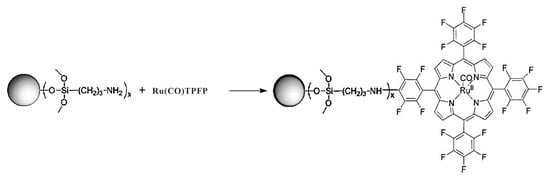
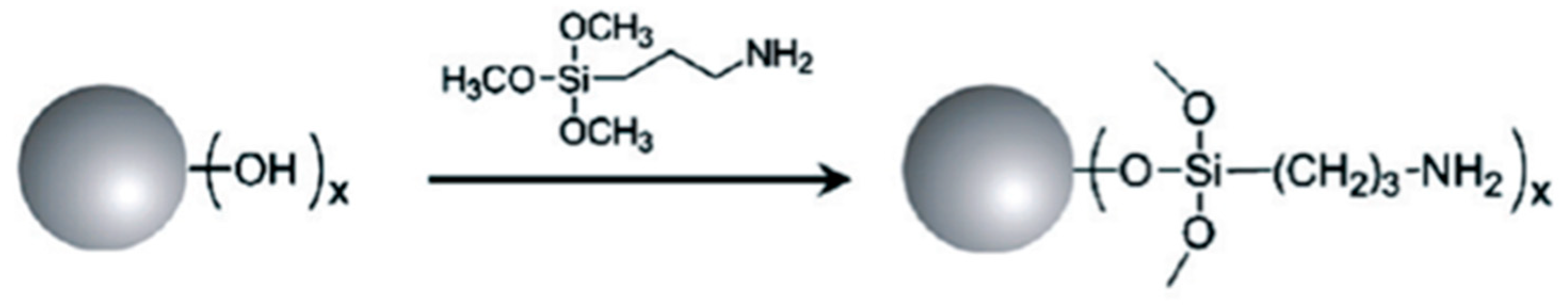
Covalent Functionalization of Nanodiamonds by Ruthenium Porphyrin, and Their Catalytic Activity in the Cyclopropanation Reaction of Olefins
Abstract
1. Introduction
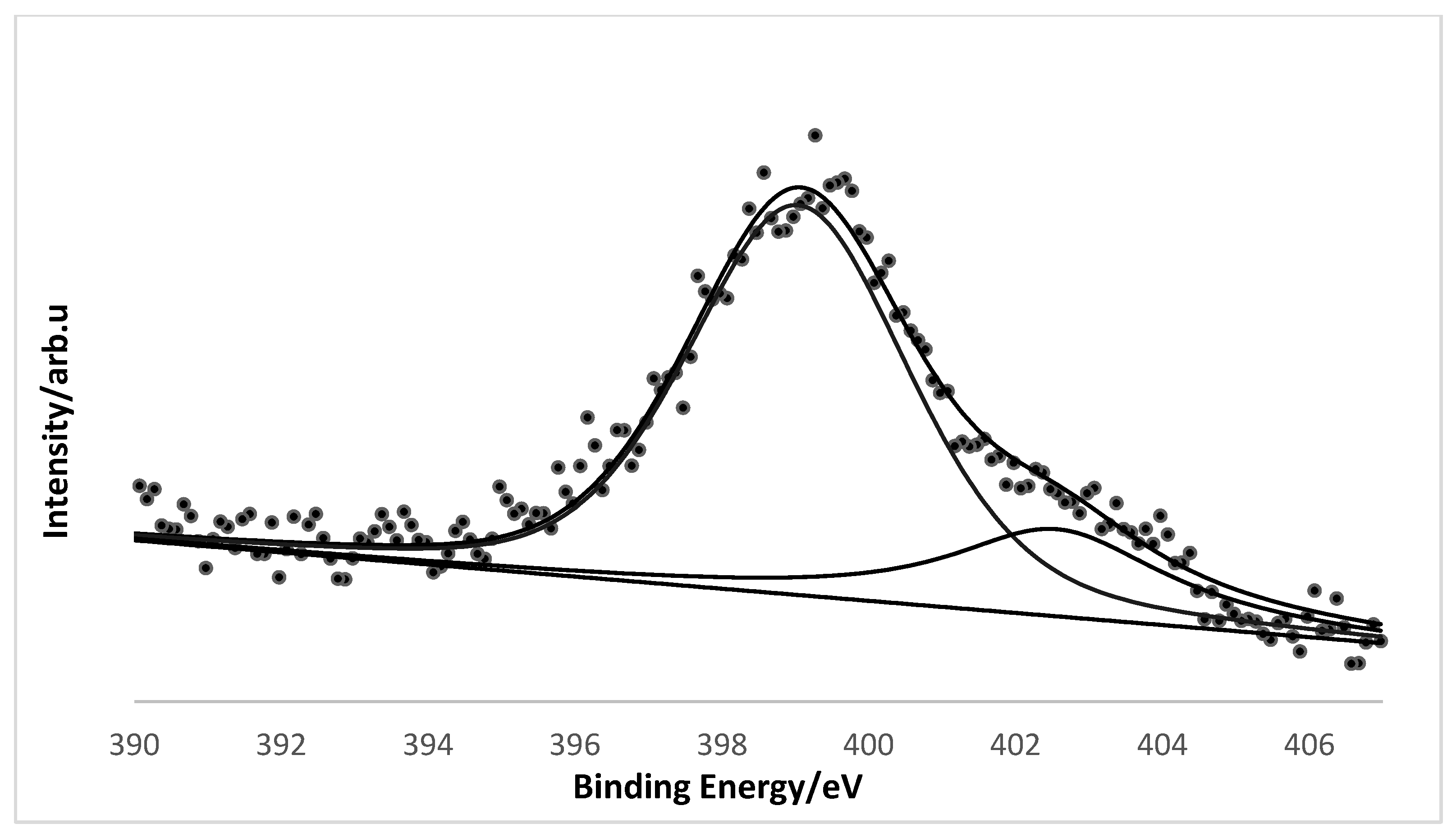
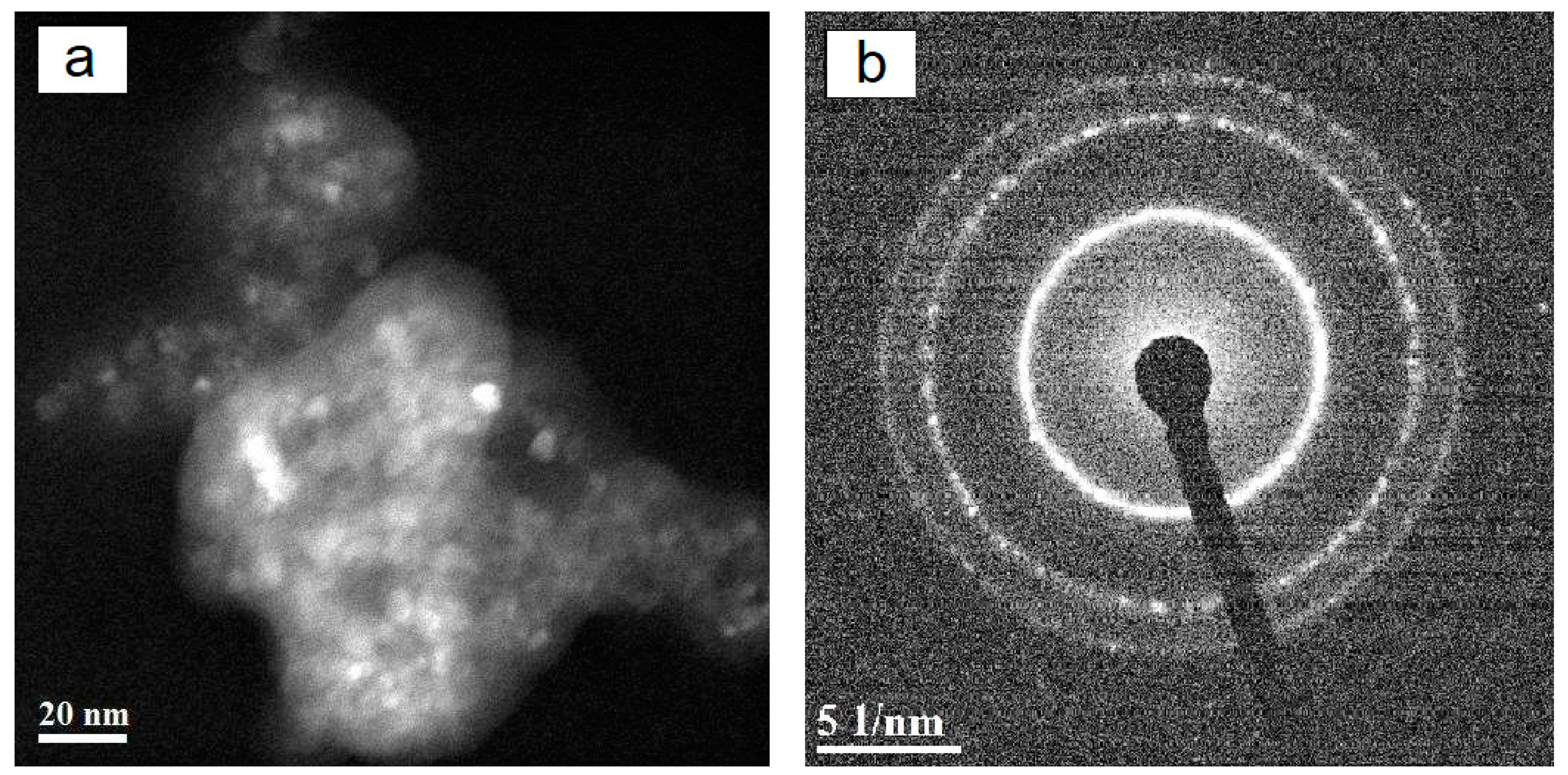
2. Results
3. Discussion
4. Materials and Methods
4.1. General
4.2. Chemicals
4.3. Synthesis
4.3.1. Reduction of DNDs
4.3.2. Covalent Functionalization of DNDs with (3-Aminopropyl)Trimethoxysilane (APS)
4.3.3. Functionalization of NDs with Ru(TF5PP)CO
4.4. Typical Cyclopropanation Reactions
4.5. Recycling of the Catalyst
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pogacic, V.; Bullock, A.N.; Federov, O.; Filippakopoulos, P.; Gasser, C.; Biondi, A.; Meyer-Monard, S.; Knapp, S.; Schwaller, J. Structural Analysis Identifies Imidazo [1,2-b]Pyridazines as PIM Kinase Inhibitors with In vitro Antileukemic. Activity Cancer Res. 2007, 67, 6916–6924. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P. Catalytic methods for metal carbene transformations. Chem. Rev. 1986, 86, 919–939. [Google Scholar] [CrossRef]
- Robbins Wolf, J.; Hamaker, C.G.; Djukic, J.-P.; Kodadek, T.; Woo, L.K. Shape and stereoselective cyclopropanation of alkenes catalyzed by iron porphyrins. J. Am. Chem. Soc. 1995, 117, 9194–9199. [Google Scholar] [CrossRef]
- Maxwell, J.L.; Brown, K.C.; Bartley, D.W.; Kodadek, T. Mechanism of the Rhodium Porphyrin-Catalyzed Cyclopropanation of Alkenes. Science 1992, 256, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, C.G.; Djukic, J.-P.; Smith, D.A.; Woo, L.K. Mechanism of Cyclopropanation Reactions Mediated by 5,10,15,20-tetra-(p-tolylporphyrinato)osmium(II) Complexes. Organometallics 2001, 20, 5189–5199. [Google Scholar] [CrossRef]
- Penoni, A.; Wanke, R.; Tollari, S.; Gallo, E.; Musella, D.; Ragaini, F.; Demartin, F.; Cenini, S. Cyclopropanation of Olefins with Diazoalkanes, Catalyzed by CoII(porphyrin) Complexes—A Synthetic and Mechanistic Investigation and the Molecular Structure of CoIII(TPP)(CH2CO2Et) (TPP = Dianion of meso-Tetraphenylporphyrin). Eur. J. Inorg. Chem. 2003, 2003, 1452–1460. [Google Scholar] [CrossRef]
- Galardon, E.; Le Maux, P.; Simonneaux, G. Cyclopropanation of Alkenes with Ethyldiazoacetate Catalysed by Ruthenium Porphyrin Complexes. Chem. Comm. 1997, 927–928. [Google Scholar] [CrossRef]
- Callot, H.J.; Metz, E.; Piechocki, C. Sterically crowded cyclopropanation catalysts. Syn-selectivity using rhodium(III)porphyrins. Tetrahedron 1982, 38, 2365–2369. [Google Scholar] [CrossRef]
- Baciocchi, E.; Boschi, T.; Cassioli, L.; Galli, C.; Jaquinod, L.; Lapi, A.; Paolesse, R.; Smith, K.M.; Tagliatesta, P. Electronic Effects on the Stereoselectivity of Epoxidation Reactions Catalyzed by Manganese Porphyrins. Eur. J. Org. Chem. 1999, 1999, 3281–3286. [Google Scholar] [CrossRef]
- Crestini, C.; Pastorini, A.; Tagliatesta, P. The Immobilized Porphyrin-Mediator System Mn(TMePyP)/clay/HBT(clay-PMS): A Lignin Peroxidase Biomimetic Catalyst in the Oxidation of Lignin and Lignin Model Compounds. J. Mol. Catal. A Chem. 2004, 208, 195–202. [Google Scholar] [CrossRef]
- Conte, V.; Elakkari, E.; Floris, B.; Mirruzzo, V.; Tagliatesta, P. The Cyclooligomerization of Arylethynes in Ionic Liquids Catalysed by Ruthenium Porphyrins: A Case of Real Catalyst Recycling. Chem. Commun. 2005, 1587–1588. [Google Scholar] [CrossRef] [PubMed]
- Tagliatesta, P.; Pastorini, A. Remarkable Selectivity in the Cyclopropanation Reactions Catalysed by an Halogenated Iron meso-Tetraphenylporphyrin. J. Mol. Catal. A Chem. 2003, 198, 57–61. [Google Scholar] [CrossRef]
- Tagliatesta, P.; Pastorini, A. Electronic and Steric Effects on the Stereoselectivity of Cyclopropanation Reactions Catalyzed by Rhodium meso-Tetraphenylporphyrins. J. Mol. Catal. A Chem. 2002, 185, 127–133. [Google Scholar] [CrossRef]
- Ciammaichella, A.; Leoni, A.; Tagliatesta, P. Ruthenium porphyrin bound to a Merrifield resin as heterogeneous catalyst for the cyclooligomerization of arylethynes. New J. Chem. 2010, 34, 2122–2124. [Google Scholar] [CrossRef]
- Ciammaichella, A.; Cardoni, V.; Leoni, A.; Tagliatesta, P. Rhodium Porphyrin Bound to a Merrifield Resin as Heterogeneous Catalyst for the Cyclopropanation Reaction of Olefins. Molecules 2016, 21, 278. [Google Scholar] [CrossRef]
- Chen, Y.; Ruppel, J.V.; Zhang, X.P.J. Cobalt-Catalyzed Asymmetric Cyclopropanation of Electron-Deficient Olefins. J. Am. Chem. Soc. 2007, 129, 12074–12075. [Google Scholar] [CrossRef]
- Anding, B.J.; Ellern, A.; Woo, L.K. Olefin Cyclopropanation Catalyzed by Iridium (III) Porphyrin Complexes. Organometallics 2012, 31, 3628–3635. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, S.; Cui, X.; Wojtas, L.; Zhang, X.P. Cobalt (II)-Catalyzed Asymmetric Olefin Cyclopropanation with α-Ketodiazoacetates. Angew. Chem. Int. Ed. 2013, 52, 11857–11861. [Google Scholar] [CrossRef]
- Otte, M.; Kuijpers, P.F.; Troeppner, O.; Ivanovic-Burmazovic, I.; Reek, J.N.H.; de Bruin, B. Encapsulated Cobalt-Porphyrin as a Catalyst for Size-Selective Radical-type Cyclopropanation Reactions. Chem. Eur. J. 2014, 20, 4880–4884. [Google Scholar] [CrossRef]
- Dzik, W.I.; Xu, X.; Zhang, X.P.; Reek, J.N.H.; de Bruin, B. ‘Carbene Radicals’ in CoII(por)-Catalyzed Olefin Cyclopropanation. J. Am. Chem. Soc. 2010, 132, 10891–10902. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotech. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Baidakova, M.; Vul, A. New prospects and frontiers of nanodiamond clusters. J. Phys. D Appl. Phys. 2007, 40, 6300–6311. [Google Scholar] [CrossRef]
- Tamburri, E.; Guglielmotti, V.; Orlanducci, S.; Terranova, M.L.; Sordi, D.; Passeri, D.; Matassa, R.; Rossi, M. Nanodiamond-mediated crystallization in fibers of PANI nanocomposites produced by template-free polymerization: Conductive and thermal properties of the fibrillar networks. Polymer 2012, 53, 4045–4053. [Google Scholar] [CrossRef][Green Version]
- Tamburri, E.; Guglielmotti, V.; Matassa, R.; Orlanducci, S.; Gay, S.; Reina, G.; Terranova, M.L.; Passeri, D.; Rossi, M. Detonation nanodiamonds tailor the structural order of PEDOT chains in conductive coating layers of hybrid nanoparticles. J. Mater. Chem. C 2014, 2, 3703–3716. [Google Scholar] [CrossRef][Green Version]
- Reina, G.; Orlanducci, S.; Cairone, C.; Tamburri, E.; Lenti, S.; Cianchetta, I.; Rossi, M.; Terranova, M.L. Rhodamine/nanodiamond as a system model for drug carrier. J. Nanosci. Nanotechnol. 2015, 15, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Orlanducci, S. Gold-Decorated Nanodiamonds: Powerful Multifunctional Materials for Sensing, Imaging, Diagnostics, and Therapy. Eur. J. Inorg. Chem. 2018, 48, 5138–5145. [Google Scholar] [CrossRef]
- Arnault, J.-C. (Ed.) Nanodiamonds: Advanced Material Analysis, Properties and Applications; Nanodiamonds for Catalytic Reactions; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 18. [Google Scholar]
- Toschi, F.; Orlanducci, S.; Guglielmotti, V.; Cianchetta, I.; Magni, C.; Terranova, M.L.; Pasquali, M.; Tamburri, E.; Matassa, R.; Rossi, M. Hybrid C-nanotubes/Si 3D nanostructures by one-step growth in a dual-plasma reactor. Chem. Phys. Lett. 2012, 539, 94–101. [Google Scholar] [CrossRef][Green Version]
- Mermouxa, M.; Chang, S.; Girard, H.A.; Arnault, J.-C. Raman spectroscopy study of detonation nanodiamond. Diam. Relat. Mater. 2018, 87, 248–260. [Google Scholar] [CrossRef]
- Krueger, A.; Liang, Y.; Jarre, G.; Stegk, J. Surface Functionalisation of Detonation Diamond Suitable for Biological Applications. J. Mater. Chem. 2006, 16, 2322–2328. [Google Scholar] [CrossRef]
- Kadish, K.M.; Han, B.C.; Franzen, M.M.; Araullo-McAdams, C. Syntheses and spectroscopic characterization of (T(p-Me2N)F4PP)H2 and (T(p-Me2N)F4PP)M where T(p-Me2N)F4PP = the dianion of meso-tetrakis(o,o,m,m-tetrafluoro-p- (dimethylamino)phenyl)porphyrin and M = cobalt(II), copper(II), or nickel(II). Structures of (T(p-Me2N)F4PP)Co and meso-tetrakis(pentafluorophenyl) porphinatocobalt(II), (TF5PP)Co. J. Am. Chem. Soc. 1990, 112, 8364–8368. [Google Scholar]
- Kadish, K.M.; Hu, Y.; Tagliatesta, P.; Boschi, T. Synthesis and electrochemical characterization of ruthenium porphyrins containing a bound PF3 axial ligand. J. Chem. Soc. Dalton Trans. 1993, 1167–1172. [Google Scholar] [CrossRef]



| Entry | Substrate | Yield% | Syn/Anti Ratio |
|---|---|---|---|
| 1 | Styrene | 35 (60 a) | 1:2.5 (1:0.9 a) |
| 2 | 4-Cl-Styrene | 25 (55 a) | 1:3.8 (1:0.9 a) |
| 3 | 4-OCH3-Styrene | 34 (59 a) | 1:3.2 (1:0.55 a) |
| 4 | Norbornene | 38 (51 a) | 1:20 (1:0.8 a) |
| 5 | 1-methylcyclopentene | - (45 a) | - |
| 6 | 2,4,4-trimethylpentene | - (70 a) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorecchio, C.; Tamburri, E.; Lazzarini, L.; Orlanducci, S.; Zanoni, R.; Tagliatesta, P. Covalent Functionalization of Nanodiamonds by Ruthenium Porphyrin, and Their Catalytic Activity in the Cyclopropanation Reaction of Olefins. Catalysts 2020, 10, 666. https://doi.org/10.3390/catal10060666
Lorecchio C, Tamburri E, Lazzarini L, Orlanducci S, Zanoni R, Tagliatesta P. Covalent Functionalization of Nanodiamonds by Ruthenium Porphyrin, and Their Catalytic Activity in the Cyclopropanation Reaction of Olefins. Catalysts. 2020; 10(6):666. https://doi.org/10.3390/catal10060666
Chicago/Turabian StyleLorecchio, Chiara, Emanuela Tamburri, Laura Lazzarini, Silvia Orlanducci, Robertino Zanoni, and Pietro Tagliatesta. 2020. "Covalent Functionalization of Nanodiamonds by Ruthenium Porphyrin, and Their Catalytic Activity in the Cyclopropanation Reaction of Olefins" Catalysts 10, no. 6: 666. https://doi.org/10.3390/catal10060666
APA StyleLorecchio, C., Tamburri, E., Lazzarini, L., Orlanducci, S., Zanoni, R., & Tagliatesta, P. (2020). Covalent Functionalization of Nanodiamonds by Ruthenium Porphyrin, and Their Catalytic Activity in the Cyclopropanation Reaction of Olefins. Catalysts, 10(6), 666. https://doi.org/10.3390/catal10060666