Abstract
Heavy fuel oils contain a high amount of sulfur. In this work, an extent amount of sulfur content waste tire pyrolysis oil (WTPO) was used as a fuel feedstock. A promising alternative oxidative desulfurization (ODS) method was applied in sulfur removal from WTPO using a S-ZrO2/SBA-15 solid acid catalyst, hydrogen peroxide (H2O2) as an oxidant and acetonitrile as an extracting solvent at varied conditions. The prepared catalyst was characterized by X-ray diffraction (XRD), Bruanuer-Emmet-Teller (BET) method and Fourier transform infrared spectroscopy (FTIR) analysis. The influence of reaction parameters such as reaction time (30-60 min), catalyst loading (0.5–1.5 wt.%), oxidant to oil mole ratio (5–15) at fixed reaction temperature 70 °C on desulfurization of WTPO were investigated. Taguchi method was selected to design the experiment for optimizing the reaction parameters by maximizing the sulfur removal efficiency. The maximum desulfurization efficiency 59.49% was obtained under optimum conditions reaction time (60 min), catalyst loading (1.0 wt.%) and oxidant to sulfur mole ratio (10:1). A catalytic S-ZrO2/SBA-15 -H2O2 oxidation system for oxidative desulfurization of waste tire pyrolysis oil using at mild reaction conditions was developed.
1. Introduction
The energy crisis and environmental pollution are the two main problems in the world today due to the increasing human population and rapid industrialization. To meet the fuel energy demand, many initiatives are being taken to find alternative fuel oils due to the depletion of fossil fuels. Fuel oil derived from waste tire pyrolysis is becoming a promising alternative energy source because of its higher heating value and availability [1,2].
A large amount of waste tires is produced every day due to the high demand for personal and commercial transportation. There are several ways to manage the disposal of waste tires, such as landfill deposition and incineration. However, these techniques have limitations due to the availability of land space, possibility of fire at the waste tire storage areas. In addition, some toxic gases and black smog are produced during the incineration of waste tires, which can cause air pollution [3,4].
Waste tires are made of natural rubber, synthetic rubber, fabric, and carbon including other chemicals. Another important component of waste tire is sulfur, which acts as a cross-linking agent. Waste tires have a higher heating value compared to waste biomass. Therefore, waste tires have huge potential for use as an alternative energy source to conventional fuel oils. Usually, waste tire oil is produced using a pyrolysis process. However, the main disadvantage of waste tire pyrolysis oil is its high sulfur content (1–1.4 wt.%) [3,4,5,6].
Sulfur is one of the major sources of air pollution, which threatens human health and the surrounding environment. Therefore, it is important to reduce the amount of sulfur in waste tire oil to make it a green fuel oil and to meet the standard for sulfur content (10–15 ppm) set by the United States Environmental Protection Agency (USEPA) [7,8,9,10].
Commercially, hydrodesulfurization (HDS) is used in the oil refinery industry to remove sulfur compounds from liquid fuel oils with a high removal efficiency in the presence of valuable metal catalysts. However, the main drawback of this method is that it needs high temperature (~400 °C) and high pressure (~100 atm), which increase the operational cost. In recent years, the catalytic oxidative desulfurization (ODS) process has drawn attraction due to its milder reaction conditions (low temperature and pressure [11,12,13,14,15,16].
The catalytic oxidative desulfurization (ODS) process consists of two steps. In the first step, sulfur containing fuel oil is subjected to catalytic oxidation to produce oxidized sulfur compounds (sulfones/sulfoxides) in the presence of an oxidant. In the second step, the oxidized sulfur compounds are removed from the reaction mixture using an adsorption or liquid/liquid extraction method (Figure 1). Highly polar solvents such as DMF, NMP, DMSO, and acetonitrile are used to extract the sulfur compounds [16,17,18,19].
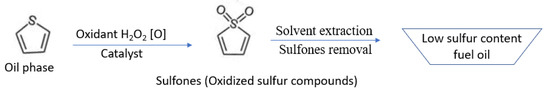
Figure 1.
Mechanism of catalytic oxidative desulfurization of fuel oil.
Catalysts play an important and effective role in the ODS process because the catalysts activate the activity of the oxidizing agent. Many catalytic-oxidation systems have been applied in the oxidation of sulfur compounds such as acetic acid/formic acid catalytic/oxidation [20,21], heteropoyacid (HPA) catalytic/oxidation [22,23], ionic liquid catalytic/oxidation [24,25], polyoxometalate catalytic/oxidation, silica and molecular sieve catalytic-oxidation [26,27] and ultrasound assisted/ oxidation [28,29]. Among them, transition-metal supported composite catalytic oxidation systems are getting more attention because of their excellent catalytic performance. The acidity of the support such as silica, alumina, silica-alumina and magnesia-alumina and the dispersion of the active sites of the catalyst show high activity on the oxidation of sulfur containing fuel oil.
In the present work, sulfate zirconium oxide (SO42−/ZrO2) supported by high surface area silica (SBA-15), S-ZrO2/SBA-15, superacid catalyst was successfully prepared using a direct wet impregnation method in acidic media and used for desulfurization of waste tire pyrolysis oil (WTPO). It is expected that when an oxidant such as H2O2 encounter the acidic sites on sulfated zirconia catalyst, the thermodynamically strong oxidant can be formed, which can oxidize sulfur compounds. The large surface area of S-ZrO2/SBA-15 super acid catalyst can enhance the contact between the sulfur compounds and H2O2 oxidant. The Taguchi method was selected as the design of the experiment (DOE) to minimize the excess experiments cost and optimize the reaction parameters. The effect of solvent to oil ratio and stirring speed on the sulfur removal of WTPO were studied. In addition, the reusability and stability of the catalyst also was investigated under mild reaction conditions.
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. X-ray Diffraction
XRD was recorded to observe the mesoporous structure of SBA-15 and crystalline ZrO2. The results depicted in Figure 2 show that there are three intense peaks observed at 30°, 50°, and 60°, and two small peaks at 35° and 62° for SO42−/ZrO2 (S-ZrO2) calcined at 540 °C. These diffraction peaks prove the presence of a bulk ZrO2 crystalline phase and the absence of other impurities. They also show that the introduction of sulfate group can stabilize the crystalline phase of ZrO2, which is a prerequisite for sulphated zirconia catalytic activity [30]. The XRD pattern of S-ZrO2/SBA-15 also shows similar diffraction peaks characteristic of ZrO2. The XRD pattern of S-ZrO2/SBA-15 also showed one broad intense peak at 20–30°, which is characteristic for the hexagonal structure of SBA-15 support [31]. From these findings, it can be concluded that the S–ZrO2/SBA-15 solid acid catalyst retains the structure of the SBA-15 support even after it was subjected to the wet impregnation process with ZrO2, the introduction of sulfuric acid, and calcination at high temperature.
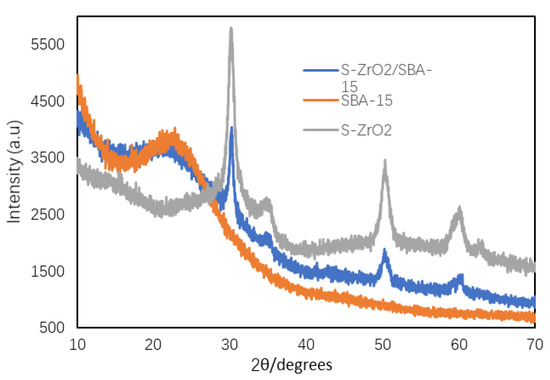
Figure 2.
XRD pattern recorded for the S-ZrO2/SBA-15 catalyst.
2.1.2. FTIR Spectroscopy
Figure 3 shows the FTIR spectra recorded for the sulfated zirconia SO42−/ZrO2 (S-ZrO2) and S-ZrO2/SBA-15 catalysts. The band observed at ~1400 cm−1 is attributed to the covalent S=O bond, which is considered as the characteristic band for SO42− for S-ZrO2 and indicate the strength of its superacidity. This band shifts to ~1530 cm−1 in the S-ZrO2/SBA-15 catalyst. The band shift can be attributed to the interaction between ZrO2 and SBA-15 [32]. All the bands observed between 700 and 900 cm−1 correspond to the Zr-O-Si bond. This indicates the successful impregnation of zirconia into SBA-15 [33].
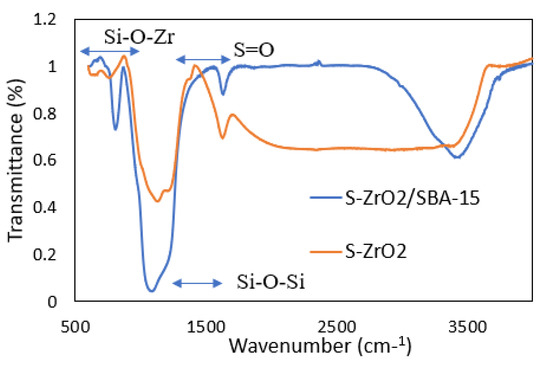
Figure 3.
FTIR spectra recorded for S-ZrO2 and S-ZrO2/SBA-15.
2.1.3. Surface Analysis of the Catalysts
The N2 adsorption-desorption isotherms and pore size distribution of the SBA-15 and S-ZrO2/SBA-15 catalysts are displayed in Figure 4A, B respectively, and their surface data (surface area, pore volume, and pore size) are presented in Table 1. Figure 4 shows both isotherms were typical IV isotherms with a H1 hysteresis loop, as defined by IUPAC. The hysteresis loop indicates the uniform mesoporous structure of SBA-15 with large dimensions [34,35]. Table 1 shows that the surface area of the SBA-15 support (805.48 m2/g) is decreased to 573.11 m2/g after the incorporation of ZrO2. Meanwhile, the pore volume and pore diameter also decreased. These results confirm that zirconia was dispersed on the surface of the SBA-15 support.
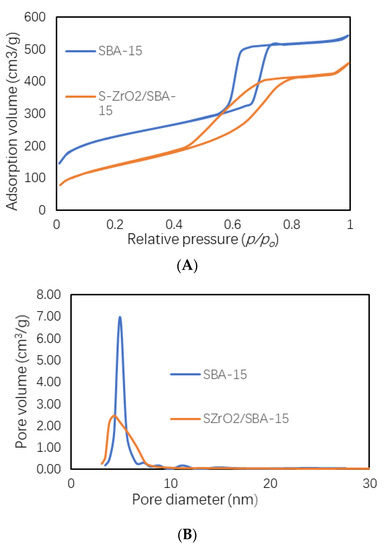
Figure 4.
(A) N2 adsorption-desorption isotherm and (B) Pore size distribution of SBA-15 and S-ZrO2/SBA-15.

Table 1.
The physiochemical properties of SBA-15 and S-ZrO2/SBA-15.
2.2. Optimization of the Reaction Parameters
The experimental data was evaluated using the signal to noise ratio (S/N) (Figure 5) according to the Taguchi (L9) orthogonal experimental design method (Table 2. S/N is the ratio between the desired value of the response variable and undesired value of the response variable. The largest S/N ratio was selected in the optimization of process parameters (reaction time, catalyst and molar ration of oxidant (H2O2) to sulfur) to maximize the sulfur removal efficiency. The S/N ratio was calculated using Equation (1).
where n is the number of observations and y is the observed value.
S/N = 10log 1/n (Σ 1/y2)
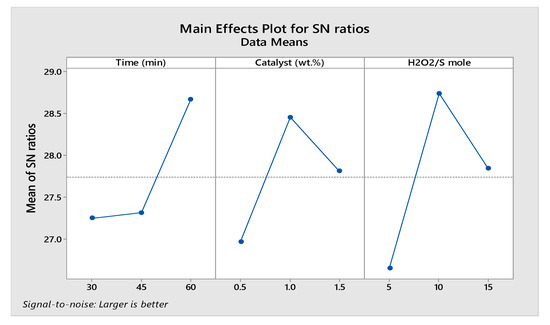
Figure 5.
The main effects plot for the S/N ratios.

Table 2.
The orthogonal array generated using the Taguchi method to optimize the reaction conditions for upgrading WTPO.
The S/N ratio and main effects plots for the S/N ratio were measured using Minitab 18 software.
The maximum value for the S/N ratio indicates the optimum for the reaction parameter. Figure 5 shows that the maximum sulfur removal efficiency can be achieved using an optimum reaction time of 60 min, 1 wt.% catalyst loading, an oxidant to sulfur (H2O2/S) molar ratio of 10:1.
2.3. Statistical Analysis
In the present study, analysis of variance (ANOVA) was performed to identify which parameter in time, catalyst loading, and H2O2/S mole ratio significantly affected desulfurization efficiency (Table 3). ANOVA was performed using Minitab 18 statistical software. F-value was used to identify the influence of process parameters [36,37]. Contribution of percentage was also calculated using contribution factor formula. The contribution factor also indicates the significant parameters, but it can be reconfirmed by F-value [38]. From the Table 3, oxidant to sulfur mole ratio (H2O2/S) was the most significant parameter with higher F-value (1.27). The other factors, Time (min) and catalyst loading, followed moderately.

Table 3.
The response table for the S/N ratios.
2.4. The Effect of the Extracting Solvent to Oil Ratio on the Desulfurization Reaction
The concentration of sulfur decreased upon increasing the solvent to oil ratio (v/v). The amount of sulfur initially decreased rapidly and then slowed down gradually. When the amount of solvent was increased, the miscibility between solvent and oxidized sulfur compounds (sulfoxides/sulfones) increased, which increased the rate of desulfurization. Figure 6 shows that the highest desulfurization efficiency was achieved at a solvent to oil ratio of 4. Therefore, a solvent to oil ratio of 4 was selected in our further experiments.
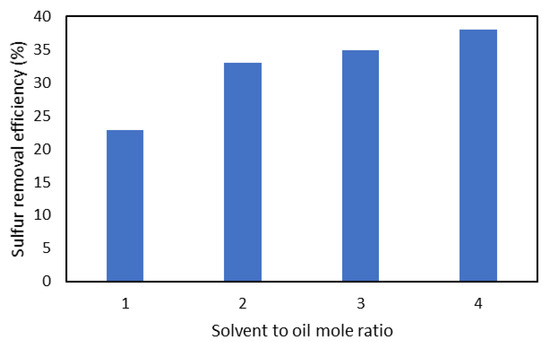
Figure 6.
The effect of the molar ratio of solvent to oil (v/v) on the desulfurization of WTPO.
2.5. The Effect of Stirring Speed on the Desulfurization Reaction
The effect of stirring rate on desulfurization of WTPO was also investigated because the sulfur removal efficiency is highly dependent on the contact between the raw oil and catalyst. The sulfur removal efficiency may be high due to efficient mixing between the oil and catalyst [39,40]. Figure 7 shows that the desulfurization efficiency increased upon increasing the stirring rate. When the stirring rate was increased up to 400 rpm, the reaction mixture (oil, catalyst, and oxidant) was mixed uniformly and the sulfur removal efficiency was increased. There was a slight change in the desulfurization efficiency when stirring speed was further increased to 450 rpm. Therefore, 400 rpm was selected as the optimum stirring rate for our further experiments.
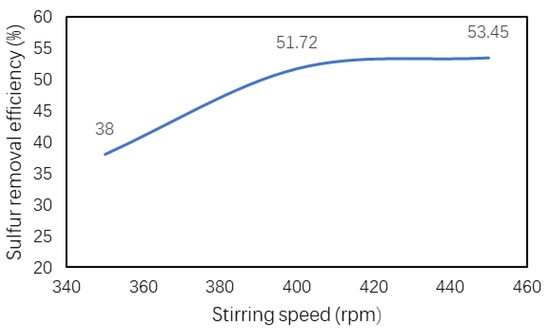
Figure 7.
The effect of the stirring rate (rpm) on the desulfurization of waste tire pyrolysis oil (WTPO).
2.6. A Comparison of Different Catalysts on the Desulfurization of Waste Tire Oil
The effects of different types of catalysts on the desulfurization of waste tire oil via oxidative desulfurization were studied. The catalytic activity of ZrO2, S-ZrO2, and S-ZrO2/SBA-15 were evaluated under the optimum conditions. Figure 8 shows that the catalytic performances of the different catalysts were in the following order: S-ZrO2/SBA-15 > SO42−/ZrO2 > ZrO2. The sulfur removal efficiency of S-ZrO2/SBA-15 was high when compared to the other bulk catalysts (ZrO2 and SO42−/ZrO2).
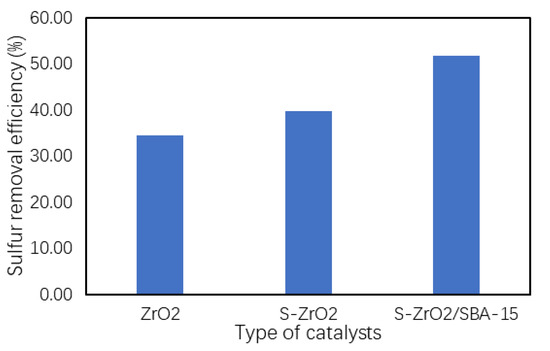
Figure 8.
The effect of different type of catalyst on the desulfurization of waste tire oil.
2.7. Reusability of the Catalyst
The reusability of synthesized heterogeneous acid catalyst was studied to investigate its stability. After each catalytic run, the catalyst was separated using filtration, washed with acetonitrile, dried at 80 °C for 1–2 h, and regenerated upon calcination at 540 °C for 6 h. The regenerated catalyst was used again in the next run under the same reaction conditions. The results obtained for the ODS reactions after recycling the catalyst several times are presented in Figure 9. They show that the sulfur removal efficiency was comparable to the fresh catalyst after three cycles. This was attributed to the high stability of the S-ZrO2/SBA-15 catalyst during the oxidative desulfurization process [41]. To observe the structural stability of the catalyst after several cycles, the XRD patterns of regenerated catalysts were recorded. The results are depicted in Figure 9.
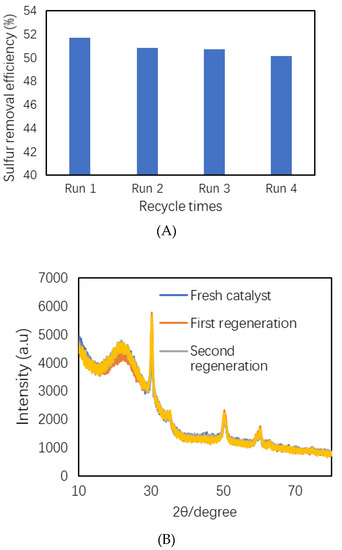
Figure 9.
(A) Recycling the S-ZrO2/SBA-15 catalyst in the ODS of WTPO. (B) A comparison of the XRD patterns recorded for the S-ZrO2/SBA-15 catalyst after three cycles.
2.8. The Effect of the Number of Extraction Steps on the Desulfurization Reaction
The number of extraction steps can play a significant role in the removal of the oxidized sulfur compounds during the extraction of waste tire oil. It is expected that the desulfurization efficiency will be increased upon increasing the number of extraction steps in order to improve the extraction efficiency of the oxidized sulfur compounds [42]. In this study, the extraction of the oxidized sulfur compounds was investigated using acetonitrile as the extracting solvent. Figure 10 shows that the sulfur removal efficiency was increased upon increasing the number of extraction steps from 1 to 3. Therefore, multiple extraction steps were very effective to reduce the sulfur content in WPTO.
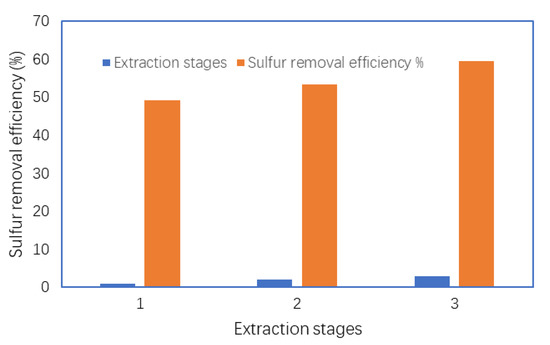
Figure 10.
The effect of multiple extraction steps on the desulfurization reaction.
2.9. FTIR Analysis
The FTIR spectra of WTPO before and after the desulfurization process are presented in Figure 11. The peaks observed in the region between 1035 to 1328 cm−1 correspond to the sulfoxide and sulfone derivatives, respectively [39,43,44]. Figure 11 shows that after the desulfurization process the peaks corresponding to the sulfur compounds were decreased, which confirms that they were partially removed after oxidation followed by extraction with acetonitrile.
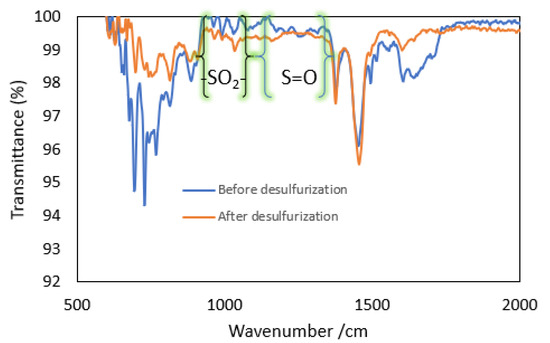
Figure 11.
FTIR spectra of waste tire pyrolysis oil before and after oxidation-extraction.
3. Materials and Methods
3.1. Collection of Crude WTPO
WTPO was produced and collected from the CECP laboratory, Department of Environmental Engineering, Yonsei University, Korea. Passenger side car tires were used as raw materials and were obtained from the waste tire disposal facility in Busan, Korea. The pyrolysis reaction was carried out in a conical spouted bed reactor. The characteristics of crude WTPO are presented in Table 4.

Table 4.
The characteristics of crude WTPO.
3.2. Materials and Feedstock
Zirconium hydroxide and Pluronic P123 were obtained from Sigma-Aldrich Co., (St. Louis, MO, USA). Hydrochloric acid (HCl) and sulfuric acid were purchased from Dae-Jung Chemical & Metals Co., Ltd., Gyeonggi-do, Korea. Tetraethyl orthosilicate (C8H20O4Si, 98%) was purchased from ACROS Organics Co., (Morris, NJ, USA). These chemicals were reagent grade. Hydrogen peroxide and acetonitrile were purchased from Daejung Chemical & Metals Co., Ltd., Gyeonggi-do, Korea.
3.3. Catalyst Preparation
Sulfated zirconium oxide supported by SBA-15 (S-ZrO2/SBA-15) was synthesized using a direct wet impregnation method using SBA-15 and the desired amount of zirconium hydroxide, according to the following method with some modification [31]. Sulphated Zr (OH)4 and Zr (OH)4/SBA-15 were filtered, dried at 80 °C for 2 h, and calcined at 540 °C for 6 h to give SO42−/ZrO2 (S-ZrO2) and S-ZrO2/SBA-15.
3.4. Catalytic ODS of WTPO
3.4.1. ODS of WTPO
All ODS reactions were carried out in a 350 mL stainless steel batch reactor equipped with a magnetic stirrer and condenser (Joyoung al-tex Co. Ltd. Model TPS20-G2, Daejon, Korea) Before running the reaction, nitrogen gas was purged due to provide an inert atmosphere inside the reaction vessel and reaction medium. In each run, 50 mL of WTPO was charged into the reaction vessel. Subsequently, a certain amount of catalyst and oxidant were added to the reaction vessel. The reaction mixture was heated to the target temperature (70 °C) and then was stirred at 400 rpm under atmospheric pressure.
3.4.2. Extraction of Desulfurized WTPO
After completing the reaction, the catalyst was recovered via filtration and then the extracting solvent was used to extract the oxidized sulfur compounds. Acetonitrile was used as the extracting solvent at a solvent /oil ratio of 2:1 in the decanter. Then, the decanter was shaken for 30 min and kept overnight for phase separation (oil-solvent phase). Two layers were formed (solvent and oil layer). Finally, the extracted oil phase was collected, and the sulfur content determined by elemental analysis (ASTM D3176). The sulfur removal efficiency was calculated using Equation (2) [7,17]
where S0 and St represent the initial and final concentration of sulfur after the ODS reaction, respectively.
Sulfur removal efficiency (%) = [1 − S0/St]
3.5. Experimental Design for the ODS of WTPO
The Taguchi method was selected to design the experiments used to optimize the sulfur removal efficiency of the ODS reaction of WTPO. The Taguchi method is a very fruitful DOE approach, which not only determines the impact of the reaction parameters, but also reduce the production cost and time [32,45]. The effect of the oxidant, catalyst loading, and reaction time on the desulfurization reaction were investigated. The experimental range and reaction parameters are presented in Table 5.

Table 5.
The design of the optimization experiments.
Using statistical software (Minitab 18), an orthogonal array was formed based on the Taguchi method to generate the number of reactions to be carried out to investigate the effect of the reaction parameters with different levels.
4. Conclusions
Solid superacid catalyst S-ZrO2/SBA-15 has been successfully prepared using a direct wet impregnation method. The analytical results obtained from XRD, BET, and FTIR confirm the structure and properties of the as-synthesized catalyst. S-ZrO2/SBA-15 was evaluated in the removal of sulfur compounds from waste tire oil by developing a moderate oxidation desulfurization process. The experiment was designed using the Taguchi method to save additional experimental cost and all reactions were run to maximize the sulfur removal efficiency using H2O2 as the oxidant, S-ZrO2/SBA-15 as the catalyst, and acetonitrile as the extracting solvent. The signal to noise ratio was selected to optimize the reaction parameters. The maximum desulfurization efficiency (59.13%) was obtained under the optimum reaction conditions (reaction time, 60 min; catalyst loading, 1.0 wt.%; H2O2 molar ratio of H2O2, 10:1; and extracting solvent acetonitrile to oil ratio, 4:1 (v/v) at fixed reaction temperature 70 °C). Multi-step extraction was effective in reducing the amount of sulfur in the fuel oil feedstock. The catalyst was successfully recycled three times, which indicates its high catalytic stability. The stability of catalyst was further confirmed using XRD after its reuse. The results indicate that S-ZrO2/SBA-15-H2O2 catalytic oxidation system has potential in the purifying process of WTPO by reducing the sulfur content under mild reaction conditions.
Author Contributions
Conceptualization, H.S.C. & M.N.H.; Methodology, M.N.H.; Software, M.N.H.; Formal Analysis, M.N.H.; Data curation, M.N.H. & M.K.C.; Writing-Original draft preparation, M.N.H.; Writing-Review & Editing, H.S.C.; Visualization, M.K.C. & H.C.P.; Supervision, H.S.C.; Funding acquisition, H.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT, and Future Planning (MSIP) of Korea (NRF-2017R1A2B4009340). This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20184030202240).
Acknowledgments
We thank Ministry of Science, ICT, and Future Planning (MSIP) of Korea and the Korea Institute of Energy Technology Evaluation and Planning (KETEP) of the Republic of Korea for their financial support.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mia, M.; Islam, A.; Rubel, R.I.; Islam, M.R. Fractional Distillation & Characterization of Tire Derived Pyrolysis Oil. Int. J. Eng. Technol. IJET 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Al-Marshed, A.; Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Optimization of Heavy Oil Upgrading Using Dispersed Nanoparticulate Iron Oxide as a Catalyst. Energy Fuels 2015, 29, 6306–6316. [Google Scholar] [CrossRef]
- Trongkaew, P.; Utistham, T.; Reubroycharoen, P.; Hinchiranan, N. Photocatalytic Desulfurization of Waste Tire Pyrolysis Oil. Energies 2011, 4, 1880–1896. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, M.I.; Naeem, K.; Humayun, M.; Faheem, F. Oxidative desulfurization of tire pyrolysis oil. Chem. Ind. Chem. Eng. Q. 2016, 22, 249–254. [Google Scholar] [CrossRef]
- Chang, Y.-M. On pyrolysis of waste tire: Degradation rate and product yields. Resour. Conserv. Recycl. 1996, 17, 125–139. [Google Scholar] [CrossRef]
- Cao, Q.; Jin, L.; Bao, W.; Lv, Y. Investigations into the characteristics of oils produced from co-pyrolysis of biomass and tire. Fuel Process. Technol. 2009, 90, 337–342. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Shaterian, M.; Aghbolagh, Z.S.; Babaei, R. Oxidative desulfurization of gasoline catalyzed by IMID@PMA@CS nanocomposite as a high-performance amphiphilic nanocatalyst. Environ. Prog. Sustain. Energy 2018, 37, 1891–1900. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Julião, D.; Cunha-Silva, L.; Domingues, V.F.; Valença, R.; Ribeiro, J.C.; de Castro, B.; Balula, S.S. Catalytic oxidative/extractive desulfurization of model and untreated diesel using hybrid based zinc-substituted polyoxometalates. Fuel 2016, 166, 268–275. [Google Scholar] [CrossRef]
- González-García, O.; Cedeño-Caero, L. V-Mo based catalysts for oxidative desulfurization of diesel fuel. Catal. Today 2009, 148, 42–48. [Google Scholar] [CrossRef]
- Hossain, M.N.; Park, H.C.; Choi, H.S. A Comprehensive Review on Catalytic Oxidative Desulfurization of Liquid Fuel Oil. Catalysts 2019, 9, 229. [Google Scholar] [CrossRef]
- Ismagilov, Z.; Yashnik, S.; Kerzhentsev, M.; Parmon, V.; Bourane, A.; Al-Shahrani, F.M.; Hajji, A.A.; Koseoglu, O. Oxidative Desulfurization of Hydrocarbon Fuels. Catal. Rev. 2011, 53, 199–255. [Google Scholar] [CrossRef]
- Choi, A.E.; Roces, S.; Dugos, N.P.; Wan, M.-W. Oxidation by H2O2 of bezothiophene and dibenzothiophene over different polyoxometalate catalysts in the frame of ultrasound and mixing assisted oxidative desulfurization. Fuel 2016, 180, 127–136. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, H.; Zhang, L.; Zhang, R.; Sun, Y.; Huang, Y. Efficient oxidative desulfurization of diesel fuel using amide-based ionic liquids. Chem. Eng. J. 2016, 283, 89–96. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Khandan, S.; Sabahi, N. Oxidative Desulfurization of Gas Oil Catalyzed by (TBA)4PW11Fe@PbO as an Efficient and Recoverable Heterogeneous Phase-Transfer Nanocatalyst. Energy Fuels 2017, 31, 5472–5481. [Google Scholar] [CrossRef]
- Dai, Y.; Qi, Y.; Zhao, D.; Zhang, H. An oxidative desulfurization method using ultrasound/Fenton’s reagent for obtaining low and/or ultra-low sulfur diesel fuel. Fuel Process. Technol. 2008, 89, 927–932. [Google Scholar] [CrossRef]
- Abdelrahman, A.A.; Betiha, M.; Rabie, A.M.; Ahmed, H.S.; El_Shahat, M. Removal of refractory Organo sulfur compounds using an efficient and recyclable {Mo132} nanoball supported graphene oxide. J. Mol. Liq. 2018, 252, 121–132. [Google Scholar] [CrossRef]
- Jalali, M.R.; Sobati, M.A. Intensification of oxidative desulfurization of gas oil by ultrasound irradiation: Optimization using Box–Behnken design (BBD). Appl. Therm. Eng. 2017, 111, 1158–1170. [Google Scholar] [CrossRef]
- Abu Bakar, W.; Ali, R.; Kadir, A.A.A.; Mokhtar, W.N.A.W. Effect of transition metal oxides catalysts on oxidative desulfurization of model diesel. Fuel Process. Technol. 2012, 101, 78–84. [Google Scholar] [CrossRef]
- Kadijani, J.A.; Narimani, E.; Kadijani, H.A. Oxidative desulfurization of organic sulfur compounds in the presence of molybdenum complex and acetone as catalysts. Pet. Coal 2014, 56, 116–123. [Google Scholar]
- Mamaghani, A.H.; Fatemi, S.; Asgari, M. Investigation of Influential Parameters in Deep Oxidative Desulfurization of Dibenzothiophene with Hydrogen Peroxide and Formic Acid. Int. J. Chem. Eng. 2013, 2013, 951045. [Google Scholar] [CrossRef]
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative Desulfurization of Light Gas Oil and Vacuum Gas Oil by Oxidation and Solvent Extraction. Energy Fuels 2000, 14, 1232–1239. [Google Scholar] [CrossRef]
- Li, S.W.; Gao, R.; Zhang, W.; Zhang, Y.; Zhao, J. Heteropolyacids supported on macroporous materials POM@MOF-199@LZSM-5: Highly catalytic performance in oxidative desulfurization of fuel oil with oxygen. Fuel 2018, 221, 1–11. [Google Scholar] [CrossRef]
- Rafiee, E.; Nobakht, N. Keggin type heteropolyacid, encapsulated in metal-organic framework: A heterogeneous and recyclable nanocatalyst for selective oxidation of sulfides and deep desulfurization of model fuels. J. Mol. Catal. A Chem. 2015, 398, 17–25. [Google Scholar] [CrossRef]
- Jiang, W.; Zhua, W.; Chang, Y.; Chao, Y.; Yin, S.; Liu, H.; Zhu, F.; Li, H. Ionic liquid extraction and catalytic oxidative desulfurization of fuels using dialkylpiperidinium tetrachloroferrates catalyst. Chem. Eng. J. 2014, 250, 48–54. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Sun, Y.; Jiang, B.; Yang, H. Deep oxidative desulfurization of fuels by superbase-derived Lewis acidic ionic liquids. Chem. Eng. J. 2017, 328, 445–453. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Naito, T.; Hirai, T. Vanadosilicate molecular sieve as a catalyst for oxidative desulfurization of light oil. Ind. Eng. Chem. Res. 2003, 42, 6034–6039. [Google Scholar] [CrossRef]
- Zhao, N.; Li, S.; Wang, J.; Zhang, R.; Gao, R.; Zhao, J.; Wang, J. Synthesis and application of different phthalocyanine molecular sieve catalyst for oxidative desulfurization. J. Solid-State Chem. 2015, 225, 347–353. [Google Scholar] [CrossRef]
- Gildo, P.J.; Dugos, N.; Wan, S.R.M. Optimized ultrasound-assisted oxidative desulfurization process of simulated fuels over activated carbon-supported phosphotungstic acid. MATEC Web Conf. 2018, 156, 03045. [Google Scholar] [CrossRef]
- Margeta, D.; Sertić-Bionda, K.; Foglar, L. Ultrasound assisted oxidative desulfurization of model diesel fuel. Appl. Acoust. 2016, 103, 202–206. [Google Scholar] [CrossRef]
- Xia, Q.-H.; Hidajat, K.; Kawi, S. Synthesis of SO42−/ZrO2/MCM-41 as a new superacid catalyst. Chem. Commun. 2000, 2229–2230. [Google Scholar] [CrossRef]
- Hossain, M.N.; Bhuyan, S.; Alam, A.H.M.A.; Seo, Y.C.; Bhuyan, S.U.S. Biodiesel from Hydrolyzed Waste Cooking Oil Using a S-ZrO2/SBA-15 Super Acid Catalyst under Sub-Critical Conditions. Energies 2018, 11, 299. [Google Scholar] [CrossRef]
- Zolfaghari, G.; Esmaili-Sari, A.; Anbia, M.; Younesi, H.; Amirmahmoodi, S.; Ghafarinazari, A. Taguchi optimization approach for Pb(II) and Hg(II) removal from aqueous solutions using modified mesoporous carbon. J. Hazard. Mater. 2011, 192, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Erdem, S.; Erdem, B.; Oksuzoglu, R.M.; Çıtak, A. Effect of calcination temperature on the structural and magnetic properties of Ni/SBA-15 nanocomposite. J. Porous Mater. 2015, 22, 689–698. [Google Scholar] [CrossRef]
- Yu, J.; Shen, A.; Cao, Y.; Lu, G. Preparation of Pd-Diimine@SBA-15 and Its Catalytic Performance for the Suzuki Coupling Reaction. Catalysts 2016, 6, 181. [Google Scholar] [CrossRef]
- Sanjini, N.S.; Velmathi, S. Iron impregnated SBA-15, a mild and efficient catalyst for the catalytic hydride transfer reduction of aromatic nitro compounds. RSC Adv. 2014, 4, 15381. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Shiao, Y.-F.; Chang, R.-F.; Yeh, Y.-T. Optimization of Microwave-Based Heating of Cellulosic Biomass Using Taguchi Method. Materials 2013, 6, 3404–3419. [Google Scholar] [CrossRef]
- Banisharif, F.; Dehghani, M.; Campos-Martin, J.M. Oxidative Desulfurization of Diesel Using Vanadium-Substituted Dawson-Type Emulsion Catalysts. Energy Fuels 2017, 31, 5419–5427. [Google Scholar] [CrossRef]
- Satya, S.; Kolakoti, A.; Rao, R. Optimization of palm methyl ester and its effect on fatty acid compositions and cetane number. Math. Models Eng. 2019, 5, 25–34. [Google Scholar] [CrossRef]
- Tang, X.-D.; Hu, T.; Li, J.-J.; Wang, F.; Qing, D.-Y. Deep desulfurization of condensate gasoline by electrochemical oxidation and solvent extraction. RSC Adv. 2015, 5, 53455–53461. [Google Scholar] [CrossRef]
- De Luna, M.D.G.; Wan, M.-W.; Golosinda, L.R.; Futalan, C.M.; Lu, M.-C. Kinetics of Mixing-Assisted Oxidative Desulfurization of Dibenzothiophene in Toluene Using a Phosphotungstic Acid/Hydrogen Peroxide System: Effects of Operating Conditions. Energy Fuels 2017, 31, 9923–9929. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Shaterian, M.; Akbarzadeh, F.; Khandan, S. Deep oxidative desulfurization of gasoline induced by PMoCu@MgCu2O4-PVA composite as a high-performance heterogeneous nanocatalyst. Chem. Eng. J. 2018, 333, 537–544. [Google Scholar] [CrossRef]
- Sobati, M.A.; Dehkordi, A.M.; Shahrokhi, M. Extraction of Oxidized Sulfur-Containing Compounds of Non-Hydrotreated Gas Oil. Chem. Eng. Technol. 2010, 33, 1515–1524. [Google Scholar] [CrossRef]
- Shakirullah, M.; Ahmad, W.; Ahmad, I.; Ishaq, M. Oxidative desulphurization study of gasoline and kerosene. Fuel Process. Technol. 2010, 91, 1736–1741. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, T.; Liu, X.; Jia, J. Rapid desulfurization of CWS via ultrasonic enhanced metal boron hydrides reduction under ambient conditions. RSC Adv. 2012, 2, 4189–4197. [Google Scholar] [CrossRef]
- Soltanieh, M.; Heidarinasab, A.; Ardjmand, M.; Ahmadpanahi, H.; Bahmani, M. Impact of support on the catalytic behavior of Mo/γ-Al2O3 and Mo/MgO catalysts on the desulfurization reaction. Appl. Catal. A Gen. 2016, 516, 41–50. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
