Performance and Stability of Wet-Milled CoAl2O4, Ni/CoAl2O4, and Pt,Ni/CoAl2O4 for Soot Combustion
Abstract
1. Introduction
2. Results and Discussion
2.1. Textural and Structural Characterization
2.2. Catalytic Activity
2.3. Stability of the Catalysts
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Setten, B.A.A.L.; Makkee, M.; Moulijn, J.A. Science and technology of catalytic diesel particulate filters. Catal. Rev. Sci. Eng. 2001, 43, 489–564. [Google Scholar] [CrossRef]
- Dang, S.S.; Serafino, A.; Müller, J.O.; Jentoft, R.E.; Schlögl, R.; Fiorito, S. Cytotoxicity and inflammatory potential of soot particles of low-emission diesel engines. Environ. Sci. Technol. 2008, 42, 1761–1765. [Google Scholar]
- Brewer, T.L. Black carbon emissions and regulatory policies in transportation. Energy Policy 2019, 129, 1047–1055. [Google Scholar] [CrossRef]
- Rich, D.Q.; Özkaynak, H.; Crooks, J.; Baxter, L.; Burke, J.; Ohman-Strickland, P.; Thevenet-Morrison, K.; Kipen, H.M.; Zhang, J.; Kostis, J.B.; et al. The triggering of myocardial infarction by fine particles is enhanced when particles are enriched in secondary species. Environ. Sci. Technol. 2013, 47, 9414–9423. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.; Schlögl, R.; Su, D.S. Diesel soot toxification. Environ. Sci. Technol. 2013, 47, 3026–3027. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.; Schuster, M.E.; Schlögl, R.; Su, D.S. Emission of highly activated soot particulate-The other side of the coin with modern diesel engines. Angew. Chem. Int. Ed. 2013, 52, 2673–2677. [Google Scholar] [CrossRef]
- Lizarraga, L.; Souentie, S.; Boreave, A.; George, C.; Danna, B.; Vernoux, P. Effect of diesel oxidation catalysts on the diesel particulate filter regeneration process. Environ. Sci. Technol. 2011, 45, 10591–10597. [Google Scholar] [CrossRef]
- Fino, D.; Fino, P.; Saracco, G.; Specchia, V. Diesel particulate traps regenerated by catalytic combustion. Korean J. Chem. Eng. 2003, 20, 445–450. [Google Scholar] [CrossRef]
- Saracco, G.; Badini, C.; Specchia, V. Catalytic traps for diesel particulate control. Chem. Eng. Sci. 1999, 54, 3035–3041. [Google Scholar] [CrossRef]
- Matarrese, R.; Castoldi, L.; Artioli, N.; Finocchio, E.; Busca, G.; Lietti, L. On the activity and stability of Pt-K/Al2O3 LNT catalysts for diesel soot and NOx abatement. Appl. Catal. B Environ. 2014, 144, 783–791. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Ran, R. NOx-assisted soot oxidation on Pt-Mg/Al2O3 catalysts: Magnesium precursor, Pt particle size, and Pt-Mg interaction. Ind. Eng. Chem. Res. 2012, 51, 2271–2279. [Google Scholar]
- Yu, X.; Zhao, Z.; Wei, Y.; Liu, J. Ordered micro/macro porous K-OMS-2/SiO2 nanocatalysts: Facile synthesis, low cost and high catalytic activity for diesel soot combustion. Sci. Rep. 2017, 7, 43894. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Li, J.H.; Zhang, Y.Q.; Hua, B.; Luo, J.L. Bifunctional catalyst of core-shell nanoparticles socketed on oxygen-deficient layered perovskite for soot combustion: In situ observation of synergistic dual active sites. ACS Catal. 2016, 6, 2710–2714. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, T.; Li, Q.; Xin, Y.; Lu, X.; Tang, W.; Zhang, Z.; Gao, P.X.; Anderson, J.A. Multiple strategies to decrease ignition temperature for soot combustion on ultrathin MnO2−x nanosheet array. Appl. Catal. B Environ. 2019, 246, 312–321. [Google Scholar] [CrossRef]
- Hatanaka, M.; Takahashi, N.; Tanabe, T.; Nagai, Y.; Dohmae, K.; Aoki, Y.; Yoshida, T.; Shinjoh, H. Ideal Pt loading for a Pt/CeO2-based catalyst stabilized by a Pt-O-Ce bond. Appl. Catal. B Environ. 2010, 99, 336–342. [Google Scholar] [CrossRef]
- Guillén-Hurtado, N.; García-García, A.; Bueno-López, A. Active oxygen by Ce-Pr mixed oxide nanoparticles outperform diesel soot combustion Pt catalysts. Appl. Catal. B Environ. 2015, 174–175, 60–66. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Li, M.; Lee, H. Combined promoting effects of platinum and MnOx-CeO2 supported on alumina on NOx-assisted soot oxidation: Thermal stability and sulfur resistance. Chem. Eng. J. 2012, 203, 25–35. [Google Scholar] [CrossRef]
- An, H.; McGinn, P.J. Catalytic behavior of potassium containing compounds for diesel soot combustion. Appl. Catal. B Environ. 2006, 62, 46–56. [Google Scholar] [CrossRef]
- Jakubek, T.; Kaspera, W.; Legutko, P.; Stelmachowski, P.; Kotarba, A. Surface versus bulk alkali promotion of cobalt-oxide catalyst in soot oxidation. Catal. Commun. 2015, 71, 37–41. [Google Scholar] [CrossRef]
- Atribak, I.; Bueno-López, A.; García-García, A.; Navarro, P.; Frías, D.; Montes, M. Catalytic activity for soot combustion of birnessite and cryptomelane. Appl. Catal. B Environ. 2010, 93, 267–273. [Google Scholar] [CrossRef]
- Legutko, P.; Stelmachowski, P.; Trębala, M.; Sojka, Z.; Kotarba, A. Role of electronic factor in soot oxidation process over tunnelled and layered potassium iron oxide catalysts. Top. Catal. 2013, 56, 489–492. [Google Scholar] [CrossRef]
- Legutko, P.; Jakubek, T.; Kaspera, W.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Soot oxidation over K-doped manganese and iron spinels-How potassium precursor nature and doping level change the catalyst activity. Catal. Commun. 2014, 43, 34–37. [Google Scholar] [CrossRef]
- Jakubek, T.; Ralphs, K.; Kotarba, A.; Manyar, H. Nanostructured potassium-manganese oxides decorated with Pd nanoparticles as efficient catalysts for low-temperature soot oxidation. Catal. Lett. 2019, 149, 100–106. [Google Scholar] [CrossRef]
- Becerra, M.E.; Arias, N.P.; Giraldo, O.H.; López Suárez, F.E.; Illán Gómez, M.J.; Bueno López, A. Soot combustion manganese catalysts prepared by thermal decomposition of KMnO4. Appl. Catal. B Environ. 2011, 102, 260–266. [Google Scholar] [CrossRef]
- Wu, X.; Liu, D.; Li, K.; Li, J.; Weng, D. Role of CeO2-ZrO2 in diesel soot oxidation and thermal stability of potassium catalyst. Catal. Commun. 2007, 8, 1274–1278. [Google Scholar] [CrossRef]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene. Chem. Eng. J. 2016, 306, 1234–1246. [Google Scholar] [CrossRef]
- Jin, B.; Wei, Y.; Zhao, Z.; Liu, J.; Li, Y.; Li, R.; Duan, A.; Jiang, G. Three-dimensionally ordered macroporous CeO2/Al2O3 -supported Au nanoparticle catalysts: Effects of CeO2 nanolayers on catalytic activity in soot oxidation. Chin. J. Catal. 2017, 38, 1629–1641. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; Da Silva, A.G.M.; Gonçalves, M.C.; Fajardo, H.V.; Balzer, R.; Probst, L.F.D.; Da Silva, A.H.M.; Assaf, J.M.; Camargo, P.H.C. Catalytic properties of AgPt nanoshells as a function of size: Larger outer diameters lead to improved performances. Langmuir 2016, 32, 9371–9379. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Ning, P.; Tang, T.; Hu, J.; Su, W. One-pot synthesis of mesoporous Al2O3-supported Pt-Pd catalysts for toluene combustion. Catal. Commun. 2018, 115, 26–30. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Jin, B.; Yu, X.; Jiao, J.; Li, K.; Liu, J. Synthesis of AuPt alloy nanoparticles supported on 3D ordered macroporous oxide with enhanced catalytic performance for soot combustion. Catal. Today 2015, 251, 103–113. [Google Scholar] [CrossRef]
- Nejar, N.; Illán-Gómez, M.J. Potassium-copper and potassium-cobalt catalysts supported on alumina for simultaneous NOx and soot removal from simulated diesel engine exhaust. Appl. Catal. B Environ. 2007, 70, 261–268. [Google Scholar] [CrossRef]
- Liao, F.; Lo, T.W.B.; Tsang, S.C.E. Recent developments in Palladium-based bimetallic catalysts. ChemCatChem 2015, 7, 1998–2014. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.; Xia, Y. Enhancing the catalytic and electrocatalytic properties of Pt-based catalysts by forming bimetallic nanocrystals with Pd. Chem. Soc. Rev. 2012, 41, 8035–8049. [Google Scholar] [CrossRef]
- Alarcón, A.; Guilera, J.; Soto, R.; Andreu, T. Higher tolerance to sulfur poisoning in CO2 methanation by the presence of CeO2. Appl. Catal. B Environ. 2020, 263, 118346. [Google Scholar] [CrossRef]
- Ricca, A.; Truda, L.; Palma, V. Study of the role of chemical support and structured carrier on the CO2 methanation reaction. Chem. Eng. J. 2019, 377, 120461. [Google Scholar] [CrossRef]
- Keghouche, N.; Chettibi, S.; Latrèche, F.; Bettahar, M.M.; Belloni, J.; Marignier, J.L. Radiation-induced synthesis of α-Al2O3 supported nickel clusters: Characterization and catalytic properties. Radiat. Phys. Chem. 2005, 74, 185–200. [Google Scholar] [CrossRef]
- Lonergan, W.W.; Vlachos, D.G.; Chen, J.G. Correlating extent of Pt-Ni bond formation with low-temperature hydrogenation of benzene and 1,3-butadiene over supported Pt/Ni bimetallic catalysts. J. Catal. 2010, 271, 239–250. [Google Scholar] [CrossRef]
- Oi-Uchisawa, J.; Wang, S.; Nanba, T.; Ohi, A.; Obuchi, A. Improvement of Pt catalyst for soot oxidation using mixed oxide as a support. Appl. Catal. B Environ. 2003, 44, 207–215. [Google Scholar] [CrossRef]
- Van Doorn, J.; Varloud, J.; Mériaudeau, P.; Perrichon, V.; Chevrier, M.; Gauthier, C. Effect of support material on the catalytic combustion of diesel soot particulates. Appl. Catal. B Environ. 1992, 1, 117–127. [Google Scholar] [CrossRef]
- Carrascull, A.; Grzona, C.; Lick, D.; Ponzi, M.; Ponzi, E. Soot combustion. Co and K catalysts supported on different supports. React. Kinet. Catal. Lett. 2002, 75, 63–68. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E. Overview of the fundamental reactions and degradation mechanisms of NOx storage/reduction catalysts. Catal. Rev. Sci. Eng. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Villani, K.; Vermandel, W.; Smets, K.; Liang, D.; Van Tendeloo, G.; Martens, J.A. Platinum particle size and support effects in NOx mediated carbon oxidation over platinum catalysts. Environ. Sci. Technol. 2006, 40, 2727–2733. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J.; Adamski, A.; Ura, B.; Trawczynski, J. Copper catalysts for soot oxidation: Alumina versus perovskite supports. Environ. Sci. Technol. 2008, 42, 7670–7675. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Liu, S.; Weng, D.; Ran, R. Effect of water vapor on sulfur poisoning of MnOx–CeO2/Al2O3 catalyst for diesel soot oxidation. RSC Adv. 2016, 6, 57033–57040. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.; Weng, D.; Lin, F.; Ran, R. MnOx-CeO2-Al2O3 mixed oxides for soot oxidation: Activity and thermal stability. J. Hazard. Mater. 2011, 187, 283–290. [Google Scholar] [CrossRef]
- Li, W.Z.; Kovarik, L.; Mei, D.; Liu, J.; Wang, Y.; Peden, C.H.F. Stable platinum nanoparticles on specific MgAl2O4 spinel facets at high temperatures in oxidizing atmospheres. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Saracco, G.; Specchia, V. Catalytic removal of NOx and diesel soot over nanostructured spinel-type oxides. J. Catal. 2006, 242, 38–47. [Google Scholar] [CrossRef]
- Zawadzki, M.; Walerczyk, W.; López-Suárez, F.E.; Illán-Gómez, M.J.; Bueno-López, A. CoAl2O4 spinel catalyst for soot combustion with NOx/O2. Catal. Commun. 2011, 12, 1238–1241. [Google Scholar] [CrossRef]
- Cauda, E.; Hernandez, S.; Fino, D.; Saracco, G.; Specchia, V. PM0.1 emissions during diesel trap regeneration. Environ. Sci. Technol. 2006, 40, 5532–5537. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Docio, C.M.; Portela, R.; Reinosa, J.J.; Rubio-Marcos, F.; Granados-Miralles, C.; Pascual, L.; Fernández, J.F. Pt-free CoAl2O4 catalyst for soot combustion with NOx/O2. Appl. Catal. A Gen. 2020, 591, 117404. [Google Scholar] [CrossRef]
- Ahmadi, M.; Mistry, H.; Roldan Cuenya, B. Tailoring the catalytic properties of metal nanoparticles via support interactions. J. Phys. Chem. Lett. 2016, 7, 3519–3533. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Docio, C.M.; Portela, R.; Reinosa, J.J.; Rubio-Marcos, F.; Fernández, J.F. Pt mechanical dispersion on non-porous alumina for soot oxidation. Catal. Commun. 2020, 140, 105999. [Google Scholar] [CrossRef]
- Lorite, I.; Martín-González, M.S.; Romero, J.J.; García, M.A.; Fierro, J.L.G.; Fernández, J.F. Electrostatic charge dependence on surface hydroxylation for different Al2O3 powders. Ceram. Int. 2012, 38, 1427–1434. [Google Scholar] [CrossRef]
- Lorite, I.; Pérez, L.; Romero, J.J.; Fernandez, J.F. Effect of the dry nanodispersion procedure in the magnetic order of the Co3O4 surface. Ceram. Int. 2013, 39, 4377–4381. [Google Scholar] [CrossRef]
- Reid, C.B.; Forrester, J.S.; Goodshaw, H.J.; Kisi, E.H.; Suaning, G.J. A study in the mechanical milling of alumina powder. Ceram. Int. 2008, 34, 1551–1556. [Google Scholar] [CrossRef]
- Álvarez-Docio, C.M.; Reinosa, J.J.; Del Campo, A.; Fernández, J.F. Investigation of thermal stability of 2D and 3D CoAl2O4 particles in core-shell nanostructures by raman spectroscopy. J. Alloys Compd. 2018, 779, 244–254. [Google Scholar] [CrossRef]
- Álvarez-Docio, C.M.; Reinosa, J.J.; del Campo, A.; Fernández, J.F. 2D particles forming a nanostructured shell: A step forward cool NIR reflectivity for CoAl2O4 pigments. Dye. Pigment. 2017, 137, 1–11. [Google Scholar] [CrossRef]
- Giménez-Mañogil, J.; García-García, A. Opportunities for ceria-based mixed oxides versus commercial platinum-based catalysts in the soot combustion reaction. Mechanistic implications. Fuel Process. Technol. 2015, 129, 227–235. [Google Scholar] [CrossRef]
- Zawadzki, M.; Staszak, W.; López-Suárez, F.E.; Illán-Gómez, M.J.; Bueno-López, A. Preparation, characterisation and catalytic performance for soot oxidation of copper-containing ZnAl2O4 spinels. Appl. Catal. A Gen. 2009, 371, 92–98. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Q.; Xiong, J.; Li, J.; Liu, J.; Zhao, Z.; Hao, S. Efficient catalysts of supported PtPd nanoparticles on 3D ordered macroporous TiO2 for soot combustion: Synergic effect of Pt-Pd binary components. Catal. Today 2019, 327, 143–153. [Google Scholar] [CrossRef]
- Zhou, X.X.; Shi, J.L.; Wang, M.; Pan, L.Y.; Zhao, H.; Chen, H.R. Facile synthesis of spinel Cu1.5Mn1.5O4 microspheres with high activity for the catalytic combustion of diesel soot. RSC Adv. 2017, 7, 20451–20459. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Saracco, G.; Specchia, V. Removal of NOx and diesel soot over catalytic traps based on spinel-type oxides. Powder Technol. 2008, 180, 74–78. [Google Scholar] [CrossRef]
- Rico-Pérez, V.; Aneggi, E.; Bueno-López, A.; Trovarelli, A. Synergic effect of Cu/Ce0.5Pr0.5O2-δ and Ce0.5Pr0.5O2-δ in soot combustion. Appl. Catal. B Environ. 2016, 197, 95–104. [Google Scholar] [CrossRef]
- Fino, D.; Bensaid, S.; Piumetti, M.; Russo, N. A review on the catalytic combustion of soot in diesel particulate filters for automotive applications: From powder catalysts to structured reactors. Appl. Catal. A Gen. 2015, 509, 75–96. [Google Scholar] [CrossRef]
- Campbell, C.T. The energetics of supported metal nanoparticles: Relationships to sintering rates and catalytic activitydcfzsd. Acc. Chem. Res. 2013, 46, 1712–1719. [Google Scholar] [CrossRef]
- Hong, S.; Rahman, T.S. Rationale for the higher reactivity of interfacial sites in methanol decomposition on Au13/TiO2 (110). J. Am. Chem. Soc. 2013, 135, 7629–7635. [Google Scholar] [CrossRef]
- Ricci, D.; Bongiorno, A.; Pacchioni, G.; Landman, U. Bonding trends and dimensionality crossover of gold nanoclusters on metal-supported MgO thin films. Phys. Rev. Lett. 2006, 97, 1–4. [Google Scholar] [CrossRef]
- Farmer, J.A.; Campbell, C.T. Ceria maintains smaller metal catalyst particles by strong metal-support bonding. Science 2010, 329, 933–936. [Google Scholar] [CrossRef]
- Hemmingson, S.L.; James, T.E.; Feeley, G.M.; Tilson, A.M.; Campbell, C.T. Adsorption and adhesion of Au on reduced CeO2 (111) surfaces at 300 and 100 K. J. Phys. Chem. C 2016, 120, 12113–12124. [Google Scholar] [CrossRef]
- Hu, C.; Creaser, D.; Grönbeck, H.; Ojagh, H.; Skoglundh, M. Selectivity and kinetics of methyl crotonate hydrogenation over Pt/Al2O3. Catal. Sci. Technol. 2015, 5, 1716–1730. [Google Scholar] [CrossRef][Green Version]
- Tuler, F.E.; Portela, R.; Ávila, P.; Banús, E.D.; Miró, E.E.; Milt, V.G. Structured catalysts based on sepiolite with tailored porosity to remove diesel soot. Appl. Catal. A Gen. 2015, 498, 41–53. [Google Scholar] [CrossRef]
- Van Setten, B.A.A.L.; Schouten, J.M.; Makkee, M.; Moulijn, J.A. Realistic contact for soot with an oxidation catalyst for laboratory studies. Appl. Catal. B Environ. 2000, 28, 253–257. [Google Scholar] [CrossRef]
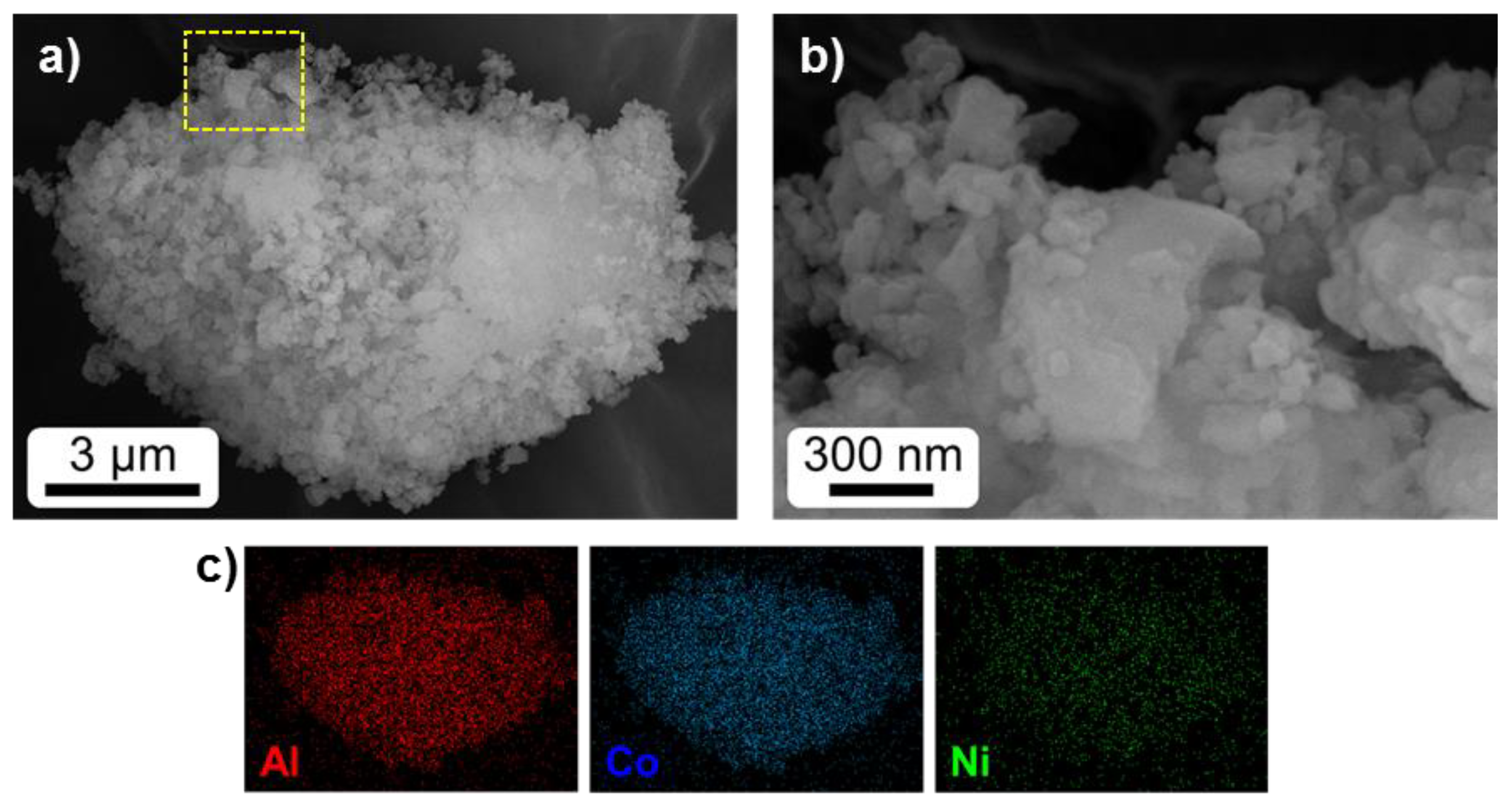
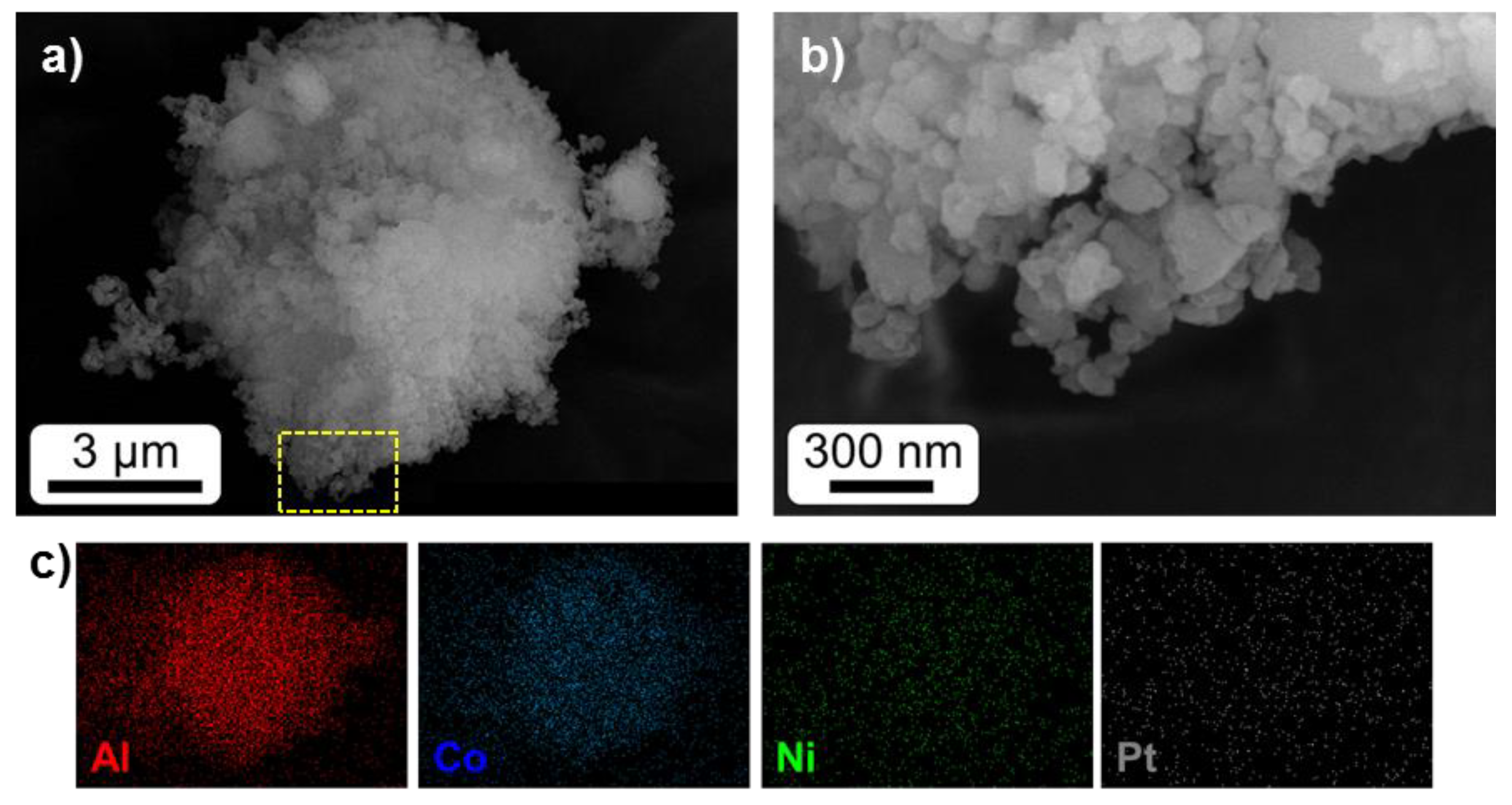


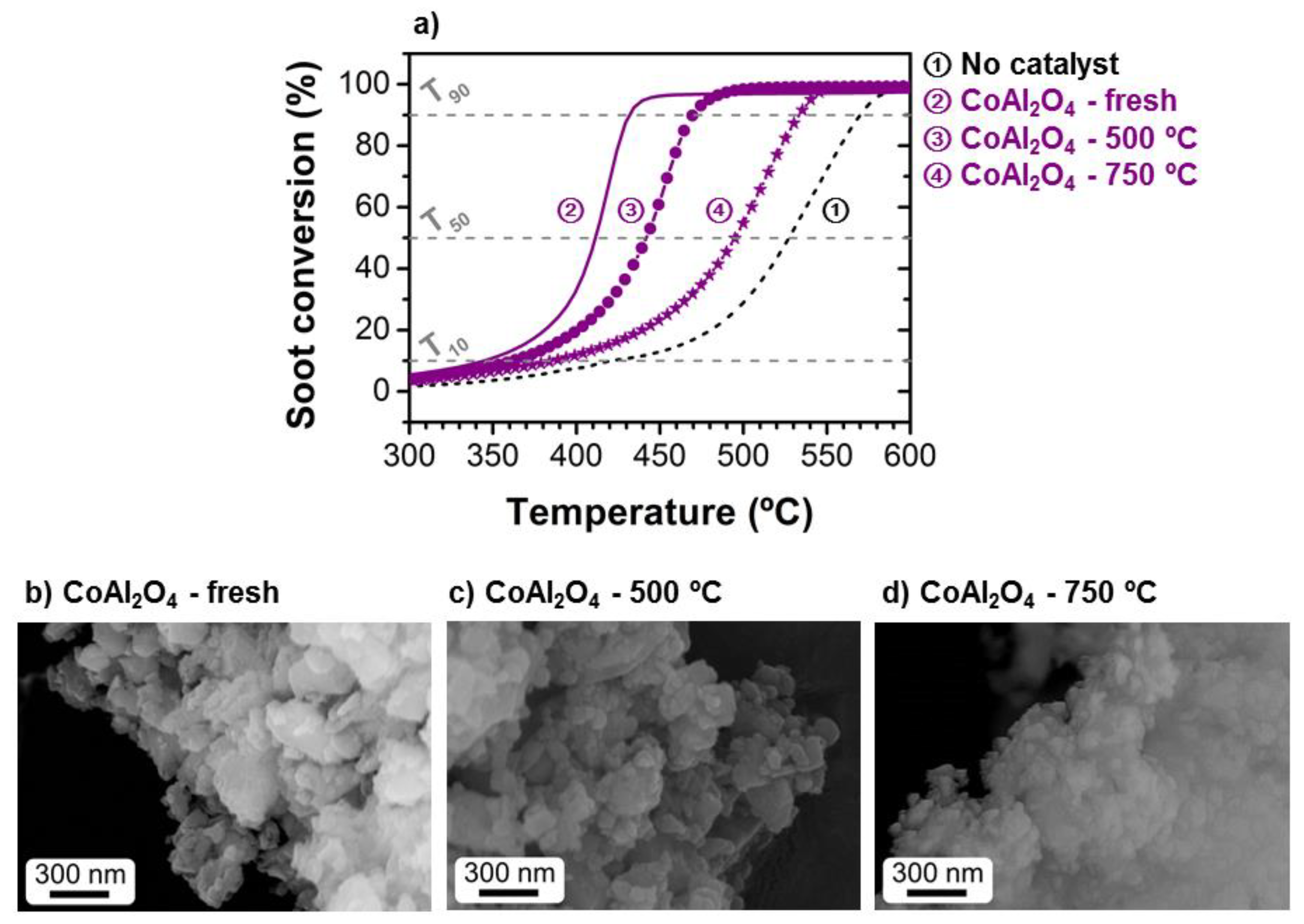
| Catalyst | Metal Content (%) | SBET (m2/g) | T50-Fresh (°C) | T50-Reused (°C) | SCO2 (%) | Reference |
|---|---|---|---|---|---|---|
| No catalyst | - | - | 527 | - | 67 | Current study |
| CoAl2O4 | - | 22.9 | 411 | 494 (750 °C) 441 (500 °C) | 100 | Current study |
| Pt,Ni/CoAl2O4 | 0.75% Pt 0.25% Ni | 19.3 | 390 | 421 | 100 | Current study |
| Ni/CoAl2O4 | 1% Ni | 22.8 | 371 | 506 | 100 | Current study |
| Pt/α-Al2O3 | 1% Pt | 15 | 398 | 422 | 100 | Current study |
| Pt/γ-Al2O3 | 1% Pt | 160 | 474 | - | 100 | [58] |
| Cu/ZnAl2O4 | 5% Cu | 111 | 600 | - | 95 | [59] |
| Pt-Pd/3DOM-TiO2 | 2% Pt-Pd | 60 | 338 | - | 99 | [60] |
| Cu1.5Mn1.5O4 | - | - | 397 | - | - | [61] |
| CoCr2O4 | - | 59 | 396 | - | - | [62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Docio, C.M.; Portela, R.; Reinosa, J.J.; Rubio-Marcos, F.; Pascual, L.; Fernández, J.F. Performance and Stability of Wet-Milled CoAl2O4, Ni/CoAl2O4, and Pt,Ni/CoAl2O4 for Soot Combustion. Catalysts 2020, 10, 406. https://doi.org/10.3390/catal10040406
Álvarez-Docio CM, Portela R, Reinosa JJ, Rubio-Marcos F, Pascual L, Fernández JF. Performance and Stability of Wet-Milled CoAl2O4, Ni/CoAl2O4, and Pt,Ni/CoAl2O4 for Soot Combustion. Catalysts. 2020; 10(4):406. https://doi.org/10.3390/catal10040406
Chicago/Turabian StyleÁlvarez-Docio, Carmen M., Raquel Portela, Julián J. Reinosa, Fernando Rubio-Marcos, Laura Pascual, and José F. Fernández. 2020. "Performance and Stability of Wet-Milled CoAl2O4, Ni/CoAl2O4, and Pt,Ni/CoAl2O4 for Soot Combustion" Catalysts 10, no. 4: 406. https://doi.org/10.3390/catal10040406
APA StyleÁlvarez-Docio, C. M., Portela, R., Reinosa, J. J., Rubio-Marcos, F., Pascual, L., & Fernández, J. F. (2020). Performance and Stability of Wet-Milled CoAl2O4, Ni/CoAl2O4, and Pt,Ni/CoAl2O4 for Soot Combustion. Catalysts, 10(4), 406. https://doi.org/10.3390/catal10040406








