Self-Nitrogen-Doped Carbon Nanosheets Modification of Anodes for Improving Microbial Fuel Cells’ Performance
Abstract
1. Introduction
2. Results and Discussion
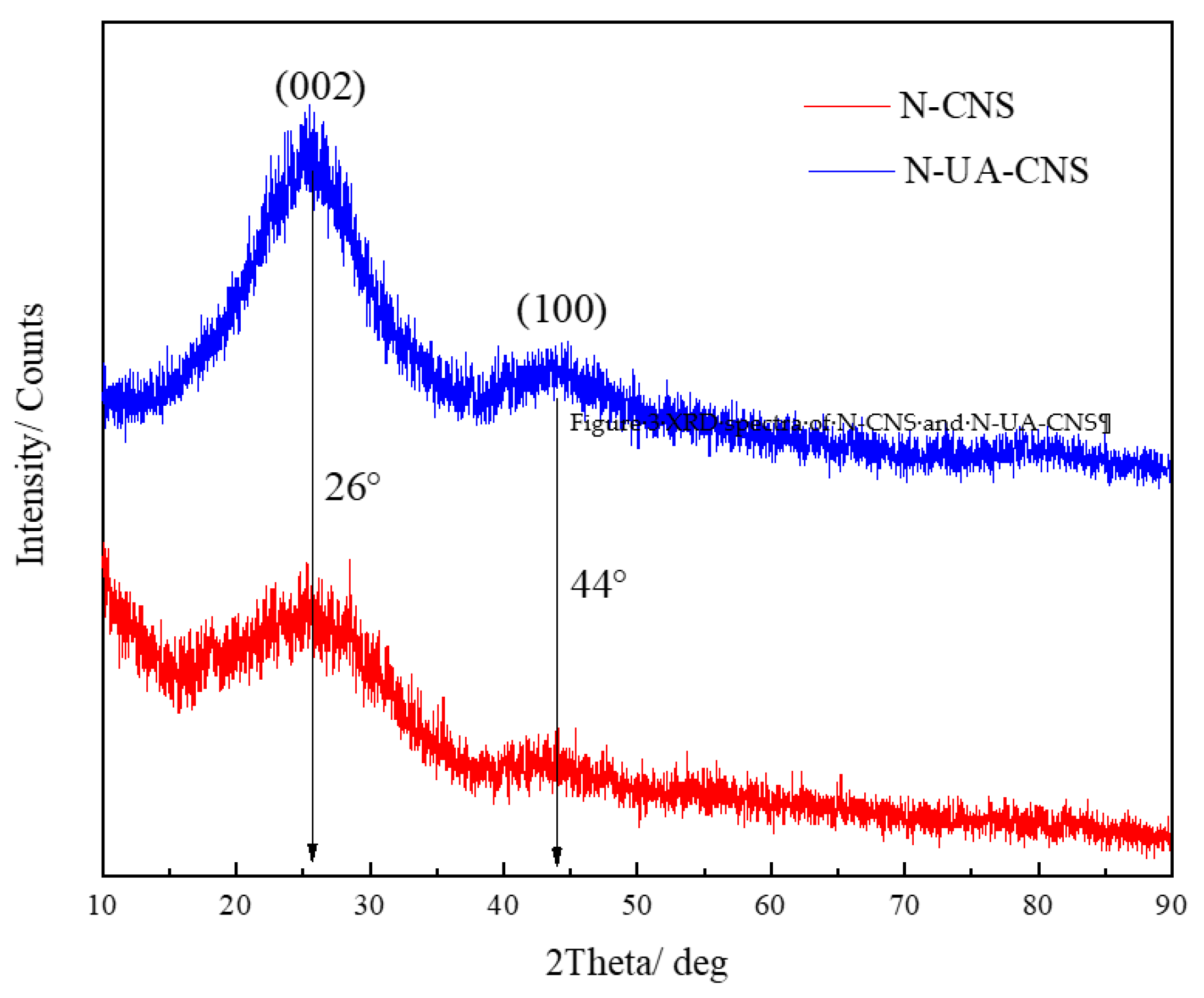
2.1. Characterization of N-CNS and N-UA-CNS
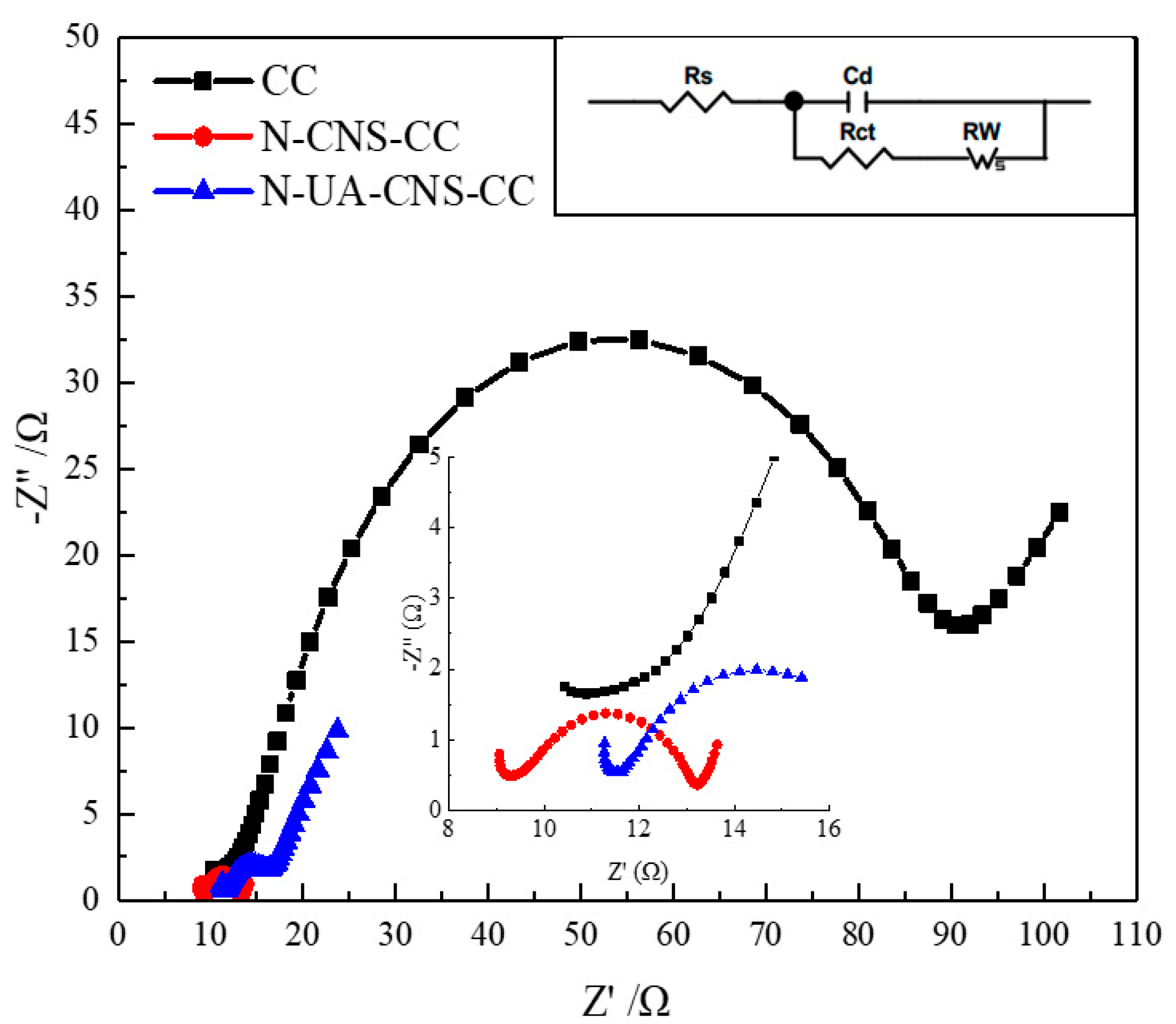
2.2. Electrochemical Characteristics of N-CNS-CC and N-UA-CNS-CC
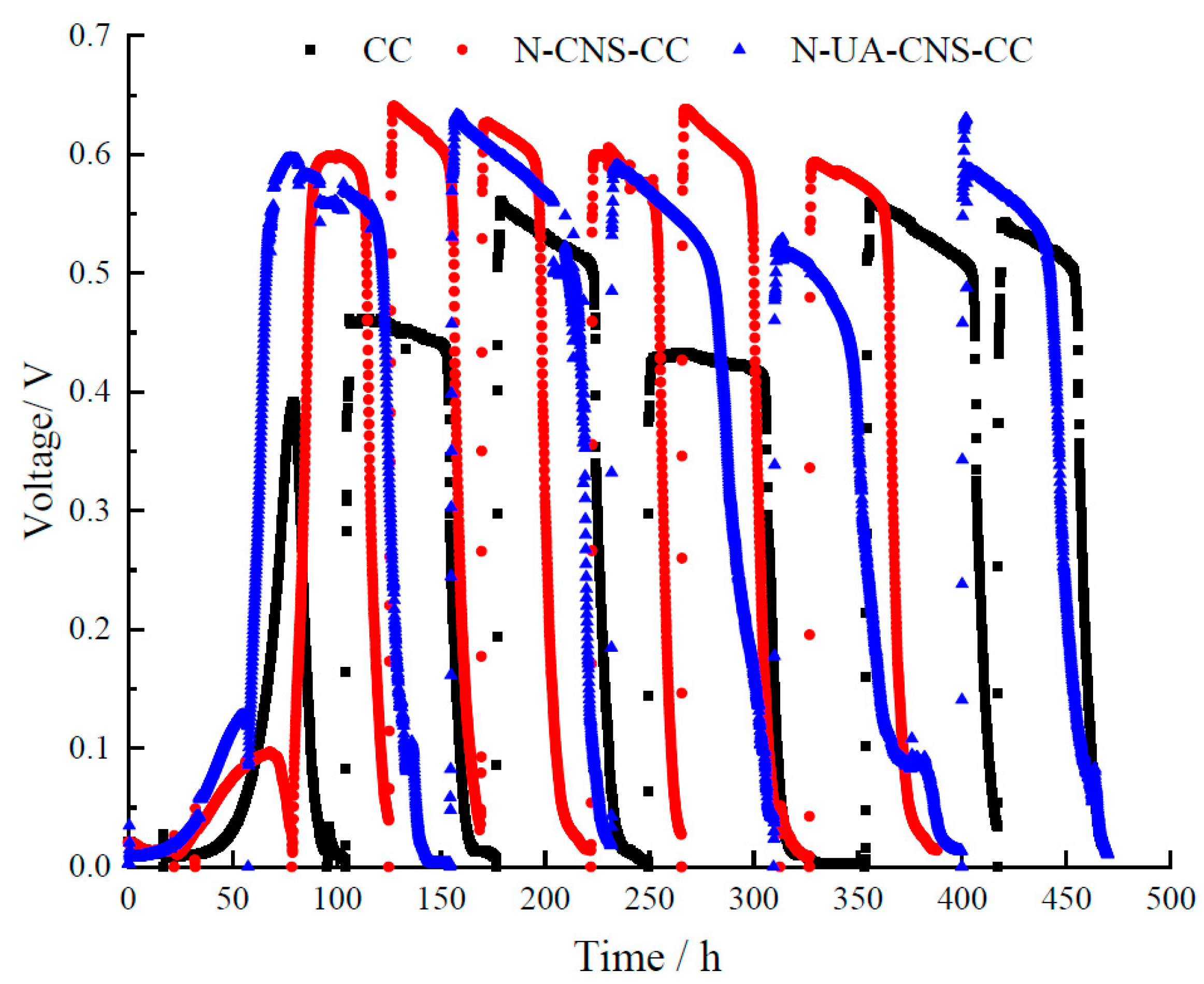
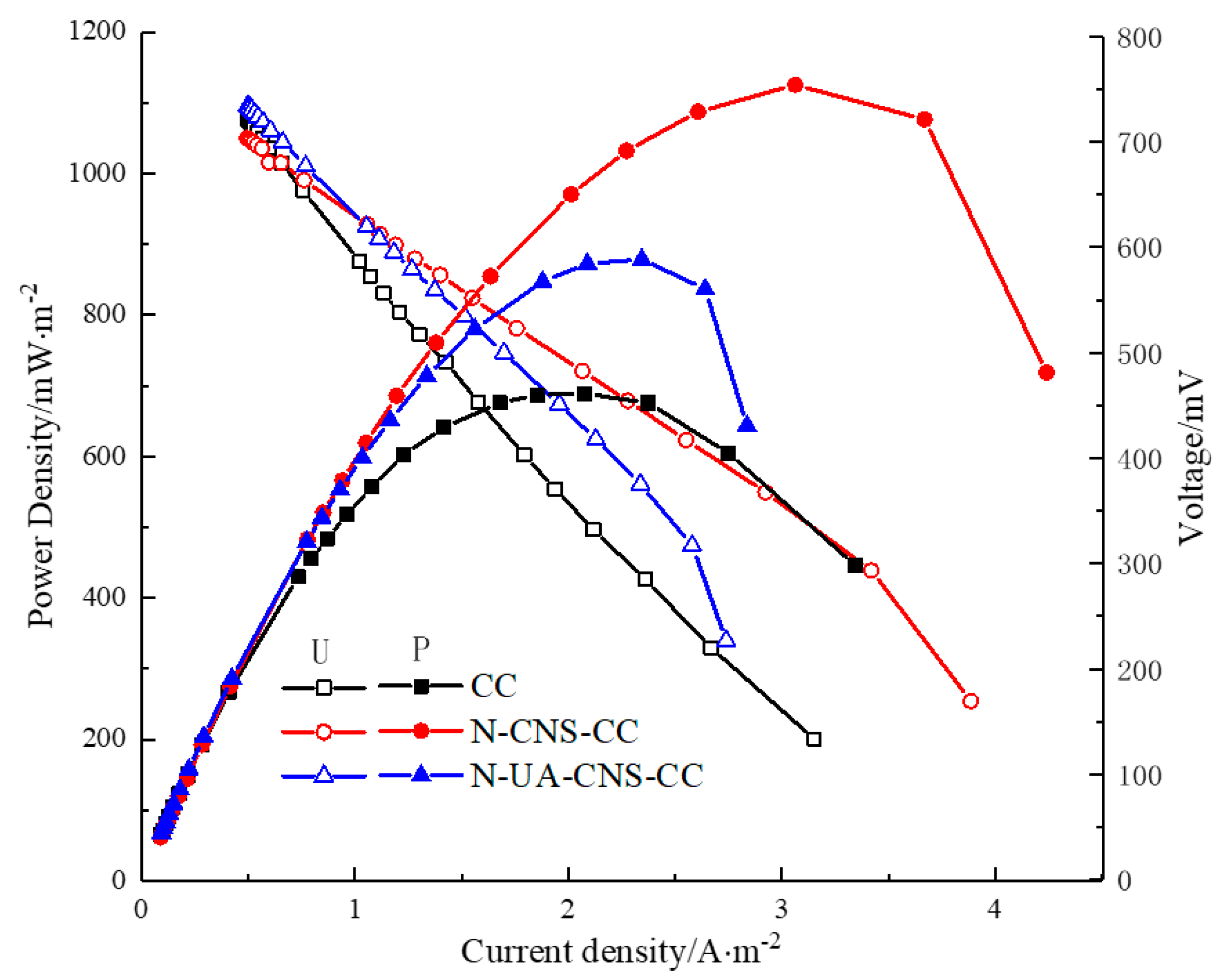
2.3. MFC Performance
3. Materials and Methods
3.1. Preparation of Self-Nitrogen-Doped Carbon
3.2. Preparation of Electrodes
3.3. MFC Setup and Operation
3.4. Material and Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Logan, B.E. Microbial Fuel Cells, (Chinese version); Chemical Industry Press: Beijing, China, 2009. [Google Scholar]
- Logan, B.E.; Rebaey, K. Conversion of wastes into bioelectricity and chemicals using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.W.; Li, H.R.; Gu, T.Y. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.A.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef]
- Logan, B.E.; Cheng, S.A.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef]
- Chen, X.F.; Cui, D.; Wang, X.J.; Wang, X.S.; Li, W.S. Porous carbon with defined pore size as anode of microbial fuel cell. Biosens. Bioelectron. 2015, 69, 135–141. [Google Scholar] [CrossRef]
- Hou, J.X.; Liu, Z.L.; Zhang, P.Y. A new method for fabrication of graphene/polyaniline nanocomplex modified microbial fuel cell anodes. J. Power Sources 2013, 224, 139–144. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Mecheri, B.; D’Epifanio, A.; Sciarria, T.P.; Adani, F.; Licoccia, S. Iron chelates as low-cost and effective electrocatalyst for oxygen reduction reaction in microbial fuel cells. Int. J. Hydrogen Energy 2014, 39, 6462–6469. [Google Scholar] [CrossRef]
- Rahmawati, I.; Ivandini, T.A.; Saepudin, E. The potency of electrical energy production from urine by microbial fuel cell using boron-doped diamond electrode. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 12001. [Google Scholar] [CrossRef]
- Khare, P.; Ranmkumar, J.; Verma, N. Carbon nanofiber-skinned three-dimensional Ni/Carbon micropillars: High performance electrodes of a microbial fuel cell. Electrochim. Acta 2016, 219, 88–98. [Google Scholar] [CrossRef]
- Guo, D.; Song, R.B.; Shao, H.H.; Zhang, J.R.; Zhu, J.J. Visible-light-enhanced power generation in microbial fuel cells coupling with 3D nitrogen-doped graphene. Chem. Commun. 2017, 53, 9949–10080. [Google Scholar]
- Wan, G.; Fu, Y.; Guo, J.N.; Xiang, Z.H. Photoelectronic porous covalent organic materials: Research progress and perspective. Acta Chim. Sin. 2015, 73, 557–578. [Google Scholar] [CrossRef]
- Ci, S.Q.; Wen, Z.H.; Chen, J.H.; He, Z. Decorating anode with bamboo-like nitrogen-doped carbon nanotubes for microbial fuel cells. Elecrtochem. Commun. 2012, 14, 71–74. [Google Scholar] [CrossRef]
- Tian, X.Y.; Zhou, M.H.; Tan, C.L.; Li, M.; Liang, L.; Li, K.R.; Su, P. KOH activated N-doped novel carbon aerogel as efficient metal-free oxygen reduction catalyst for microbial fuel cells. Chem. Eng. J. 2018, 348, 775–785. [Google Scholar] [CrossRef]
- Yong, Y.; Zhou, S.G.; Liu, Y.; Tang, J.H. Nanostructured macroporous bioanode based on polyaniline-modified natural loofah sponge for high-performance microbial fuel cells. Environ. Sci. Technol. 2013, 47, 14525–14532. [Google Scholar]
- Pan, F.P.; Cao, Z.Y.; Zhao, Q.P.; Liang, H.Y.; Zhang, J.Y. Nitrogen-doped porous carbon nanosheets made from biomass as highly active electrocatalyst for oxygen reduction reaction. J. Power Sources 2014, 272, 8–15. [Google Scholar] [CrossRef]
- Zhao, J.; Gong, J.W.; Li, Y.J.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; Cao, D.X.; Wang, G.L. Self N-doped porous interconnected carbon nanosheets materials for supercapacitors. Acta Chim. Sin. 2018, 76, 107–112. [Google Scholar] [CrossRef]
- Tay, T.; Ucar, S.; Karagoz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef]
- Deng, H.; Li, G.X.; Yang, H.B.; Tang, J.P.; Tang, J.Y. Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation. Chem. Eng. J. 2010, 163, 373–381. [Google Scholar] [CrossRef]
- Zhang, T.; Zeng, Y.L.; Chen, S.L.; Ai, X.P.; Yang, H.X. Improved performances of E. coli-catalyzed microbial fuel cells with composite graphite/PTFE anodes. Electrochem. Commun. 2007, 9, 349–353. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.X.; Ge, B.C.; Pu, L.T.; Liu, Z.Q. Influence of micropore and mesoporous in activated carbon air-cathode catalysts on oxygen reduction reaction in microbial fuel fells. Electrochim. Acta 2016, 214, 110–118. [Google Scholar] [CrossRef]
- Wang, J.Q.; Han, Z.D. The combustion behavior of polyacrylate ester/graphite oxide composites. Polym. Adv. Technol. 2006, 17, 335–340. [Google Scholar] [CrossRef]
- He, Z.; Mansfeld, F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ. Sci. 2009, 2, 215–219. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Leropoulos, I. Microbial fuel cells: From fundamentals to applications. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Z.L.; Hou, J.X.; Chen, W.W.; Lou, X.G.; Li, Y.X. Study of urine-powered microbial fuel cell anode modified by graphene-type novel materials. CIESC J. 2018, 69, 2790–2796. (In Chinese) [Google Scholar]
- Wang, Y.Q.; Li, B.; Dan Cui, D.; Xiang, X.D.; Li, W.S. Nano-molybdenum carbide/carbon nanotubes composite as bifunctional anode catalyst for high-performance Escherichia coli-based microbial fuel cell. Biosens. Bioelectron. 2014, 51, 349–355. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Wang, B.; Xuan, J.; Wong, J.W.; Lee, P.K.; Leung, M.K. Interfacial electron transfer and bioelectrocatalysis of carbonized plant material as effective anode of microbial fuel cell. Electrochim. Acta 2015, 157, 314–323. [Google Scholar] [CrossRef]
- Chen, W.W.; Liu, Z.L.; Hou, J.X.; Zhou, Y.; Lou, X.G.; Li, Y.X. Supercapacitor materials anodes for enhancing urine-powered microbial fuel cell performance. J. Eng. Thermophys. (Gongcheng Rewuli Xuebao) 2018, 39, 1818–1823. (In Chinese) [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, X.; Liu, Z.; Chen, W.; Lou, X.; Li, Y.; Liao, Q. Self-Nitrogen-Doped Carbon Nanosheets Modification of Anodes for Improving Microbial Fuel Cells’ Performance. Catalysts 2020, 10, 381. https://doi.org/10.3390/catal10040381
Xing X, Liu Z, Chen W, Lou X, Li Y, Liao Q. Self-Nitrogen-Doped Carbon Nanosheets Modification of Anodes for Improving Microbial Fuel Cells’ Performance. Catalysts. 2020; 10(4):381. https://doi.org/10.3390/catal10040381
Chicago/Turabian StyleXing, Xiaoye, Zhongliang Liu, Wenwen Chen, Xiaoge Lou, Yanxia Li, and Qiang Liao. 2020. "Self-Nitrogen-Doped Carbon Nanosheets Modification of Anodes for Improving Microbial Fuel Cells’ Performance" Catalysts 10, no. 4: 381. https://doi.org/10.3390/catal10040381
APA StyleXing, X., Liu, Z., Chen, W., Lou, X., Li, Y., & Liao, Q. (2020). Self-Nitrogen-Doped Carbon Nanosheets Modification of Anodes for Improving Microbial Fuel Cells’ Performance. Catalysts, 10(4), 381. https://doi.org/10.3390/catal10040381




