1. Introduction
Due to the fact that supported gold catalysts exhibit well-documented high activity in low temperature CO oxidation [
1,
2,
3,
4] they are commonly studied for automotive applications, despite the fact that the platinum, palladium, and ruthenium catalysts are highly active in the combustion of hydrocarbons [
5]. It has been shown that there is a substantial influence of the support, gold precursor, and the method of synthesis on the activity of the resulting catalytic system [
6]. Since the reducibility of the support is a key factor in CO oxidation this reaction was selected as the model reaction, but additional testing in a stream whose composition simulated the lean-burn natural gas engine exhaust has also been performed. The sensitivity of gold catalyst activity to changes in support reducibility means that non-stoichiometric oxides such as ceria and cerium-zirconium mixed oxides are commonly used as supports. However, the change of the support composition has a substantial impact not only on the reducibility of the system, but also on variables such as: lattice parameter of the support and hence the interaction of gold with the support, etc. This is why the goal of this study was to prepare a specific fraction of one batch of the calcined support with a specific composition, onto which first gold was deposited, followed by the deposition of potassium ions, so that the obtained systems would differ mainly in the potassium loading.
Potassium carbonate has been used in the gasification of coal for decades [
7,
8,
9]. Its activity is attributed to the mobility of the potassium ions [
7]. Studies of a Cu-V-K-Cl/TiO
2 catalyst by Ciambelli et al. have shown that the catalyst “is able to strongly lower the ignition temperature of different carbon materials, from fullerene to graphite, to carbon particulate from diesel engine and from oil fired power plants” [
10] and the activity of such catalysts in soot oxidation was observed to depend on the reducibility of the system [
11]. The addition of potassium ions on ceria [
12] or ceria-zirconia [
13] has also been successfully applied in diesel soot catalysts. Upon comparison of the activity of alkali and alkaline earth metal oxides in soot combustion [
14] it was concluded that alkaline earth metals are worse catalysts due to a lower mobility of the surface species, which is in line with the reports on coal gasification [
8]. In the current work, potassium carbonate was deposited onto the surface of gold catalysts supported on ceria-zirconia as a novel method of testing how the suppression of the reducibility of the support influences the activity of the catalytic system in CO oxidation and other reactions in a multicomponent stream.
Currently, research in gold catalysts is carried out in the hope to keep the superior activity in CO oxidation while increasing the activity in the oxidation of VOCs. Therefore, tests were carried out in a multicomponent stream which included CO, saturated hydrocarbons, water vapor, NO, CO
2, and nitrogen and the gas flow rate and volume of the catalyst bed (gas hourly space velocity, GHSV) was similar to normal reaction conditions in a catalytic converter. Among volatile organic compounds propene is very commonly studied in research on gold catalysts [
15,
16,
17]. The results indicate that the complete combustion of propene can take place at temperatures above 200 °C [
3]. As shown by comparative studies of propane and propene combustion, the combustion of saturated hydrocarbons is relatively more difficult than that of unsaturated ones [
3,
6]. This is why propane was used in our tests. Complete oxidation of propane on nano-gold catalysts supported on ceria, zirconia, and ceria-rich ceria-zirconia was studied by Ali et al. [
18]. The activity tests were carried out in a stream which contained only propane (0.5 vol%) in helium. Their results show that the performance of Au/CeO
2 was superior to that of Au/ZrO
2. The activity of Au supported on the ceria doped with zirconium ions was an intermediate between those of gold on each of the two pure supports. However, the two pure oxides and the mixed oxide have different structures and properties, so a meaningful comparison which could contribute to the understanding of how reducibility of the support is not possible with these systems. Therefore, the focus of our study was one kind of support, Ce
0.85Zr
0.15O
2, which came from the same batch and the large, 150–250 µm, fraction was used for catalyst preparation. The differences between the support in each of the samples are negligible and hence the differences in reducibility can be attributed to the species deposited onto the surface of these particles (gold, potassium, or both) and their interaction with the support.
2. Results
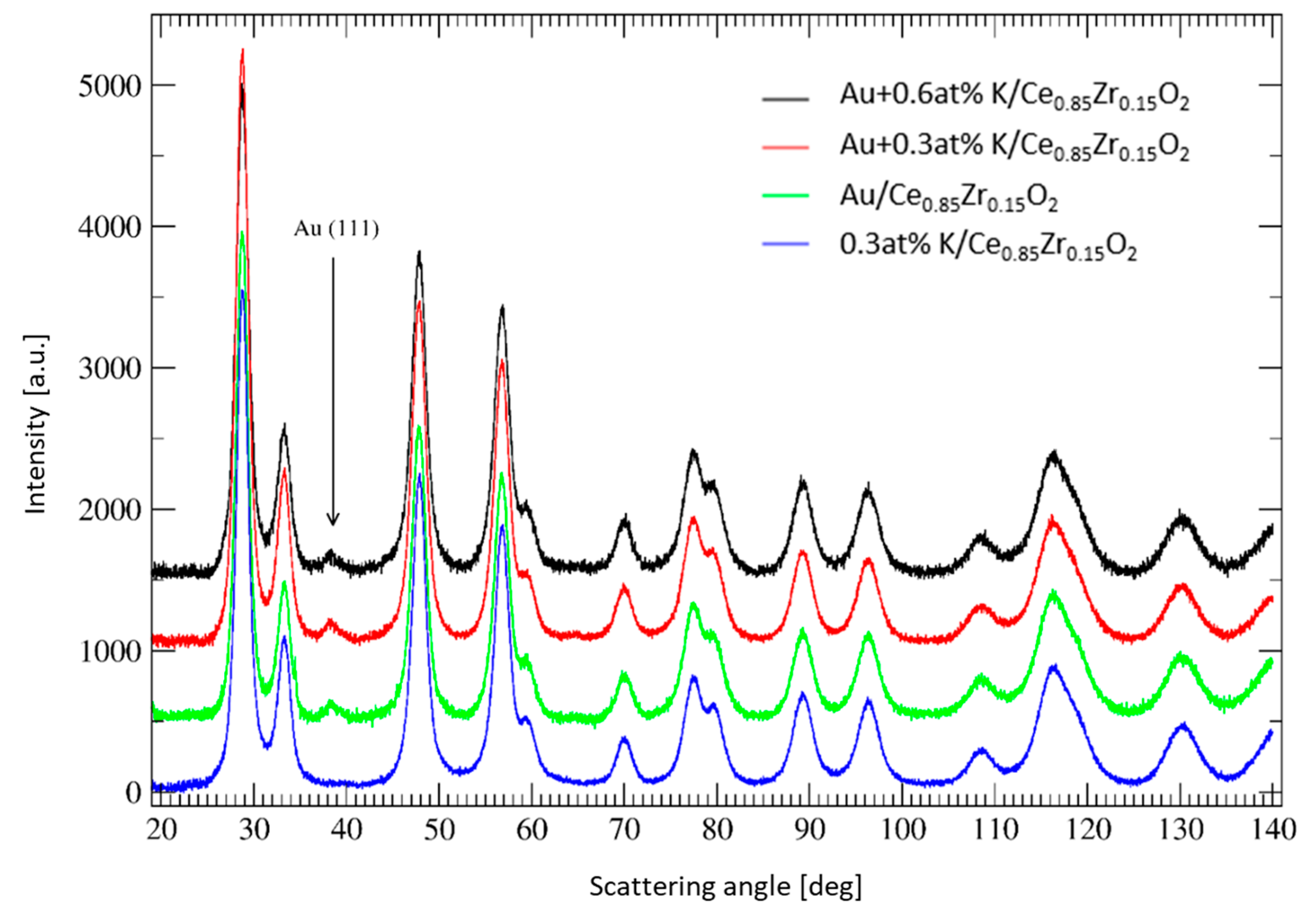
X-ray Diffraction patterns of the Au catalysts without and with 0.3 and 0.6 at% potassium ions, respectively, are shown in
Figure 1 along with that of the ceria-zirconia doped with 0.3 at% potassium ions. They show that the main phase present in these systems is the ceria-zirconia solid solution with the fluorite type structure. The values of the lattice constant of the support for these four samples were comparable within the experimental error (
Table 1). The strain of the support lattice is small in the case of all samples (
Table 1). The Ce:Zr ratio determined using XRD was 85:15, which is in accordance with the nominal composition of the cerium-zirconium mixed oxide. No signals from a potassium-containing phase have been detected in the XRD patterns. The Au catalysts contain a slightly visible Au (111) peak at a scattering angle of approximately 38.2° that was used to estimate the size of the gold nanoparticles. They are approximately 4 nm in diameter. The gold loading was equal to 0.2 at% (1 wt%) for all catalysts and the potassium concentration corresponds to 0.3 and 0.6 at%, respectively, as determined by the ICP analysis. The specific surface area values of the samples were between 70–75 m
2/g, regardless of the presence of Au and K.
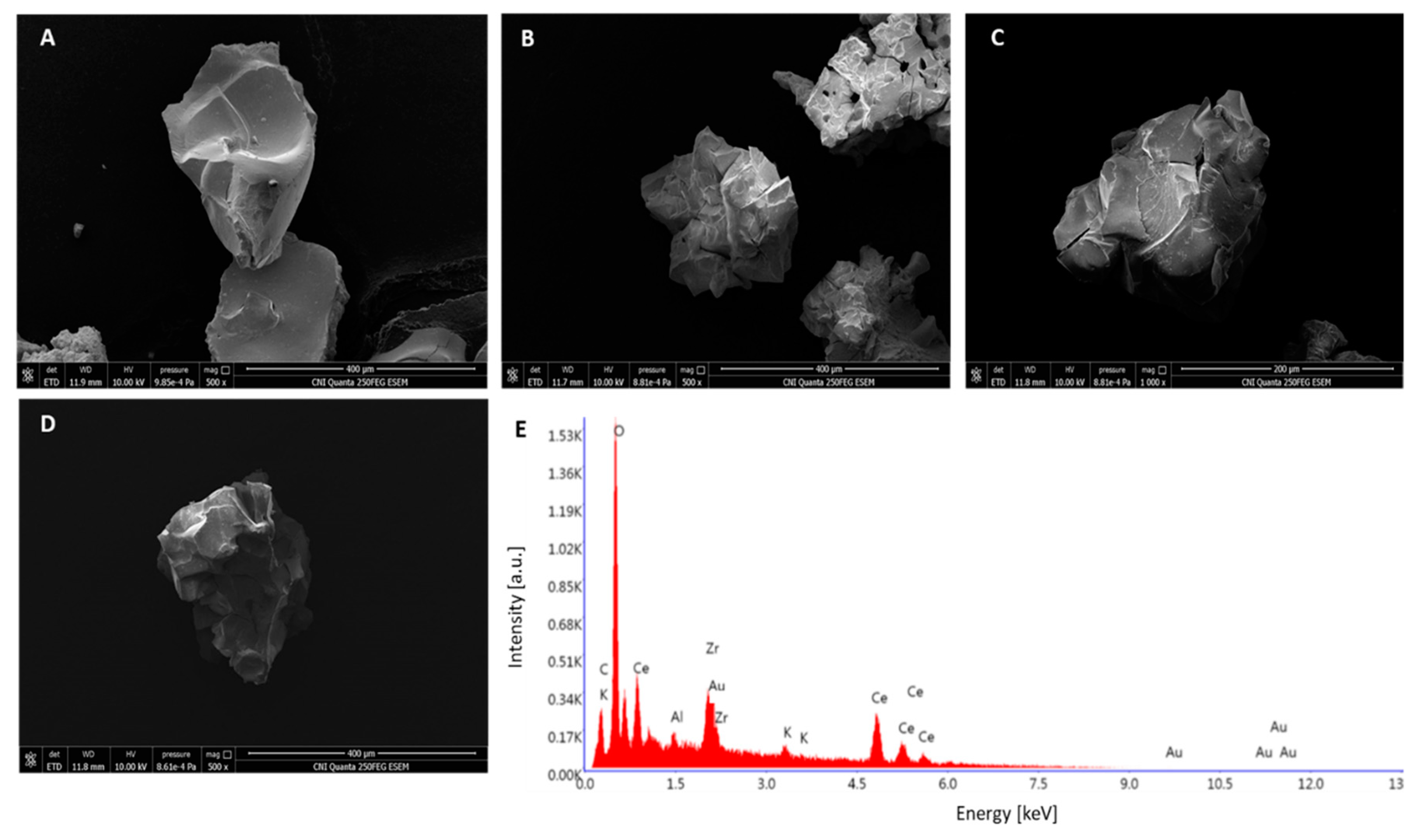
The SEM results revealed no substantial differences in the topographies of the samples (
Figure 2A–D). The SEM-EDX for the catalyst with 0.6 at% potassium ions can be seen in
Figure 2E. Visible signals from all components can be seen, including a pronounced potassium signal. The Zr/(Zr + Ce) ratio is 17 at%, which is much closer to the value obtained from XRD than those obtained by XPS (
Table 2). Hence, to sum up this portion of results, it can be stated that there are no substantial differences between the topography of the catalysts and their composition (other than the potassium ion content), which enables meaningful comparisons and analysis of activity results.
X-ray Photoelectron Spectroscopy measurements were conducted on at least four spots of each catalyst due to the possibility of surface inhomogeneity and hence ranges are given in
Table 2. The XPS results have shown that all of the catalysts had only the elements which they were designed to have. Signals from all of them are visible in the survey spectra (not shown). The amount of zirconium on the surface is higher than the value found using XRD (
Table 2). This is in agreement with previous findings about Zr enrichment occurring in ceria-zirconia solutions after thermal treatment at high temperature [
19]. Moreover, the gold and potassium loadings were also higher than those determined by ICP, which was expected due to the fact they were deposited onto the support after support synthesis. The reason for using this method of catalyst preparation was to have all the catalysts made with the 150–250 µm fraction of the support onto which gold and potassium carbonate were deposited in sequence without changing the bulk properties. Hence, comparisons made with the potassium ion loaded support are valid.
The Au surface content as determined by XPS seems unaffected by the presence of K and the same conclusion can be drawn for K surface concentration (
Table 2). These experiments revealed heterogeneity of the surface in terms of both the gold and potassium distribution. However, in several of the tested spots of the Au + 0.6 at% K/Ce
0.85Ze
0.15O
2 catalyst an Au:K ratio very close to 1:1 has been observed. This may indicate a formation of a compound with such stoichiometry, e.g., aurate or an alloy. Nevertheless, a carbonate signal in the C 1s detailed regions suggests that at least a part of the potassium is in the form of the carbonate, although the phase was not detected by XRD.
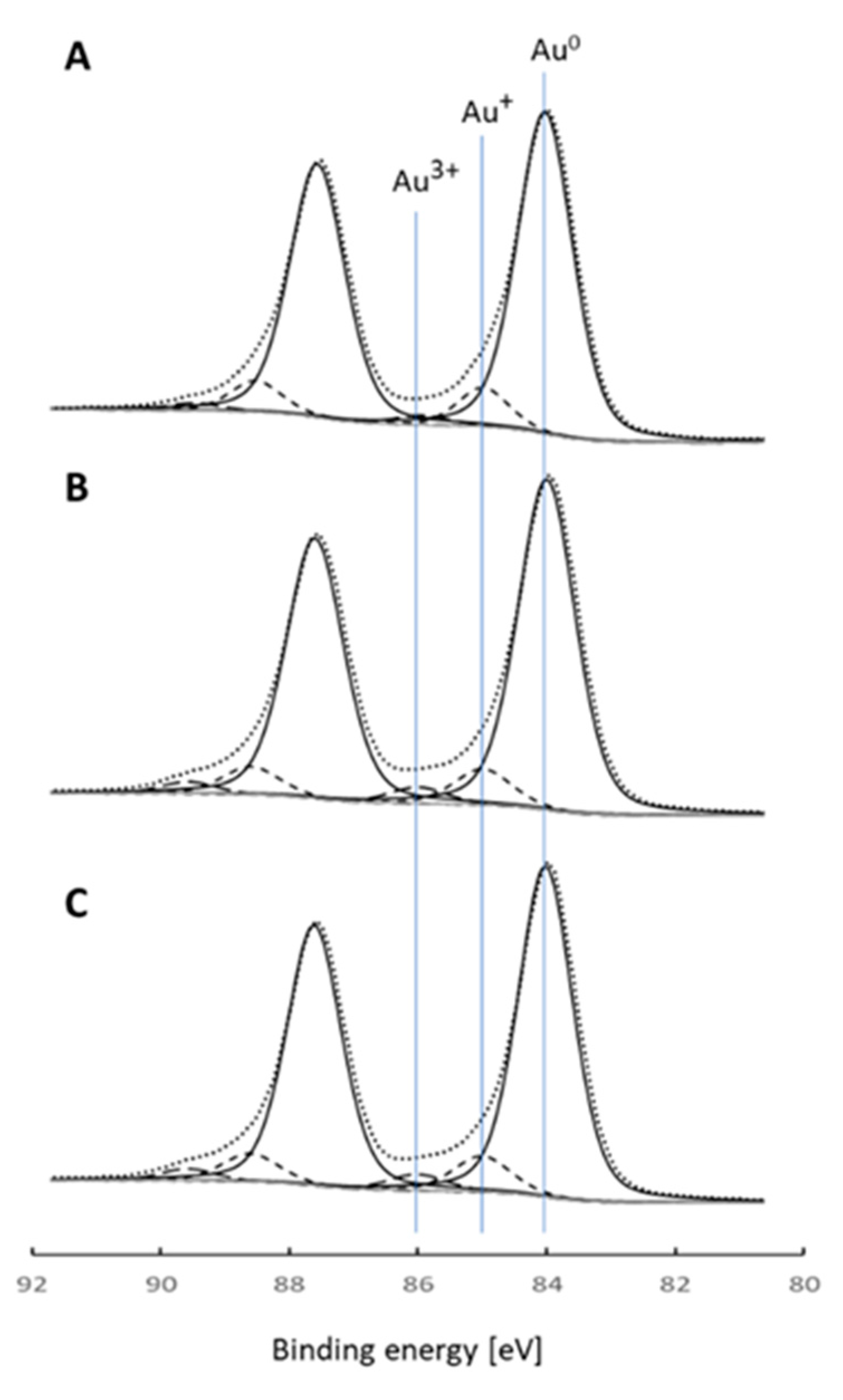
The Au 4f detailed regions of the XPS spectra collected for the catalysts are presented in
Figure 3. The Au 4f peaks were fitted with three doublets with a spin orbital splitting of 3.7 eV. The Au 4f
7/2 components were located at the binding energy of 84.0, 85.0, and 86.0 eV (
Figure 3), which corresponded to Au
0, Au
+, and Au
3+, respectively, although there are some discrepancies in the literature regarding the position of the binding energy of the Au
3+ component [
20,
21]. The distribution of Au shows that the majority of the gold is found in the metallic form (
Table 2), which is in line with the results of the XRD measurements. This can be expected considering the relatively high calcination temperature. Usually, the calcination treatment reduces ionic gold species to the metallic state [
22]. The reason why the calcination of the catalysts was carried out at 550 °C is that the catalytic tests were to be performed up to that temperature. Therefore, such a calcination procedure ensures that the analysis of the catalytic tests is not obscured by additional sintering of the support.
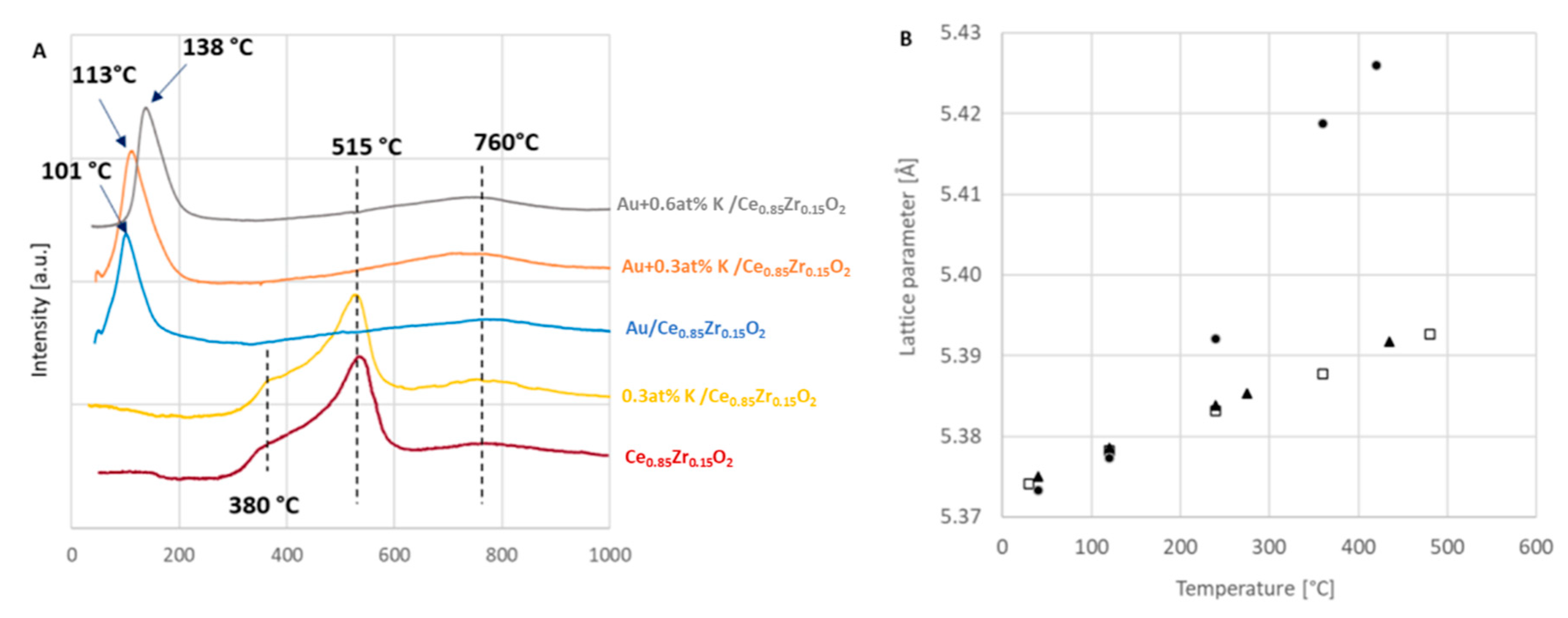
The Temperature Programmed Reduction profiles obtained for the investigated systems are depicted in
Figure 4A. The reduction of the potassium doped support (yellow curve) is characterized by a main peak with maximum at 515 °C that is preceded by a shoulder at 380 °C and followed by a broad signal at 760 °C. A similar profile was registered for the potassium-free ceria-zirconia (red curve) and it is in agreement with the typical reduction of cerium-zirconium oxides, the number and position of reduction peaks depending on the exact composition and oxygen mobility of the solid solutions [
23,
24]. For the potassium-doped support, the hydrogen consumption of the main peak is centered at 515 °C and it corresponds to 24 mL·g
CeZr×−1, while the broad feature at 760 °C corresponds to 10 mL·g
CeZr−1. Within the experimental error (± 10%) no differences were observed for the hydrogen consumption values associated with the TPR curve of the potassium-free ceria-zirconia. Such values corresponding to an overall hydrogen consumption of 34 mL·g
CeZr−1, or 38.3 mL·g
CeO2−1 are in good agreement with values ranging between 40–50 mL·g
CeO2−1 previously observed for samples with composition Ce
0.6Zr
0.4O
2 and improved oxygen mobility [
23].
The presence of gold results in a low temperature reduction peak, between 100–140 °C, that is due to the shift of the main peaks at 515 °C detected for the CeZr supports (with and without potassium). That peak is followed by the same very broad signal of hydrogen uptake at 760 °C, common to the supports. TPR curves with similar features are typical for ceria and cerium-zirconium mixed oxides doped with Au [
25,
26], as well as Pt [
27] and Pd [
28]. According to the literature [
25,
26,
27,
28] the noble metal increases the oxygen mobility of the part of ceria or ceria-zirconia where the metallic nanoparticles are well dispersed, leaving the part with low oxygen mobility unaffected and reducible at around 800 °C. The influence of the potassium loading can be seen as shifting the maximum of the low temperature reduction signal to slightly higher values. For the Au/support system the maximum of the reduction occurs at 101 °C. In the systems with potassium the maximum takes place at 113 and 138 °C, for the 0.3 at% and 0.6% K loading, respectively.
The increased reducibility of the Au/ceria-zirconia catalysts (with and without K), associated with the low-temperature peak between 100–140°C, implies, according to the literature [
25], hydrogen activation on the Au metal nanoparticles and subsequent spillover on the support, with promotion of the reduction at low temperature. It is evident that increasing K loading from 0.3 to 0.6 at% inhibits such a spillover effect with a consequent shift of the maximum of the low temperature reduction peak to slightly higher values. Based on this it can be supposed that Au nanoparticles and K ions are in close contact. Together with a 1:1 ratio of Au:K in some of the spots of the Au + 0.6 at% K/Ce
0.85Zr
0.15O
2 catalyst in the XPS measurement, it may be indicative of the formation of a compound or another type of interaction, which is why the difference of T
max of the low-temperature reduction signal of Au/Ce
0.85Zr
0.15O
2 and Au + 0.6 at% K/Ce
0.85Zr
0.15O
2 is larger than twice the difference between Au/Ce
0.85Zr
0.15O
2 and Au + 0.3at%K/Ce
0.85Zr
0.15O
2. However, it should be kept in mind that this ratio was only observed with the most surface-sensitive technique and that an enrichment in Au with the increase of probing depth was shown by EDX.
The overall hydrogen consumption registered for the three Au supported catalysts was unaffected by the K presence, at the two atomic concentrations herein used, with total values around 34–37 mL·gCeZr−1 and the hydrogen consumed at around 760 °C being the same for all three samples and equal to 10 mL gCeZr−1.
Another way to study reduction is by the application of in situ X-ray Diffraction studies in hydrogen. The information acquired from such a measurement can easily differentiate between a small surface reduction and a substantial reduction by the extent of the change in the lattice parameter of the support. The tests were conducted in three atmospheres: hydrogen, helium, and oxygen. The aim was to investigate how the interaction of gold with the support changes the reduction behavior of the catalysts and to see if the influence of the presence of potassium can be observed, as in the case of the TPR measurements. The results of X-ray Diffraction studies carried out on the gold catalyst with 0.3 at% potassium are shown in
Figure 4B. The squares correspond to measurements carried out in oxygen and the triangles to those performed in helium. These two sets of points fall on one line, which corresponds to thermal expansion of the support lattice. The difference between the value noted at the lowest temperature and 480 °C is 0.018 Å. In contrast, in the results obtained during treatment with hydrogen it can be seen that only at the two lower temperatures, i.e., 40 and 120 °C, the results are the same as those measured in the other two atmospheres, but the third value is substantially higher than the corresponding values of the support lattice parameter in He and O
2. The two values obtained at the two highest temperatures, namely 360 and 420 °C, are even further away from the values typical for thermal expansion. The support lattice parameter at the highest temperature with hydrogen is 0.034 Å higher than those in the oxygen and in helium. Therefore, the reduction of the catalysts is not a superficial one, but a structural change occurs. Similar results have been noted for the catalyst with the higher potassium loading. Since this reduction is already visible at temperatures around 240 °C, which is much below the temperature at which the support + 0.3 at% K releases its oxygen, these results indicate that this substantial reduction is facilitated by the presence of gold. In situ XRD studies in hydrogen performed by Bekheet et al. have also shown pronounced changes in the ceria lattice parameter and the lattice parameter of Sm-doped ceria [
29]. That study found no CeH
x phase, but instead the formation of ordered oxygen vacancies and changes of both the stoichiometry and crystallographic system for pure CeO
x, whereas the Sm stabilized the structure and no transition to a rhombohedral phase despite the change of the stoichiometry. A similar situation of a substantial support lattice parameter change without a phase change was noted by us for the ceria-zirconia lattice. Investigation of the lattice parameter of cerium-zirconium mixed oxides has been conducted by some of us in a CO/He mixture [
30].
At all temperatures the activity in CO oxidation of the gold catalyst without potassium was the highest in both the multicomponent stream and the stream with only 1 vol% CO and 1 vol% O
2 in helium (
Figure 5A,B, respectively), whereas the activity of the potassium-doped support is negligible up to the temperature of approximately 350 °C. In
Figure 5B the dotted lines represent extrapolation of the curves up to 100% of conversion not registered in our experimental conditions.
The comparison of the two sets of results clearly shows the negative impact of the presence of other inlet stream components in the feed on the CO conversion. Presumably, the most pronounced effect is that of the high content of water vapor, as the presence of water has been shown to inhibit CO oxidation [
31]. The activity of the catalyst with 0.3 at% potassium was lower than that of Au/Ce
0.85Zr
0.15O
2 in the whole range of studied temperatures. The activity of the catalyst with 0.6 at% potassium was consistently lower than that of the catalyst with 0.3 at%. This indicates that the increase of potassium loading further hinders the reducibility of the support, which is responsible for activity in CO oxidation in accordance with the Mars-van Krevelen mechanism [
4] and, as we have demonstrated, from the linear relationship between the reducibility of Au/ceria catalysts and CO oxidation activity [
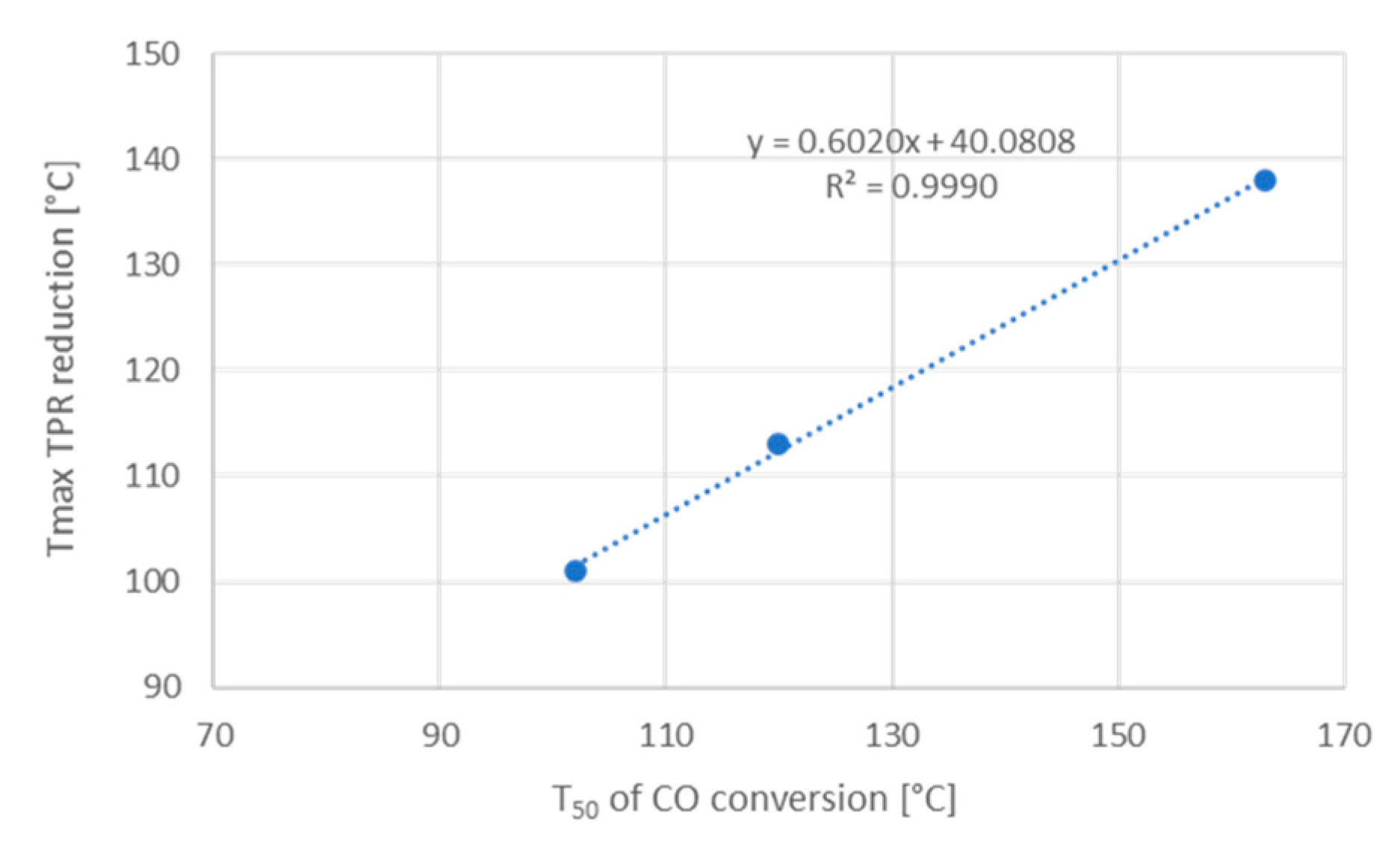
25]. This agrees with our TPR results, which have shown that the addition of 0.3 and 0.6 at% potassium shift the low temperature reduction peak to higher temperatures. In fact, a linear dependence with excellent correlation between the T
50 determined in the tests with 1 vol% CO, 1 vol% O
2, balance He, and the T
max of the reduction peak was found (
Figure 6).
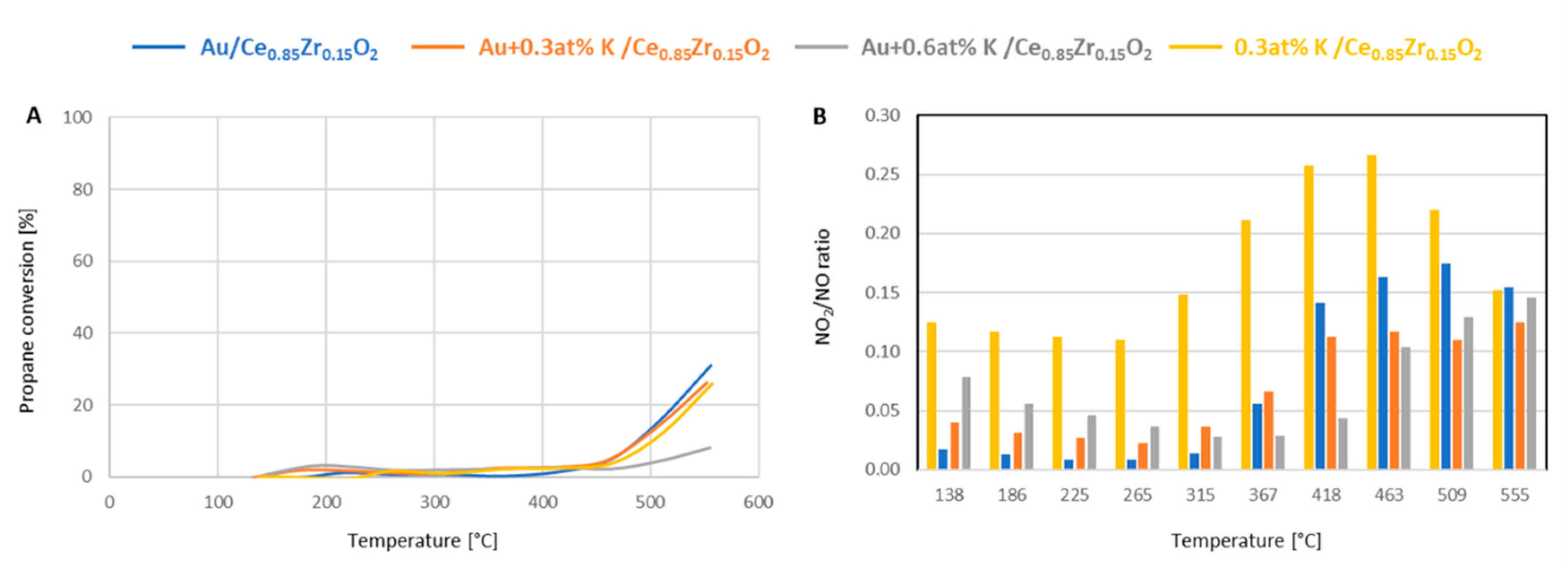
The activity of these systems in the propane combustion and NO oxidation are summed up in
Figure 7. It is noteworthy that the results of the activity tests revealed that under the studied conditions the gold catalysts are not superior to the potassium-doped support in propane oxidation, i.e., their activity is comparable in the studied range of temperatures (
Figure 7A). Both systems with potassium, i.e., with and without gold, exhibited the same activity. When interpreting this in the light of the results of our TPR and in situ XRD studies, it can be stated that the interaction of gold and the support does not impact the activity of the system in this reaction. The only catalyst with a substantially lower activity in propane combustion is the catalyst with 0.6 at% potassium ions deposited onto its surface. In contrast to both CO oxidation and propane combustion, the conversion of NO to NO
2 at temperatures up to 550 °C is the highest on the potassium-doped support.
Figure 7B shows the NO
2/NO ratio in the outlet streams. The effect of potassium on the activity of gold catalysts in this reaction is visible, but changes in the studied temperature range: at temperatures up to 265 °C the Au + 0.6 at%/Ce
0.85Zr
0.15O
2 system exhibits the highest activity and Au/Ce
0.85Zr
0.15O
2 has the lowest activity, whereas in the range 418–509 °C the Au + 0.6 at%/Ce
0.85Zr
0.15O
2 exhibits the highest activity in NO oxidation (
Figure 7B). At the highest studied temperature, the activity of the potassium-doped support and the gold-containing systems are all similar.
3. Materials and Methods
The catalysts were prepared from ceria-zirconia which was obtained via the aqueous route from ammonium ceric nitrate (analytical grade, BDH, Randor, PA, USA) and zirconyl nitrate (analytical grade, Fisher, Ottawa, ON, Canada). The mixture of 26.63 g of zirconyl nitrate and 48.42 g of ammonium ceric nitrate was dissolved in 100 mL of water and a concentrated ammonium hydroxide solution was slowly added (analytical grade, Caledon, Georgetown, ON, Canada). The solid was filtered, dried, calcined at 550 °C for 4 h, and crushed. The 150–250 µm fraction was divided into four parts and three of them were placed in a beaker containing an appropriate volume of a solution of HAuCl4·H2O (0.01 mol·L−1) (analytical grade, Sigma-Aldrich, Oakville, ON, Canada) in order to prepare catalysts with Au loading equal to 1 wt%. The pH value of the suspension was adjusted to 8.5 with a potassium carbonate solution (0.5 mol·L−1) and the mixture was stirred overnight at 65 °C. The precipitate was washed several times until the pH of the filtrate was neutral and the test with AgNO3 came out negative. The material was calcined at 350 °C for 3 h and divided into three parts (6 g each). Two of them were dosed with a potassium carbonate solution (analytical grade, Fisher, Ottawa, ON, Canada), 0.750 and 1.50 mL, respectively, so as to obtain catalysts with a final potassium ion loading of 0.3 and 0.6 at%. All three catalysts were then calcined at 550 °C for 4 h. The support also underwent the same thermal treatment as the others and was then dosed with the solution of potassium carbonate to a final loading of 0.3 at%.
Two sets of activity tests were carried out. Both were performed in tubular flow-through reactors using 0.5 g of the catalyst. The first were performed with a gas flow rate of 1.5 L min−1 in the temperature range 110–600 °C. The inlet stream contained a mixture of hydrocarbons (1000 ppm CH4, 150 ppm C2H6, and 50 ppm C3H8), 200 ppm NO, 1000 ppm CO, 7% water vapor, 5% CO2, 10% O2, and balance N2. The outlet stream composition was monitored using a MultiGas 2030 FTIR Continuous Gas Analyzer, MKS Instruments Inc. (Rochester, NY, USA). The second set of activity tests was conducted in a stream containing 1 vol% CO + 1 vol% O2 in He (50 mL·min−1).
Inductively Coupled Plasma measurements were performed using an Optima 7000 Inductively Coupled Plasma Optical Emission Spectrometer (Perkin-Elmer Inc., Waltham, MA, USA). They were used to determine the amount of gold and potassium ions present in the solutions of the samples obtained from digesting 0.3 g in sulfuric acid and then in aqua regia. The concentrations were determined using external standard calibration curves.
The specific surface area of the catalysts and potassium doped support were determined using a Micromeritics ASAP 2010 instrument (Micromeritics Instrument Corp., Norcross, GA, USA). Nitrogen physisorption was performed at 77 K. The standard pressure used was in the p/po range of 0.01–0.50. The samples (approx. 0.4 g) were outgassed for 30 min at 150 °C under a vacuum prior to the measurement. The Brunauer-Emmet-Teller equation was used to calculate the specific surface area of the samples.
The Secondary Emission Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDX) experiments were performed on a Quanta FEG 250 instrument (Field Electron and Ion Company, FEI, Hillsboro, OR, USA). The working distance was 10 mm. Images were taken at magnifications of 500, 1000, as well as 10,000 (for EDX analysis). EDX measurements were carried out for three spots (spot size 4) of each sample using the beam energy of 10 kV.
Reduction properties of the gold catalysts and the ceria-zirconia mixed oxide were studied by Temperature Programmed Reduction (TPR) measurements. Experiments were carried out with a Micromeritics Autochem 2910 apparatus equipped with a thermal conductivity detector (TCD) (Micromeritics Instrument Corp., Norcross, GA, USA). Before starting the analyses, the catalyst (ca. 100 mg) was pretreated in a flow of 5% vol O2 in He gas mixture (30 mL· min−1) at 150 °C (10 °C min−1) for 10 min to clean the surface and then cooling down in a flow of He (30 mL·min−1) up to room temperature. A 5 vol% H2 in Ar gas mixture (30 mL·min−1) was used to reduce the sample by heating from room temperature to 1050 °C at the rate of 10 °C min−1. The hydrogen consumption values associated with the TPR profile were determined by applying a calibration curve to the TCD signals and are quoted with a precision of ±10%.
X-ray Diffraction studies were carried out with a D5000 horizontal goniometer (Bruker AXS GmbH, Karlsruhe, Germany) equipped with a Cu anode sealed tube (Kα radiation λ = 1.5418 Å) which operated at 40 kV, 40 mA, using Bragg-Brentano divergent beam optics with a Kβ filter and a LynxEye strip detector. Approximately 50 mg of the sample was smeared over the surface of porous glass, mounted to the measurement environmental chamber, exposed to oxygen (5.0 purity, flow 20 mL·min−1), and measured in a sequence of rising temperatures up to approx. 420 °C, then exposed to helium (5.6 purity, flow 20 mL·min−1) and measured in a sequence of temperatures down to 40 °C. Next, it was exposed to hydrogen (5.0 purity, flow 20 mL·min−1) and measured in a sequence of temperatures rising to approx. 420 °C, then flushed with helium and cooled down. Each XRD measurement encompassed the range of 20–140° of scattering angle and lasted 106 min. The analysis involved averaging of several datasets, background subtraction, and fitting of the measured peaks with doublets of Voigt functions (Kα1,2) (program Fityk v.1.3.0). The support peak parameters were analyzed via the Williamson-Hall plot, lattice parameter extrapolation using Nelson-Riley function and Debye-Waller factor determination. The following parameters were calculated: volume weighted average of atomic rows length D (interpreted as a support crystal size), support microstrain parameter ε corresponding to a range of local variations of the support lattice parameter (resulting in additional peak broadening rising with angle as 2θ° and the ceria-zirconia lattice parameter, as well as the average gold particle size and the Ce:Zr ratio.
The X-ray Photoelectron Spectroscopy measurements were carried out on a K-Alpha instrument (Thermo Scientific, Waltham, MA, USA). Survey spectra were obtained with a pass energy of 200 eV, 1 eV step and detailed regions, i.e., C 1s + K 2p, Au 4f, Ce 3d, Zr 3d, and O 1s were collected with a pass energy of 50 eV, 0.1 eV step. Each sample was measured at least four times and the values were averaged. The quantitative analysis of the spectra was performed after background subtraction and calibration using the C 1s signal. For quantification the K 2p doublet was fitted with a single pair of curves with a spin orbital splitting of 2.8 ± 0.1 eV.