Theoretical and Experimental Studies on the Visible Light Activity of TiO2 Modified with Halide-Based Ionic Liquids
Abstract
1. Introduction
2. Results
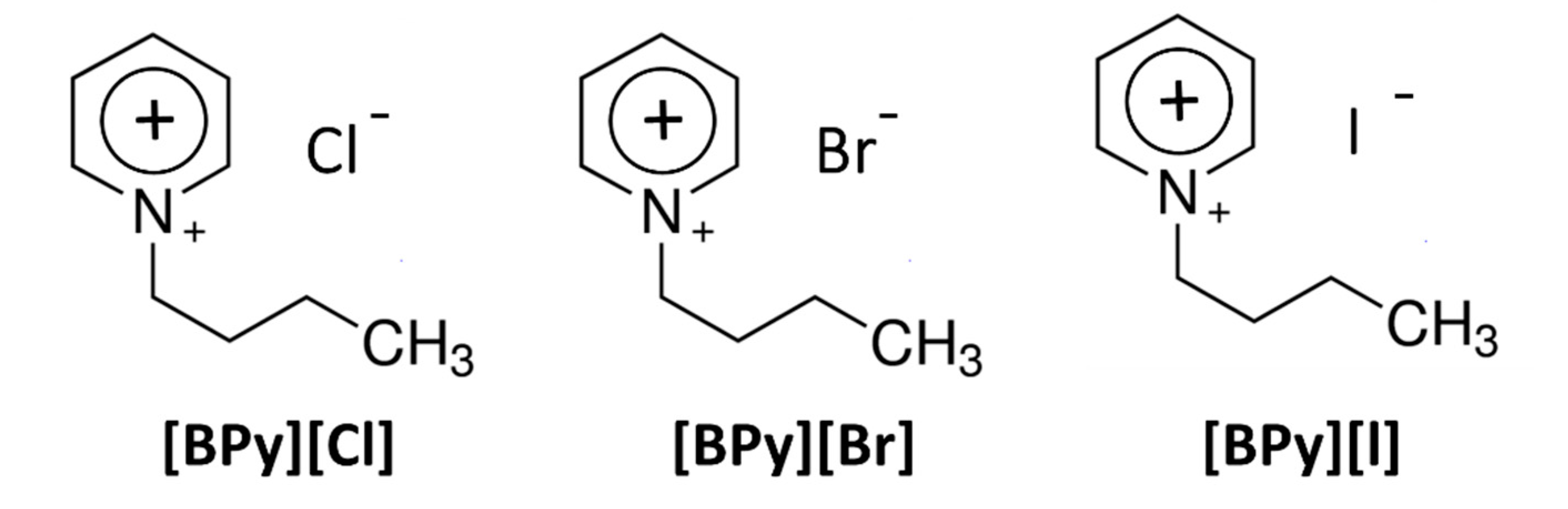
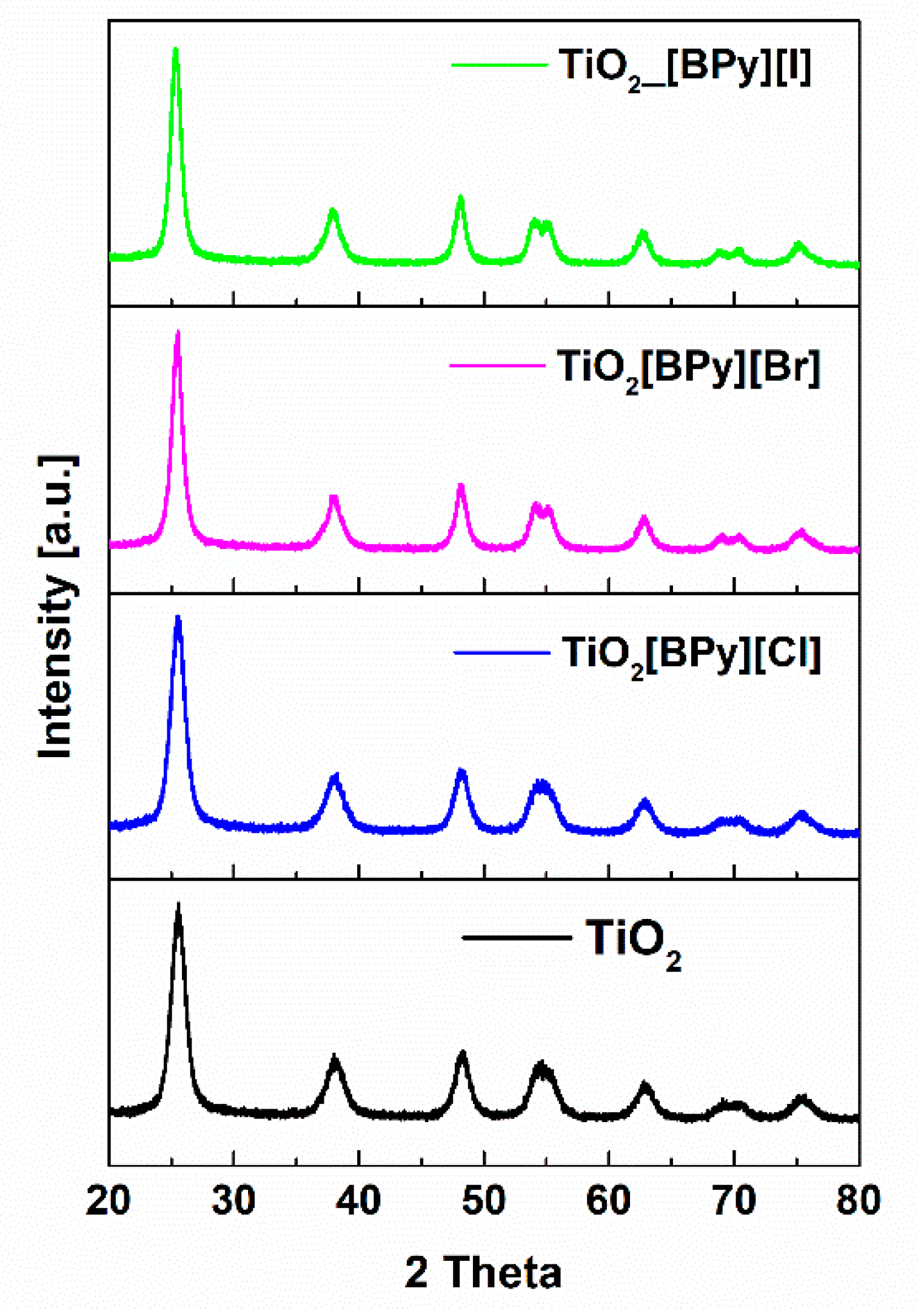
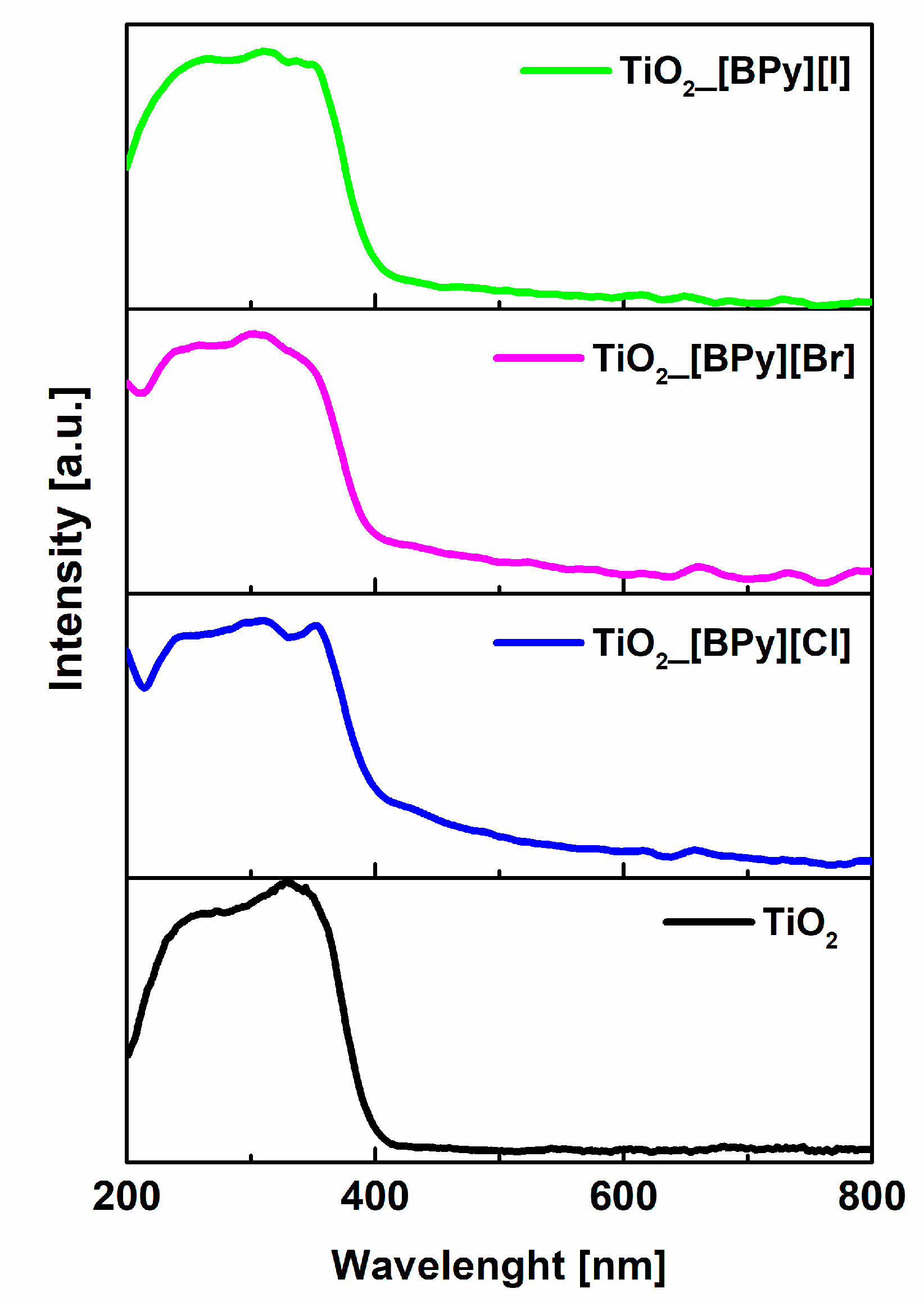
2.1. Structure, Morphology and Optical Properties
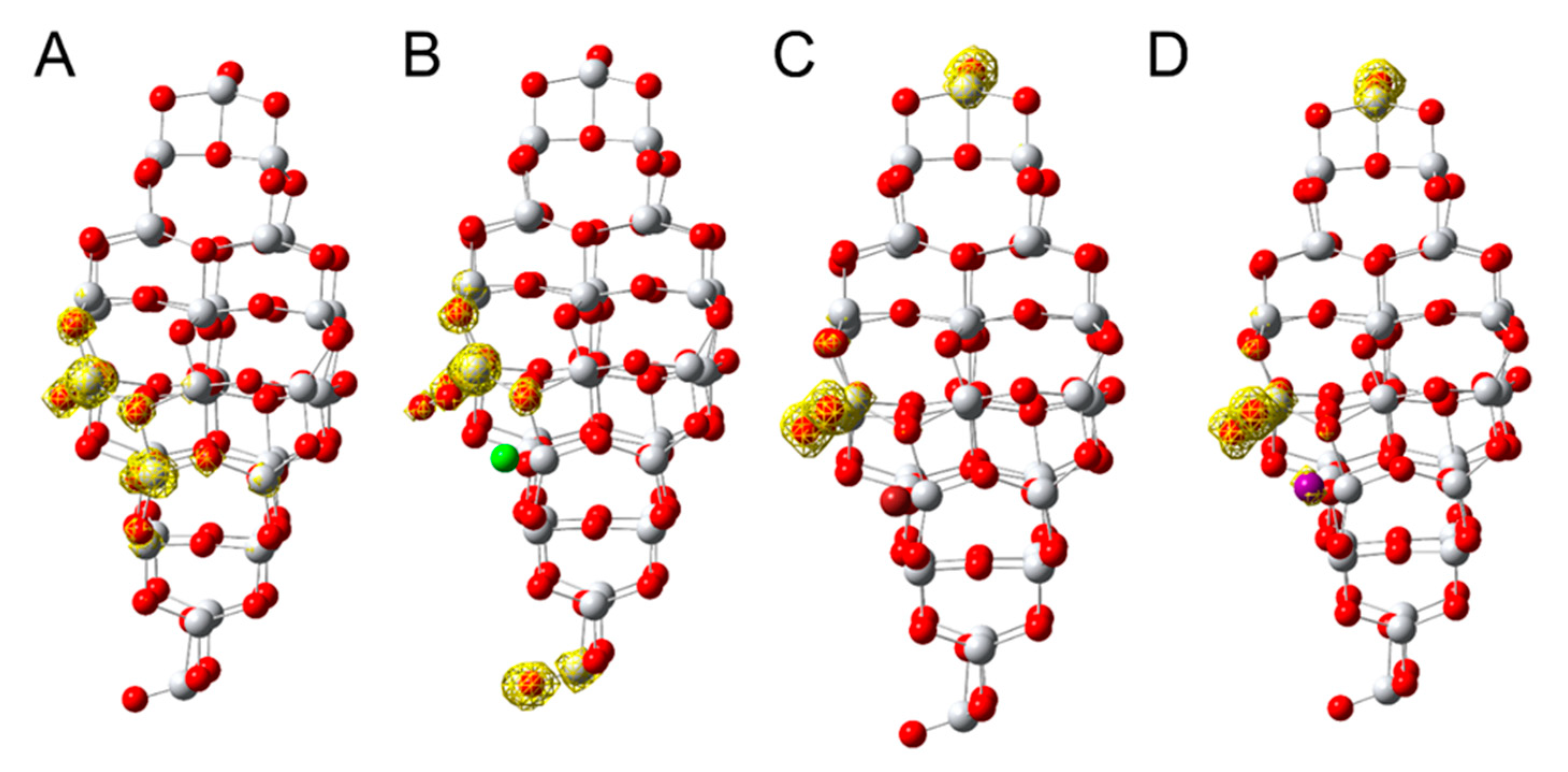
2.2. Photocatalytic Activity and Mechanism
3. Materials and Methods
3.1. Samples Preparation Method
3.2. Characteristic of the Material
3.3. Photocatalytic Testes (Screening Tests)
3.4. Irradiations (Action Spectral Measurements)
3.5. Computations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yun, E.-T.; Yoo, H.-Y.; Kim, W.; Kim, H.-E.; Kang, G.; Lee, H.; Lee, S.; Park, T.; Lee, C.; Kim, J.-H.; et al. Visible-light-induced activation of periodate that mimics dye-sensitization of TiO2: Simultaneous decolorization of dyes and production of oxidizing radicals. Appl. Catal. B Environ. 2017, 203, 475–484. [Google Scholar] [CrossRef]
- Samsudin, E.M.; Hamid, S.B.A.; Juan, J.C.; Basirun, W.J.; Centi, G. Enhancement of the intrinsic photocatalytic activity of TiO2 in the degradation of 1,3,5-triazine herbicides by doping with N,F. Chem. Eng. J. 2015, 280, 330–343. [Google Scholar] [CrossRef]
- Park, Y.; Singh, N.J.; Kim, K.S.; Tachikawa, T.; Majima, T.; Choi, W. Fullerol–Titania Charge-Transfer-Mediated Photocatalysis Working under Visible Light. Chem. Eur. J. 2009, 15, 10843–10850. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. Enhanced visible light driven photocatalytic application of Ag2O decorated ZnO nanorods heterostructures. Sep. Purif. Technol. 2017, 183, 341–349. [Google Scholar] [CrossRef]
- Gołąbiewska, A.; Zielińska-Jurek, A.; Zaleska, A. Characterization of TiO2 modified with bimetallic Ag/Au nanoparticles obtained in microemulsion system. J. Adv. Oxid. Technol. 2012, 15, 71–77. [Google Scholar]
- Liu, H.; Liang, Y.; Hu, H.; Wang, M. Hydrothermal synthesis of mesostructured nanocrystalline TiO 2 in an ionic liquid–water mixture and its photocatalytic performance. Solid State Sci. 2009, 11, 1655–1660. [Google Scholar] [CrossRef]
- Hardacre, C.; Holbrey, J.D.; McMath, S.E.J.; Bowron, D.T.; Soper, A.K. Structure of molten 1,3-dimethylimidazolium chloride using neutron diffraction. J. Chem. Phys. 2003, 118, 273–278. [Google Scholar] [CrossRef]
- Łuczak, J.; Paszkiewicz, M.; Krukowska, A.; Malankowska, A.; Zaleska-Medynska, A. Ionic liquids for nano- and microstructures preparation. Part 1 Properties and multifunctional role. Adv. Colloid Interface Sci. 2016, 230, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, J.; Paszkiewicz, M.; Krukowska, A.; Malankowska, A.; Zaleska-Medynska, A. Ionic liquids for nano- and microstructures preparation. Part 2: Application in synthesis. Adv. Colloid Interface Sci. 2016, 227, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, M.; Łuczak, J.; Lisowski, W.; Patyk, P.; Zaleska-Medynska, A. The ILs-assisted solvothermal synthesis of TiO2 spheres: The effect of ionic liquids on morphology and photoactivity of TiO2. Appl. Catal. B Environ. 2016, 184, 223–237. [Google Scholar] [CrossRef]
- Gołąbiewska, A.; Checa-Suárez, M.; Paszkiewicz-Gawron, M.; Lisowski, W.; Raczuk, E.; Klimczuk, T.; Polkowska, Ż.; Grabowska, E.; Zaleska-Medynska, A.; Łuczak, J. Highly Active TiO2 Microspheres Formation in the Presence of Ethylammonium Nitrate Ionic Liquid. Catalysts 2018, 8, 279. [Google Scholar] [CrossRef]
- Ramanathan, R.; Bansal, V. Ionic liquid mediated synthesis of nitrogen, carbon and fluorine-codoped rutile TiO2 nanorods for improved UV and visible light photocatalysis. RSC Adv. 2015, 5, 1424–1429. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Liu, S.; Jaroniec, M. Ionic-Liquid-Assisted Synthesis of Uniform Fluorinated B/C-Codoped TiO2 Nanocrystals and Their Enhanced Visible-Light Photocatalytic Activity. Chem. Eur. J. 2013, 19, 2433–2441. [Google Scholar] [CrossRef]
- Łuczak, J.; Paszkiewicz-Gawron, M.; Długokęcka, M.; Lisowski, W.; Grabowska, E.; Makurat, S.; Rak, J.; Zaleska-Medynska, A. Visible light photocatalytic activity of ionic liquid-TiO2 spheres: Effect of the ionic liquid’s anion structure. ChemCatChem 2017, 9, 4377–4388. [Google Scholar] [CrossRef]
- Qi, L.; Yu, J.; Jaroniec, M. Enhanced and suppressed effects of ionic liquid on the photocatalytic activity of TiO2. Adsorption 2013, 19, 557–561. [Google Scholar] [CrossRef]
- Paszkiewicz-Gawron, M.; Długokȩcka, M.; Lisowski, W.; Paganini, M.C.; Giamello, E.; Klimczuk, T.; Paszkiewicz, M.; Grabowska, E.; Zaleska-Medynska, A.; Łuczak, J. Dependence between Ionic Liquid Structure and Mechanism of Visible-Light-Induced Activity of TiO2 Obtained by Ionic-Liquid-Assisted Solvothermal Synthesis. ACS Sustain. Chem. Eng. 2018, 6, 3927–3937. [Google Scholar] [CrossRef]
- Ratke, L.; Voorhees, P.V. Growth and Coarsening: Ostwald Ripening in Material Processing; Springer Science & Business Media: Berlin/Heilderberg, Germany; New York, NY, USA, 2002. [Google Scholar]
- Gołąbiewska, A.; Paszkiewicz-Gawron, M.; Sadzińska, A.; Lisowski, W.; Grabowska, E.; Zaleska-Medynska, A.; Łuczak, J. Fabrication and photoactivity of ionic liquid–TiO2 structures for efficient visible-light-induced photocatalyticdecomposition of organic pollutants in aqueous phase. Beilstein J. Nanotechnol. 2018, 9, 580–590. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Kim, S.; Ko, K.C.; Lee, J.Y.; Illas, F. Single oxygen vacancies of (TiO2)35 as a prototype reduced nanoparticle: Implication for photocatalytic activity. Phys. Chem. Chem. Phys. 2016, 18, 23755–23762. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab Initio Study of Solvated Molecules: A New Implementation of the Polarizable Continuum Model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Reszczyńska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Visible light activity of rare earth metal doped (Er3+, Yb3+ or Er3+/Yb3+) titania photocatalysts. Appl. Catal. B Environ. 2015, 163, 40–49. [Google Scholar] [CrossRef]
- Reszczyńska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Lanthanide co-doped TiO2: The effect of metal type and amount on surface properties and photocatalytic activity. Appl. Surf. Sci. 2014, 307, 333–345. [Google Scholar] [CrossRef]
- Rybińska-Fryca, A.; Mikołajczyk, A.; Łuczak, J.; Paszkiewicz-Gawron, M.; Paszkiewicz, M.; Zaleska-Medynska, A.; Puzyn, T. How thermal stability of ionic liquids lead to more efficient TiO2-based nanophotocatalysts: Theoretical and experimental studies. J. Colloid Interface Sci. 2020, in press. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Jacquemin, D.; Wathelet, V.; Perpète, E.A.; Adamo, C. Extensive TD-DFT Benchmark: Singlet-Excited States of Organic Molecules. J. Chem. Theory Comput. 2009, 5, 2420–2435. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, ver. D.01.; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]


| Sample | Ti 2p3/2 Fraction (%) | ||
|---|---|---|---|
| ∑ Ti (at.%) | Ti4+ 458.7 ± 0.3 eV (%) | Ti3+ 457 ± 0.3 eV (%) | |
| TiO2 | 29.44 | 97.59 | 2.41 |
| TiO2_[BPy][Cl] | 24.58 | 92.64 | 7.36 |
| TiO2_[BPy][Br] | 25.67 | 95.86 | 4.14 |
| TiO2_[BPy][I] | 24.79 | 95.92 | 4.08 |
| Sample | Phenol Degradation Reaction Rate under UV-Vis Irradiation λ > 420 nm (µmol·dm−3·min−1) |
|---|---|
| TiO2 | 0.22 |
| TiO2_[BPy][Cl] | 0.87 |
| TiO2_[BPy][I] | 0.96 |
| TiO2_[BPy][Br] | 1.19 |
| TiO2_[BPy][Cl] | TiO2_[BPy][Br] | TiO2_[BPy][I] | |||
|---|---|---|---|---|---|
| λ * (nm) | AQE (%) | λ (nm) | AQE (%) | λ (nm) | AQE (%) |
| 370 | 6.43 | 420 | 5.29 | 550 | 0.87 |
| 403 | 4.88 | 448 | 3.16 | 582 | 1.31 |
| 430 | 4.22 | 480 | 3.07 | 610 | 0.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszkiewicz-Gawron, M.; Makurat, S.; Rak, J.; Zdrowowicz, M.; Lisowski, W.; Zaleska-Medynska, A.; Kowalska, E.; Mazierski, P.; Łuczak, J. Theoretical and Experimental Studies on the Visible Light Activity of TiO2 Modified with Halide-Based Ionic Liquids. Catalysts 2020, 10, 371. https://doi.org/10.3390/catal10040371
Paszkiewicz-Gawron M, Makurat S, Rak J, Zdrowowicz M, Lisowski W, Zaleska-Medynska A, Kowalska E, Mazierski P, Łuczak J. Theoretical and Experimental Studies on the Visible Light Activity of TiO2 Modified with Halide-Based Ionic Liquids. Catalysts. 2020; 10(4):371. https://doi.org/10.3390/catal10040371
Chicago/Turabian StylePaszkiewicz-Gawron, Marta, Samanta Makurat, Janusz Rak, Magdalena Zdrowowicz, Wojciech Lisowski, Adriana Zaleska-Medynska, Ewa Kowalska, Paweł Mazierski, and Justyna Łuczak. 2020. "Theoretical and Experimental Studies on the Visible Light Activity of TiO2 Modified with Halide-Based Ionic Liquids" Catalysts 10, no. 4: 371. https://doi.org/10.3390/catal10040371
APA StylePaszkiewicz-Gawron, M., Makurat, S., Rak, J., Zdrowowicz, M., Lisowski, W., Zaleska-Medynska, A., Kowalska, E., Mazierski, P., & Łuczak, J. (2020). Theoretical and Experimental Studies on the Visible Light Activity of TiO2 Modified with Halide-Based Ionic Liquids. Catalysts, 10(4), 371. https://doi.org/10.3390/catal10040371












