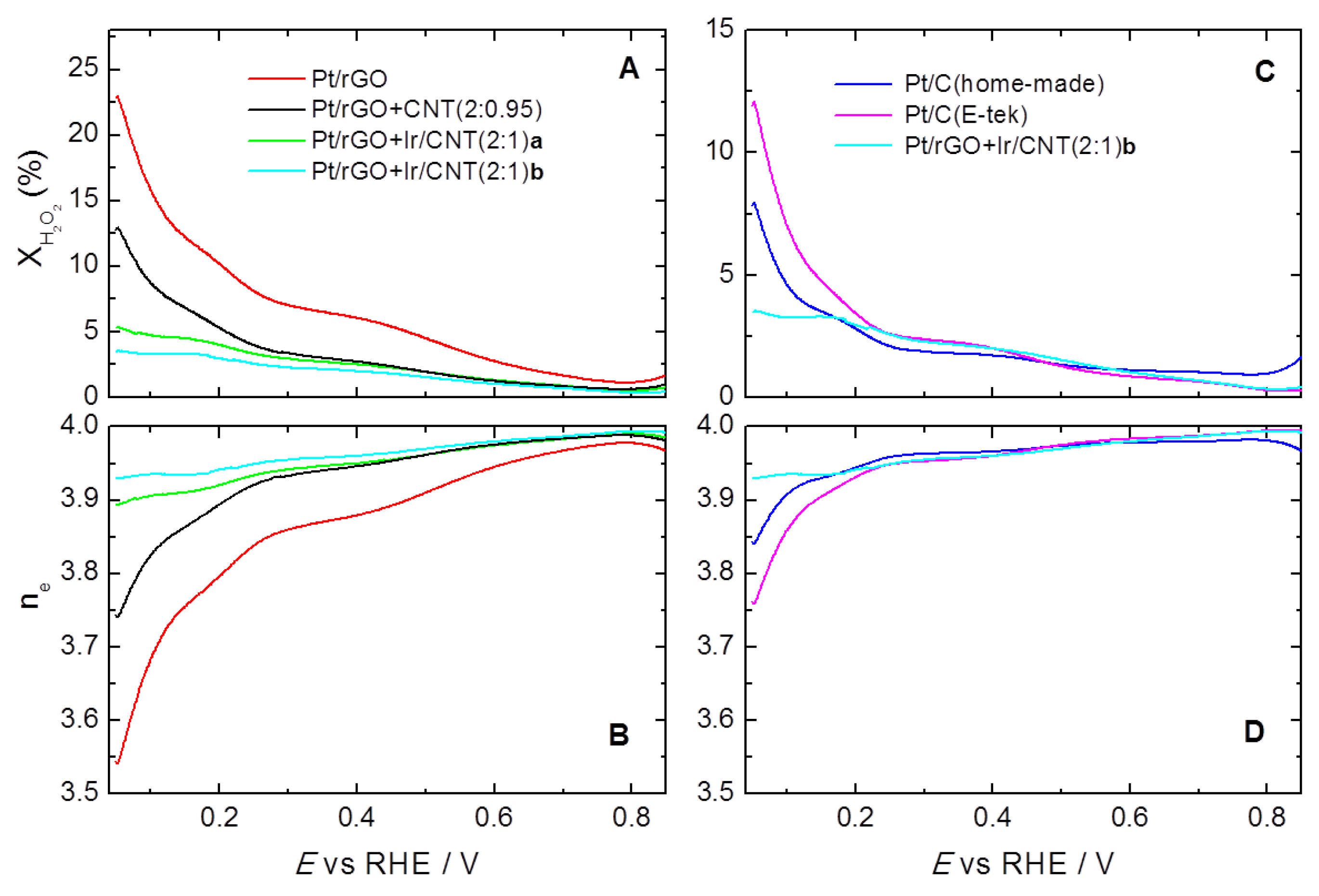
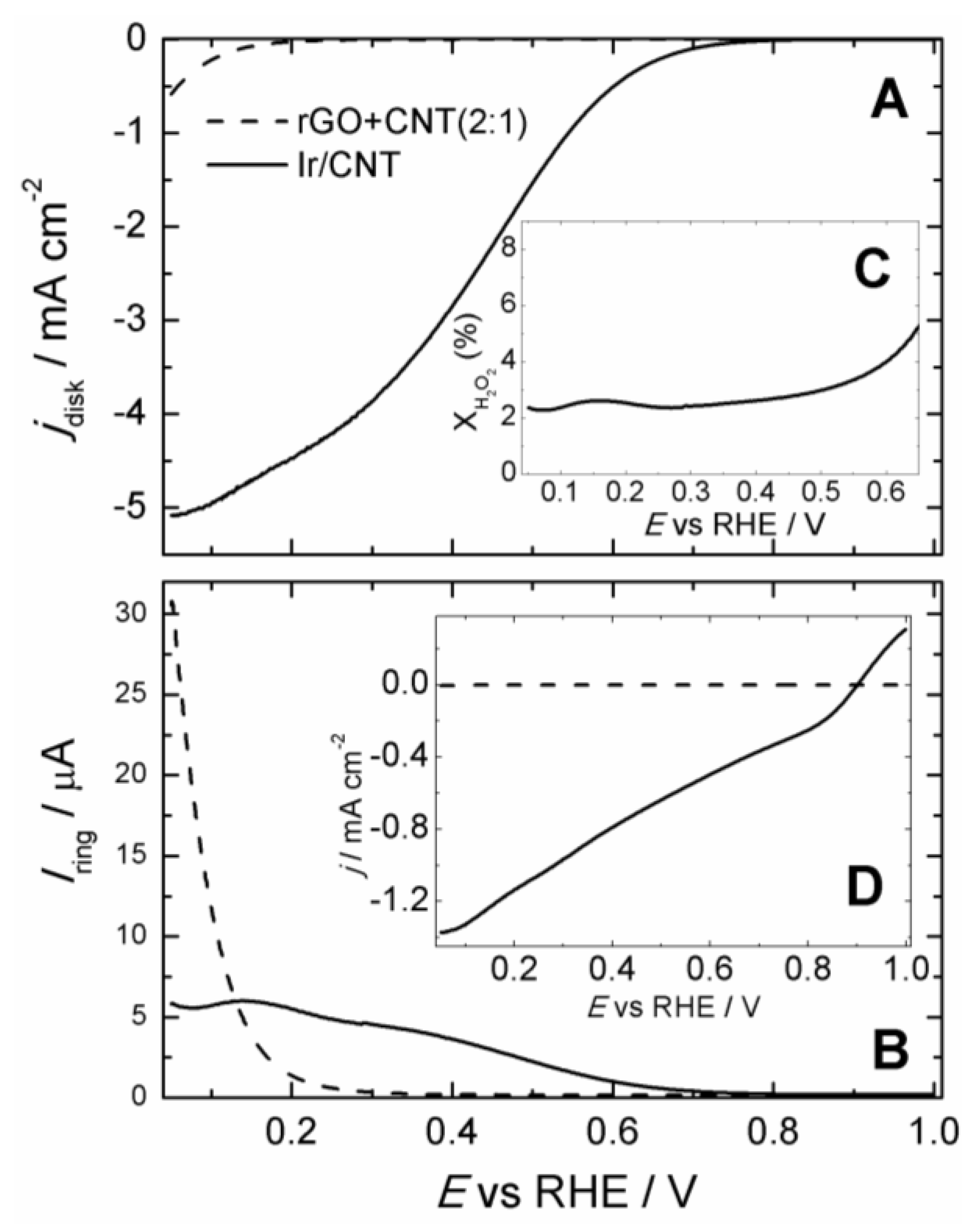
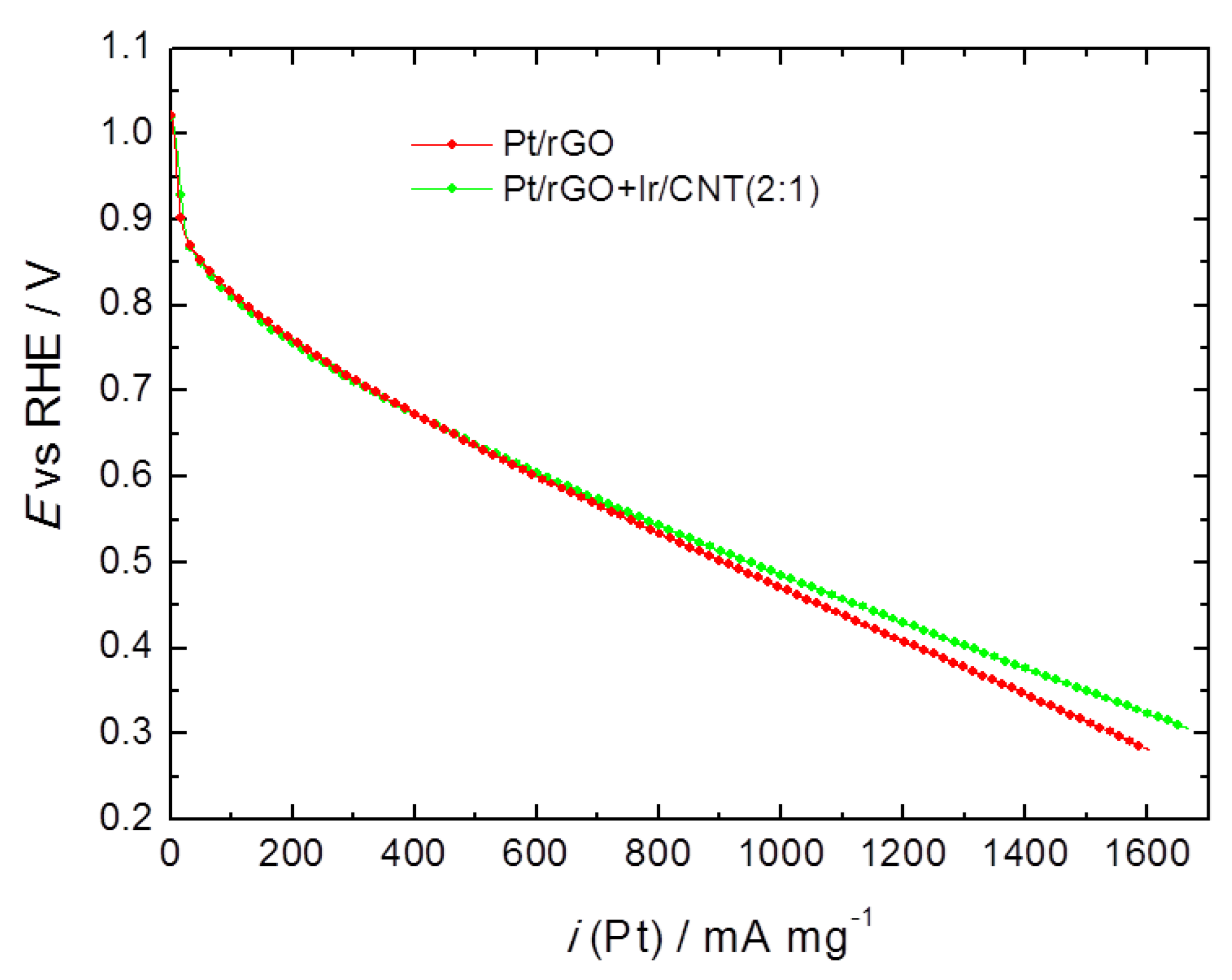
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Materials Preparation
3.3. Equipment and Characterization of Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prabhakaran, V.; Arges, C.G.; Ramani, V. In situ fluorescence spectroscopy correlates ionomer degradation to reactive oxygen species generation in an operating fuel cell. Phys. Chem. Chem. Phys. 2013, 15, 18965–18972. [Google Scholar] [CrossRef] [PubMed]
- Zlotorowicz, A.; Jayasayee, K.; Dahl, P.I.; Thomassen, M.S.; Kjelstrup, S. Tailored porosities of the cathode layer for improved polymer electrolyte fuel cell performance. J. Power Sources 2015, 287, 472–477. [Google Scholar] [CrossRef]
- Jayasayee, K.; Zlotorowicz, A.; Clos, D.P.; Dahl, Ø.; Thomassen, M.S.; Dahl, P.I.; Kjelstrup, S. Improved cathode catalyst layers for proton exchange membrane fuel cells. ECS Trans. 2014, 64, 321–339. [Google Scholar] [CrossRef]
- Roen, L.M.; Paik, C.H.; Jarvi, T.D. Electrocatalytic corrosion of carbon support in PEMFC cathodes. Electrochem. Solid State Lett. 2004, 7, A19–A22. [Google Scholar] [CrossRef]
- Schonvogel, D.; Hulstede, J.; Wagner, P.; Kruusenberg, I.; Tammeveski, K.; Dyck, A.; Agert, C.; Wark, M. Stability of Pt nanoparticles on alternative carbon supports for oxygen reduction reaction. J. Electrochem. Soc. 2017, 164, F995–F1004. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Wang, C.; Nie, Z.; Liu, J.; Wang, Y.; Lin, Y. Highly durable graphene nanoplatelets supported Pt nanocatalysts for oxygen reduction. J. Power Sources 2010, 195, 4600–4605. [Google Scholar] [CrossRef]
- Higgins, D.; Zamani, P.; Yu, A.; Chen, Z. The application of graphene and its composites in oxygen reduction electrocatalysis: A perspective and review of recent progress. Energy Environ. Sci. 2016, 9, 357–390. [Google Scholar] [CrossRef]
- He, D.; Cheng, K.; Peng, T.; Sun, X.; Pan, M.; Mu, S. Bifunctional effect of reduced graphene oxides to support active metal nanoparticles for oxygen reduction reaction and stability. J. Mater. Chem. 2012, 22, 21298–21304. [Google Scholar] [CrossRef]
- Lee, J.-S.; Jo, K.; Lee, T.; Yun, T.; Cho, J.; Kim, B.-S. Facile synthesis of hybrid graphene and carbon nanotubes as a metal-free electrocatalyst with active dual interfaces for efficient oxygen reduction reaction. J. Mater. Chem. A 2013, 1, 9603–9607. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, T.-Y.; Qian, Y.-H.; Li, S.-S.; Yu, S.-H. A nitrogen-doped graphene/carbon nanotube nanocomposite with synergistically enhanced electrochemical activity. Adv. Mater. 2013, 25, 3192–3196. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Vikkisk, M.; Joost, U.; Shulga, E.; Kink, I.; Kallio, T.; Tammeveski, K. Highly active nitrogen-doped few-layer graphene/carbon nanotube composite electrocatalyst for oxygen reduction reaction in alkaline media. Carbon 2014, 73, 361–370. [Google Scholar] [CrossRef]
- Shui, J.; Wang, M.; Du, F.; Dai, L. N-doped carbon nanomaterials are durable catalysts for oxygen reduction reaction in acidic fuel cells. Sci. Adv. 2015, 1, e1400129. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Park, H.J.; Kim, J.; Hur, S.H. Highly durable Pt/graphene oxide and Pt/C hybrid catalyst for polymer electrolyte membrane fuel cell. J. Power Sources 2014, 248, 1156–1162. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhu, E.; McLouth, T.; Chiu, C.-Y.; Huang, X.; Huang, Y. Stabilization of high-performance oxygen reduction reaction Pt electrocatalyst supported on reduced graphene oxide/carbon black composite. J. Am. Chem. Soc. 2012, 134, 12326–12329. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Cheng, K.; Peng, T.; Pan, M.; Mu, S. Graphene/carbon nanospheres sandwich supported PEM fuel cell metal nanocatalysts with remarkably high activity and stability. J. Mater. Chem. A 2013, 1, 2126–2132. [Google Scholar] [CrossRef]
- Pham, K.-C.; McPhail, D.S.; Mattevi, C.; Wee, A.T.S.; Chu, D.H.C. Graphene-carbon nanotube hybrids as robust catalyst supports in proton exchange membrane fuel cells. J. Electrochem. Soc. 2016, 163, F255–F263. [Google Scholar] [CrossRef]
- Jyothirmayee Aravind, S.; Imran Jafri, R.; Rajalakshmi, N.; Ramaprabhu, S. Solar exfoliated graphene–carbon nanotube hybrid nanocomposites as efficient catalyst supports for proton exchange membrane fuel cells. J. Mater. Chem. 2011, 21, 18199–18204. [Google Scholar] [CrossRef]
- Yoo, E.J.; Kim, J.; Hosono, E.; Zhou, H.-S.; Kudo, T.; Honma, I. Large reversible li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef]
- Antolini, E. Iridium as catalyst and cocatalyst for oxygen evolution/reduction in acidic polymer electrolyte membrane electrolyzers and fuel cells. ACS Catal. 2014, 4, 1426–1440. [Google Scholar] [CrossRef]
- Ioroi, T.; Yasuda, K. Platinum-iridium alloys as oxygen reduction electrocatalysts for polymer electrolyte fuel cells. J. Electrochem. Soc. 2005, 152, A1917–A1924. [Google Scholar] [CrossRef]
- Holt-Hindle, P.; Yi, Q.; Wu, G.; Koczkur, K.; Chen, A. Electrocatalytic activity of nanoporous Pt-Ir materials toward methanol oxidation and oxygen reduction. J. Electrochem. Soc. 2008, 155, K5–K9. [Google Scholar] [CrossRef]
- Zhang, G.; Shao, Z.-G.; Lu, W.; Li, G.; Liu, F.; Yi, B. One-pot synthesis of Ir@Pt nanodendrites as highly active bifunctional electrocatalysts for oxygen reduction and oxygen evolution in acidic medium. Electrochem. Commun. 2012, 22, 145–148. [Google Scholar] [CrossRef]
- Zhang, J.; Vukmirovic, M.B.; Sasaki, K.; Nilekar, A.U.; Mavrikakis, M.; Adzic, R.R. Mixed-metal Pt monolayer electrocatalysts for enhanced oxygen reduction kinetics. J. Am. Chem. Soc. 2005, 127, 12480–12481. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Eid, K.; Deng, Y.; Guo, J.; Wang, L.; Wang, H.; Gu, H. One-pot synthesis of Pt-Ir tripods with a dendritic surface as an efficient catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 9107–9112. [Google Scholar] [CrossRef]
- Topalov, G.; Ganske, G.; Lefterova, E.; Schnakenberg, U.; Slavcheva, E. Preparation and properties of thin Pt-Ir films deposited by dc magnetron co-sputtering. Int. J. Hydrogen Energy 2011, 36, 15437–15445. [Google Scholar] [CrossRef]
- Radev, I.; Topalov, G.; Lefterova, E.; Ganske, G.; Schnakenberg, U.; Tsotridis, G.; Slavcheva, E. Optimization of platinum/iridium ratio in thin sputtered films for PEMFC cathodes. Int. J. Hydrogen Energy 2012, 37, 7730–7735. [Google Scholar] [CrossRef]
- Wesselmark, M.; Wickman, B.; Lagergrena, C.; Lindbergh, G. The impact of iridium on the stability of platinum on carbon thin-film model electrodes. Electrochim. Acta 2013, 111, 152–159. [Google Scholar] [CrossRef]
- Kim, I.G.; Nah, I.W.; Oh, I.-H.; Park, S. Crumpled rGO-supported Pt-Ir bifunctional catalyst prepared by spray pyrolysis for unitized regenerative fuel cells. J. Power Sources 2017, 364, 215–225. [Google Scholar] [CrossRef]
- Kuttiyiel, K.A.; Sasaki, K.; Choi, Y.M.; Su, D.; Liu, P.; Adzic, R.R. Bimetallic IrNi core platinum monolayer shell electrocatalysts for the oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 5297–5304. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Ganesan, P.; Jung, H.-Y.; Popov, B.N. Development of supported bifunctional oxygen electrocatalysts and corrosion-resistant gas diffusion layer for unitized regenerative fuel cell applications. J. Power Sources 2012, 198, 23–29. [Google Scholar] [CrossRef]
- Diodati, S.; Negro, E.; Vezzù, K.; Di Noto, V.; Gross, S. Oxygen reduction reaction and X-ray photoelectron spectroscopy characterisation of carbon nitride-supported bimetallic electrocatalysts. Electrochim. Acta 2016, 215, 398–409. [Google Scholar] [CrossRef]
- Ioroi, T.; Kitazawa, N.; Yasuda, K.; Yamamoto, Y.; Takenaka, H. Iridium oxide/platinum electrocatalysts for unitized regenerative polymer electrolyte fuel cells. J. Electrochem. Soc. 2000, 147, 2018–2022. [Google Scholar] [CrossRef]
- Yao, W.; Yang, J.; Wang, J.; Nuli, Y. Chemical deposition of platinum nanoparticles on iridium oxide for oxygen electrode of unitized regenerative fuel cell. Electrochem. Commun. 2007, 9, 1029–1034. [Google Scholar] [CrossRef]
- da Silva, G.C.; Fernandes, M.R.; Ticianelli, E.A. Activity and stability of Pt/IrO2 bifunctional materials as catalysts for the oxygen evolution/reduction reactions. ACS Catal. 2018, 8, 2081–2092. [Google Scholar] [CrossRef]
- Takimoto, D.; Fukuda, K.; Miyasaka, S.; Ishida, T.; Ayato, Y.; Mochizuki, D.; Shimizu, W.; Sugimoto, W. Synthesis and oxygen electrocatalysis of iridium oxide nanosheets. Electrocatalysis 2017, 8, 144–150. [Google Scholar] [CrossRef]
- Jang, S.-E.; Kim, H. Effect of water electrolysis catalysts on carbon corrosion in polymer electrolyte membrane fuel cells. J. Am. Chem. Soc. 2010, 132, 14700–14701. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-G.; Lee, W.H.; Kim, H. The inhibition of electrochemical carbon corrosion in polymer electrolyte membrane fuel cells using iridium nanodendrites. Int. J. Hydrogen Energy 2012, 37, 2455–2461. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, H. Optimization of electrode structure to suppress electrochemical carbon corrosion of gas diffusion layer for unitized regenerative fuel cell. J. Electrochem. Soc. 2014, 161, F729–F733. [Google Scholar] [CrossRef]
- Dembinska, B.; Dobrzeniecka, A.; Pisarek, M.; Kulesza, P.J. Selenourea-assisted synthesis of selenium-modified iridium catalysts: Evaluation of their activity toward reduction of oxygen. Electrochim. Acta 2015, 185, 162–171. [Google Scholar] [CrossRef]
- Zhang, T.; Li, S.-C.; Zhu, W.; Zhang, Z.-P.; Gu, J.; Zhang, Y.-W. Shape-tunable Pt-Ir alloy nanocatalysts with high performance in oxygen electrode reactions. Nanoscale 2017, 9, 1154–1165. [Google Scholar] [CrossRef]
- Jovanovič, P.; Hodnik, N.; Ruiz-Zepeda, F.; Arčon, I.; Jozinović, B.; Zorko, M.; Bele, M.; Šala, M.; Šelih, V.S.; Hočevar, S.; et al. Electrochemical dissolution of iridium and iridium oxide particles in acidic media: Transmission electron microscopy, electrochemical flow cell coupled to inductively coupled plasma mass spectrometry, and X-ray absorption spectroscopy study. J. Am. Chem. Soc. 2017, 139, 12837–12846. [Google Scholar] [CrossRef]
- Choi, H.C.; Shim, M.; Bangsaruntip, S.; Dai, H. Spontaneous reduction of metal ions on the sidewalls of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 9058–9059. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, G.; Chen, J.; Chen, X.; Xie, Z.; Wang, X. Synthesis of “clean” and well-dispersive Pd nanoparticles with excellent electrocatalytic property on graphene oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Tang, H.; Wang, D.; Gao, Y.; Tang, Z. Facile synthesis of surfactant-free au cluster/graphene hybrids for high-performance oxygen reduction reaction. ACS Nano 2012, 6, 8288–8297. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ciganda, R.; Yate, L.; Tuninetti, J.; Shalabaeva, V.; Salmon, L.; Moya, S.; Ruiz, J.; Astruc, D. Redox synthesis and high catalytic efficiency of transition-metal nanoparticle-graphene oxide nanocomposites. J. Mater. Chem. A 2017, 5, 21947–21954. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Cryst. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Lesiak, B.; Mazurkiewicz, M.; Malolepszy, A.; Stobinski, L.; Mierzwa, B.; Mikolajczuk-Zychora, A.; Juchniewicz, K.; Borodzinski, A.; Zemec, J.; Jiricek, P. Effect of the Pd/MWCNTs anode catalysts preparation methods on their morphology and activity in a direct formic acid fuel cell. Appl. Surf. Sci. 2016, 387, 929–937. [Google Scholar] [CrossRef]
- Platinum Transition Metal. Available online: https://xpssimplified.com/elements/platinum.php (accessed on 10 February 2020).
- van Spronsen, M.A.; Frenken, J.W.M.; Groot, I.M.N. Observing the oxidation of platinum. Nat. Commun. 2017, 8, 42. [Google Scholar] [CrossRef]
- El Sawy, E.N.; Birss, V.I. Nano-porous iridium and iridium oxide thin films formed by high efficiency electrodeposition. J. Mater. Chem. 2009, 19, 8244–8252. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Birss, V.I.; Andreas, H.; Serebrennikova, I.; Elzanowska, H. Electrochemical characterization of sol-gel formed Ir metal nanoparticles. Electrochem. Solid State Lett. 1999, 2, 326–329. [Google Scholar] [CrossRef]
- Lee, K.; Zhang, L.; Zhang, J. A novel methanol-tolerant Ir-Se chalcogenide electrocatalyst for oxygen reduction. J. Power Sources 2007, 165, 108–113. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H. Facile synthesis of carbon-supported IrxSey chalcogenide nanoparticles and their electrocatalytic activity for the oxygen reduction reaction. J. Phys. Chem. C 2008, 112, 2058–2065. [Google Scholar] [CrossRef]
- Neergat, M.; Gunasekar, V.; Singh, R.K. Oxygen reduction reaction and peroxide generation on Ir, Rh, and their selenides—A comparison with Pt and RuSe. J. Electrochem. Soc. 2011, 158, B1060–B1066. [Google Scholar] [CrossRef]
- Masa, J.; Batchelor-McAuley, C.; Schuhmann, W.; Compton, R.G. Koutecky-Levich analysis applied to nanoparticle modified rotating disk electrodes: Electrocatalysis or misinterpretation? Nano Res. 2014, 7, 71–78. [Google Scholar] [CrossRef]
- Chatenet, M.; Genies-Bultel, L.; Aurousseau, M.; Durand, R.; Andolfatto, F. Oxygen reduction on silver catalysts in solutions containing various concentrations of sodium hydroxide—Comparison with platinum. J. Appl. Electrochem. 2009, 32, 1131–1140. [Google Scholar] [CrossRef]
- Antoine, O.; Durand, R. RRDE Study of oxygen reduction on Pt nanoparticles inside Nafion®: H2O2 production in PEMFC cathode conditions. J. Appl. Electrochem. 2009, 30, 839–844. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Paulus, U.A.; Gasteiger, H.A.; Behm, R.J. The oxygen reduction reaction on a Pt/carbon fuel cell catalyst in the presence of chloride anions. J. Electroanal. Chem. 2001, 508, 41–47. [Google Scholar] [CrossRef]
- Hoare, J.P. Oxygen overvoltage on bright iridium. J. Electroanal. Chem. 1968, 18, 251–259. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Mingers, A.; Mayrhofer, K.J.J. Oxygen evolution activity and stability of iridium in acidic media. Part 1.—Metallic iridium. J. Electroanal. Chem. 2016, 773, 69–78. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lindau, I.; Pianetta, P.; Yu, K.Y.; Spicer, W.E. Photoemission of gold in the energy range 30–300 eV using synchrotron radiation. Phys. Rev. B 1976, 13, 492–495. [Google Scholar] [CrossRef]
- CasaXPS. Processing Software for XPS, AES, SIMS and More. Available online: http://www.casaxps.com (accessed on 10 February 2020).
- Kulesza, P.J.; Zak, J.K.; Rutkowska, I.A.; Dembinska, B.; Zoladek, S.; Miecznikowski, K.; Negro, E.; Di Noto, V.; Zelanay, P. Elucidation of role of graphene in catalytic designs for electroreduction of oxygen. Curr. Opin. Electrochem. 2018, 9, 257–264. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembinska, B.; Zlotorowicz, A.; Modzelewska, M.; Miecznikowski, K.; Rutkowska, I.A.; Stobinski, L.; Malolepszy, A.; Krzywiecki, M.; Zak, J.; Negro, E.; et al. Low-Noble-Metal-Loading Hybrid Catalytic System for Oxygen Reduction Utilizing Reduced-Graphene-Oxide-Supported Platinum Aligned with Carbon-Nanotube-Supported Iridium. Catalysts 2020, 10, 689. https://doi.org/10.3390/catal10060689
Dembinska B, Zlotorowicz A, Modzelewska M, Miecznikowski K, Rutkowska IA, Stobinski L, Malolepszy A, Krzywiecki M, Zak J, Negro E, et al. Low-Noble-Metal-Loading Hybrid Catalytic System for Oxygen Reduction Utilizing Reduced-Graphene-Oxide-Supported Platinum Aligned with Carbon-Nanotube-Supported Iridium. Catalysts. 2020; 10(6):689. https://doi.org/10.3390/catal10060689
Chicago/Turabian StyleDembinska, Beata, Agnieszka Zlotorowicz, Magdalena Modzelewska, Krzysztof Miecznikowski, Iwona A. Rutkowska, Leszek Stobinski, Artur Malolepszy, Maciej Krzywiecki, Jerzy Zak, Enrico Negro, and et al. 2020. "Low-Noble-Metal-Loading Hybrid Catalytic System for Oxygen Reduction Utilizing Reduced-Graphene-Oxide-Supported Platinum Aligned with Carbon-Nanotube-Supported Iridium" Catalysts 10, no. 6: 689. https://doi.org/10.3390/catal10060689
APA StyleDembinska, B., Zlotorowicz, A., Modzelewska, M., Miecznikowski, K., Rutkowska, I. A., Stobinski, L., Malolepszy, A., Krzywiecki, M., Zak, J., Negro, E., Di Noto, V., & Kulesza, P. J. (2020). Low-Noble-Metal-Loading Hybrid Catalytic System for Oxygen Reduction Utilizing Reduced-Graphene-Oxide-Supported Platinum Aligned with Carbon-Nanotube-Supported Iridium. Catalysts, 10(6), 689. https://doi.org/10.3390/catal10060689