Synergistic Effect of Dielectric Barrier Discharge Plasma and TiO2-Pillared Montmorillonite on the Degradation of Rhodamine B in an Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
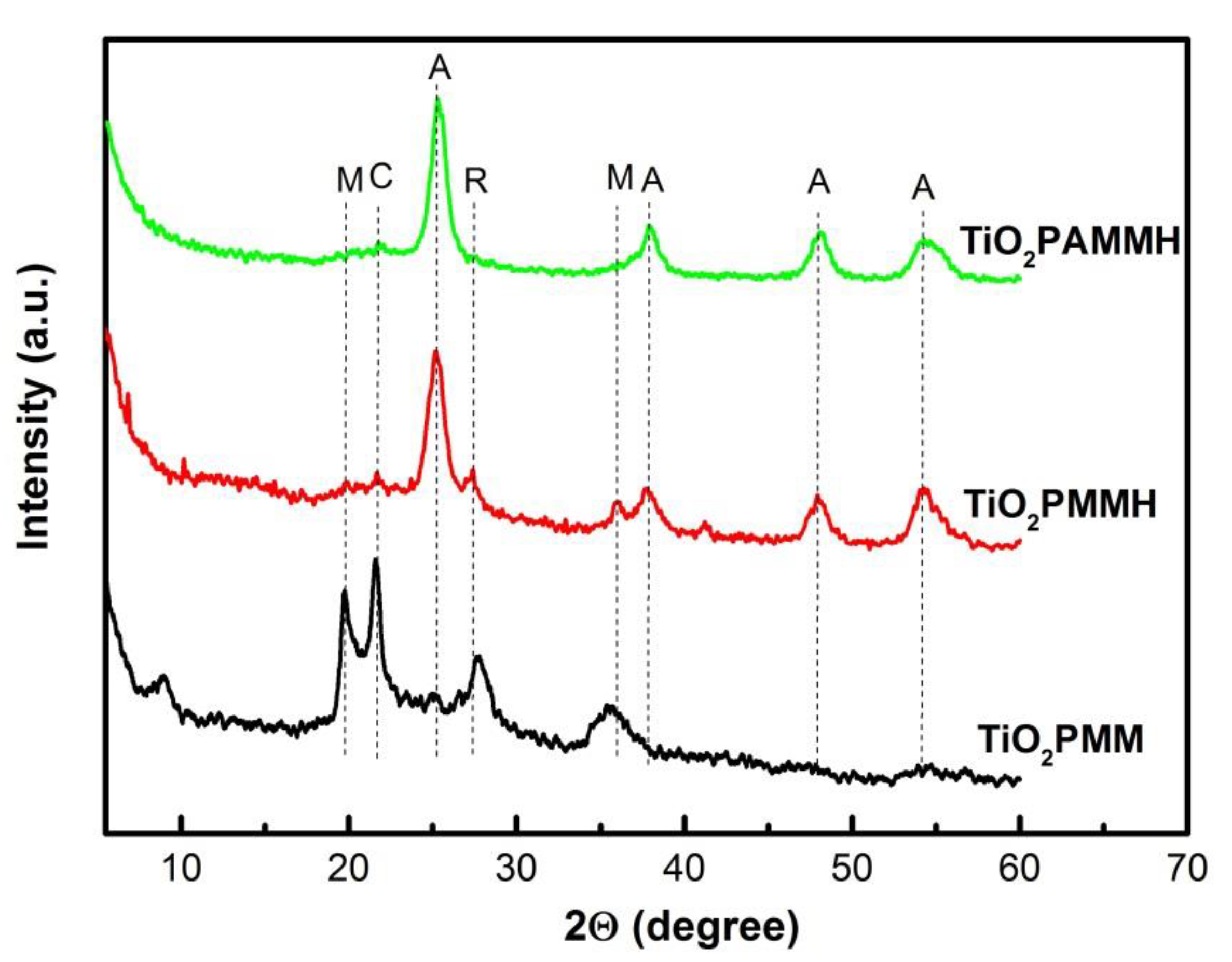
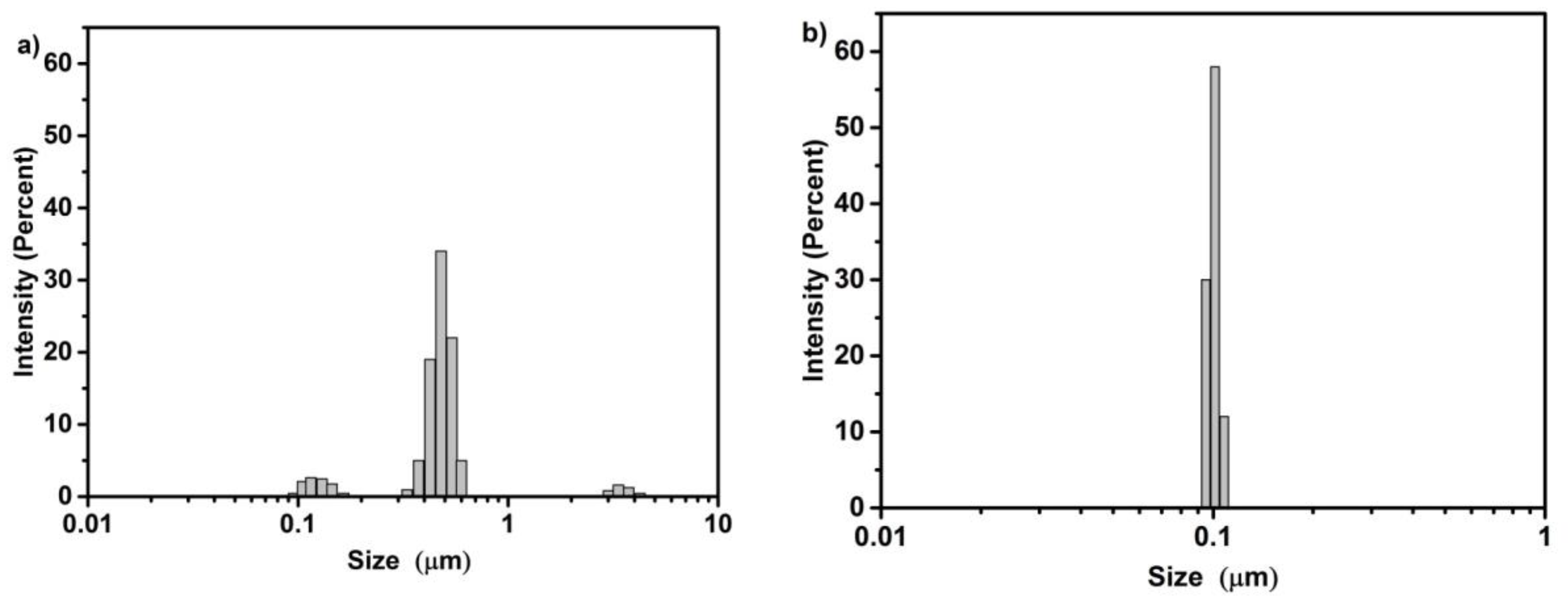
2.1. X-ray Diffraction, Textural Characterization and Particle Size Distribution
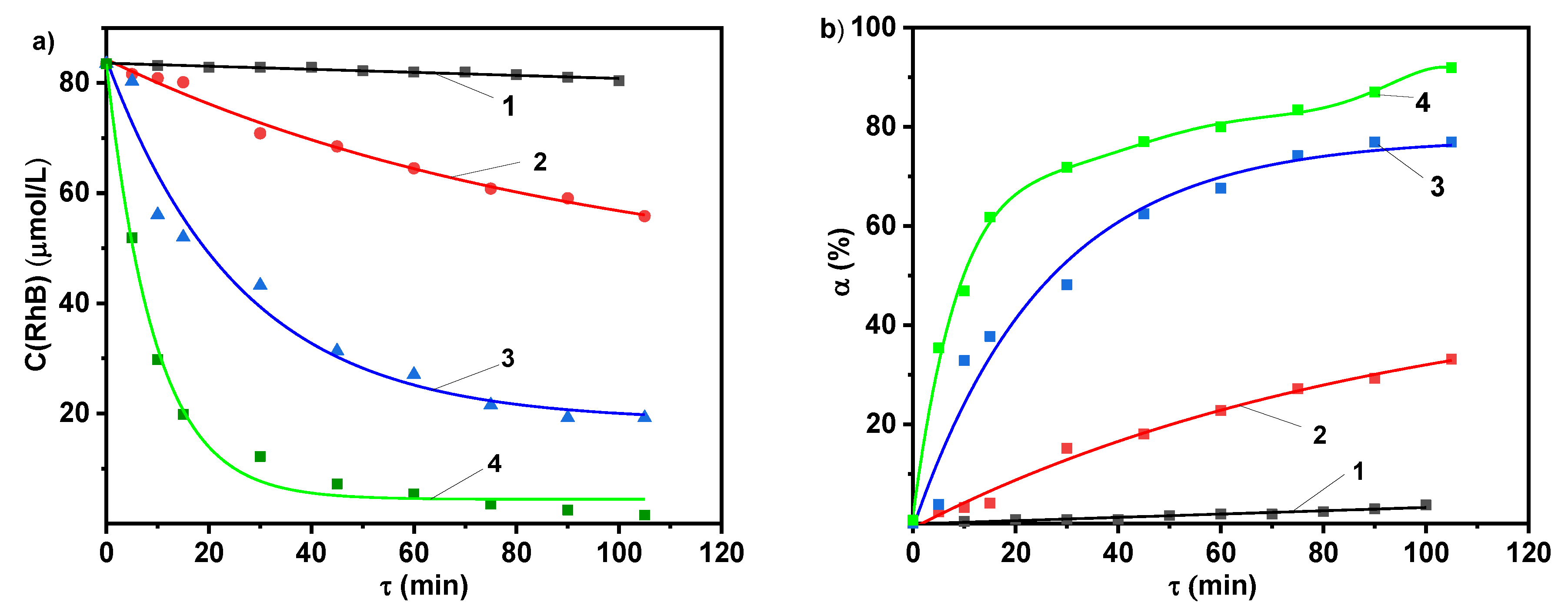
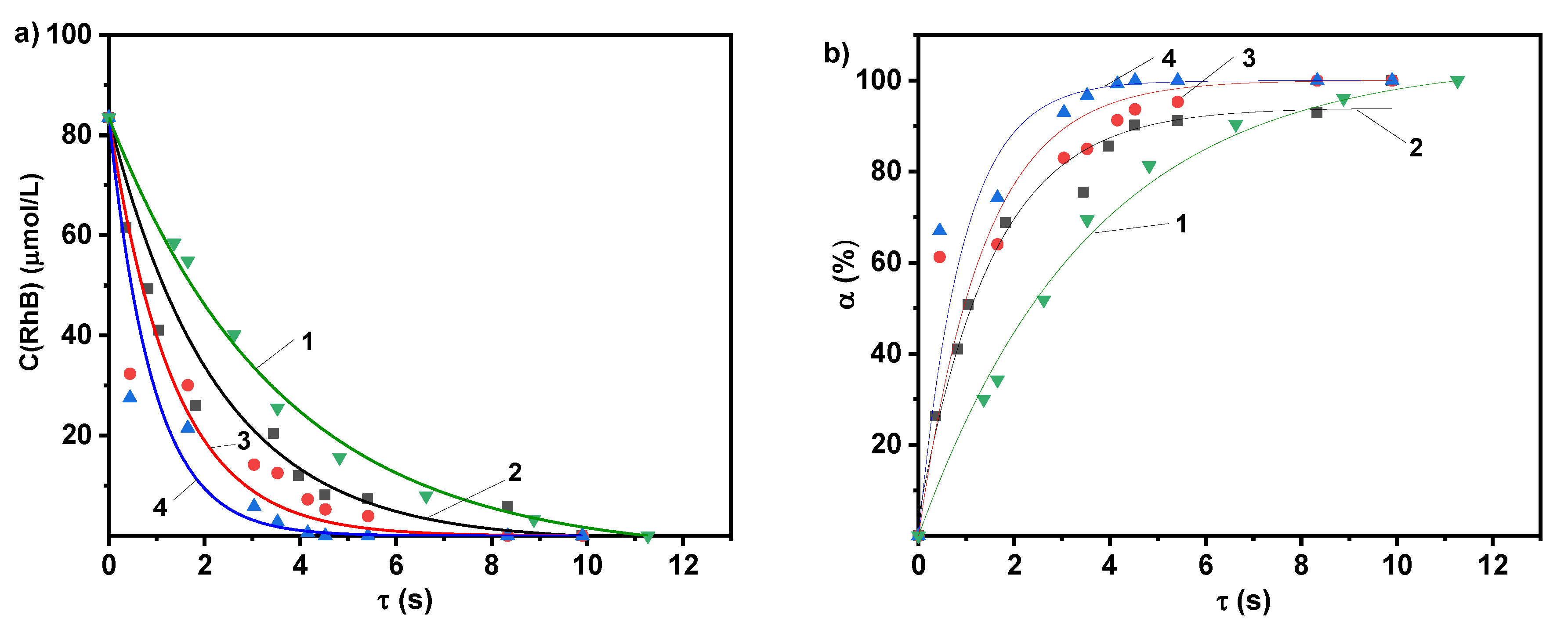
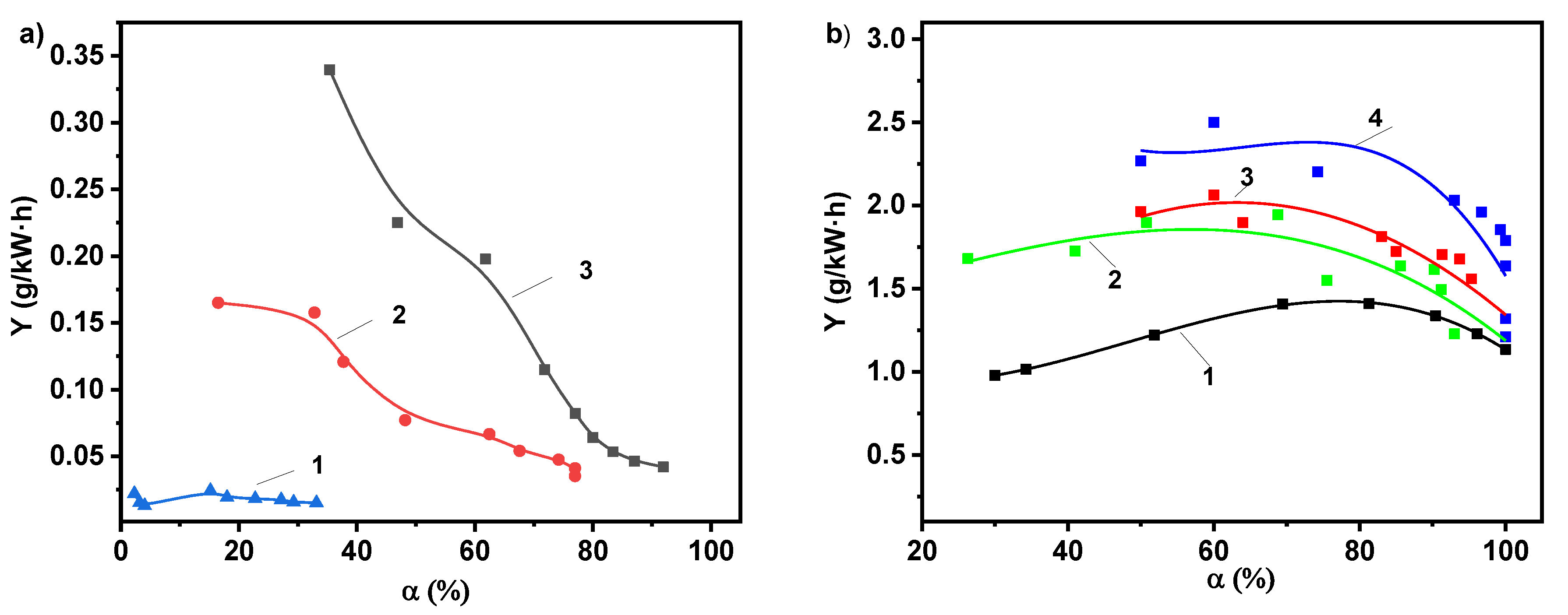
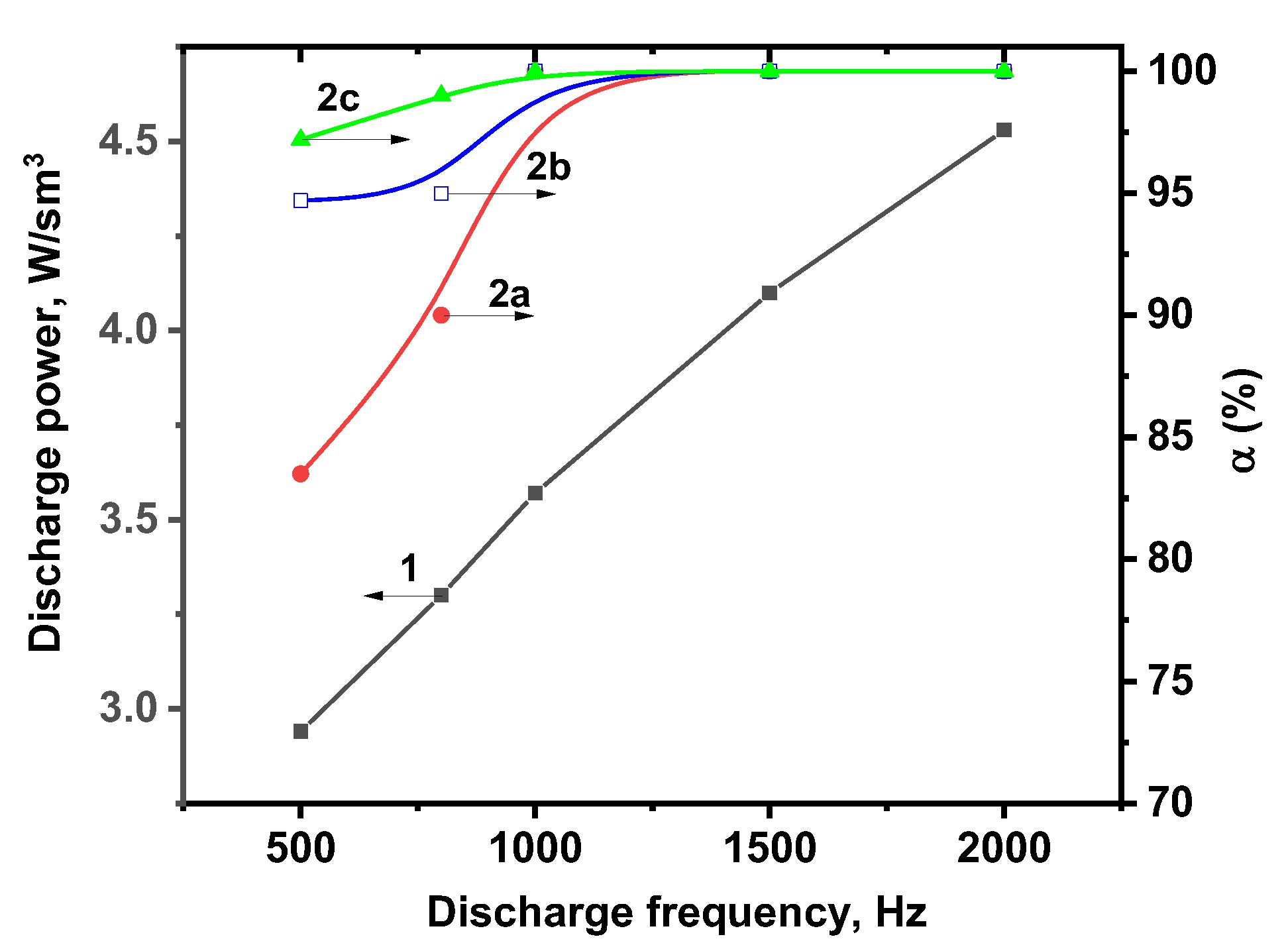
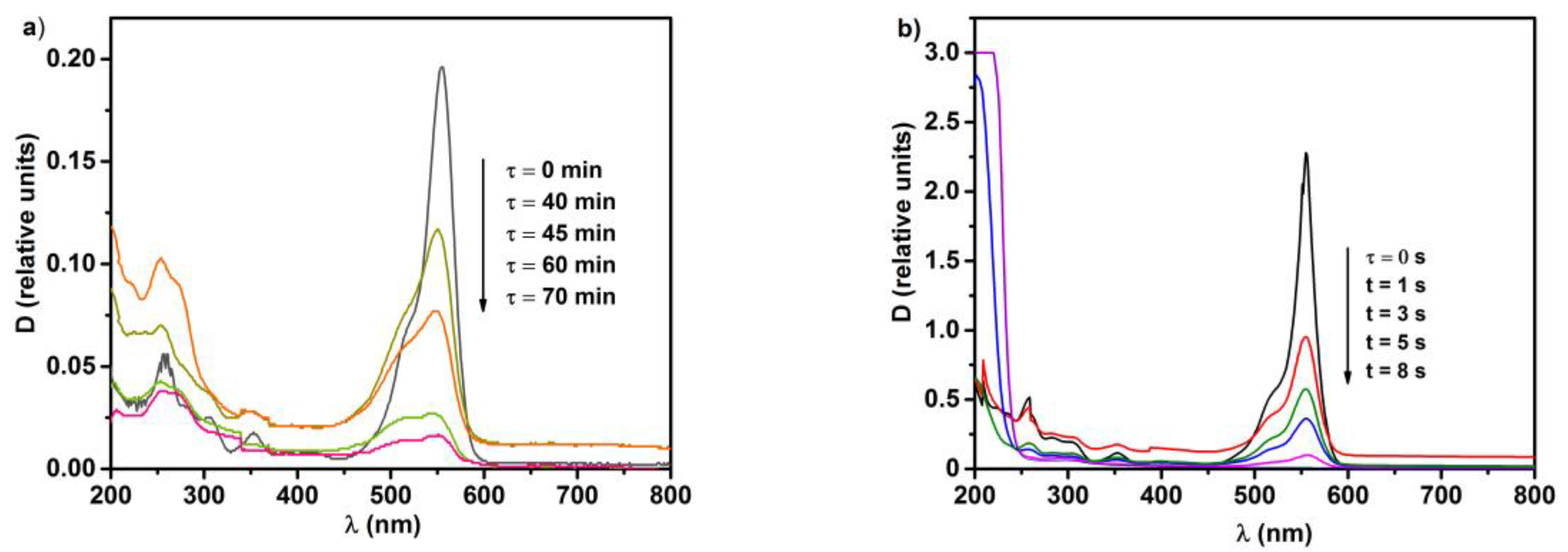
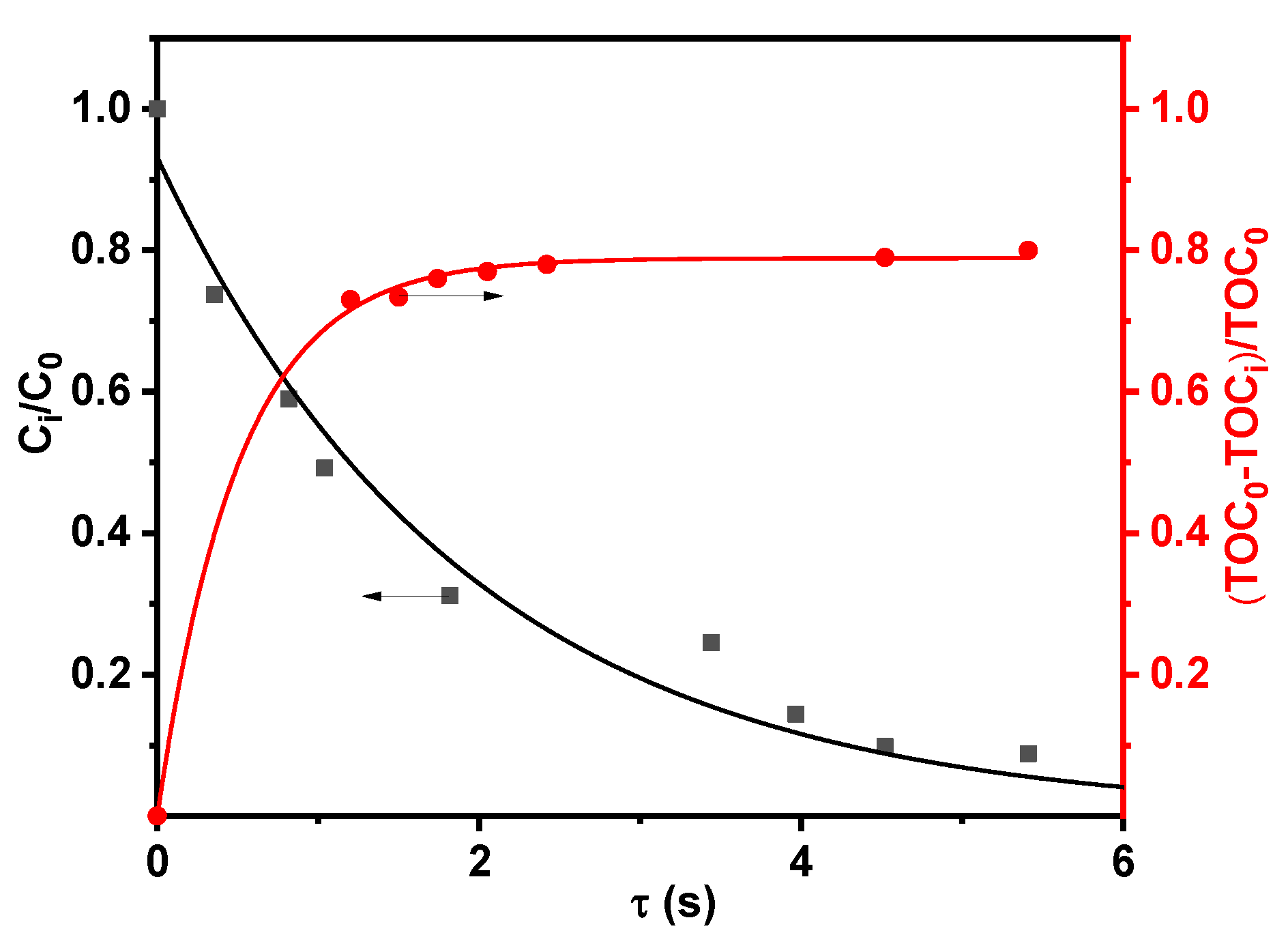
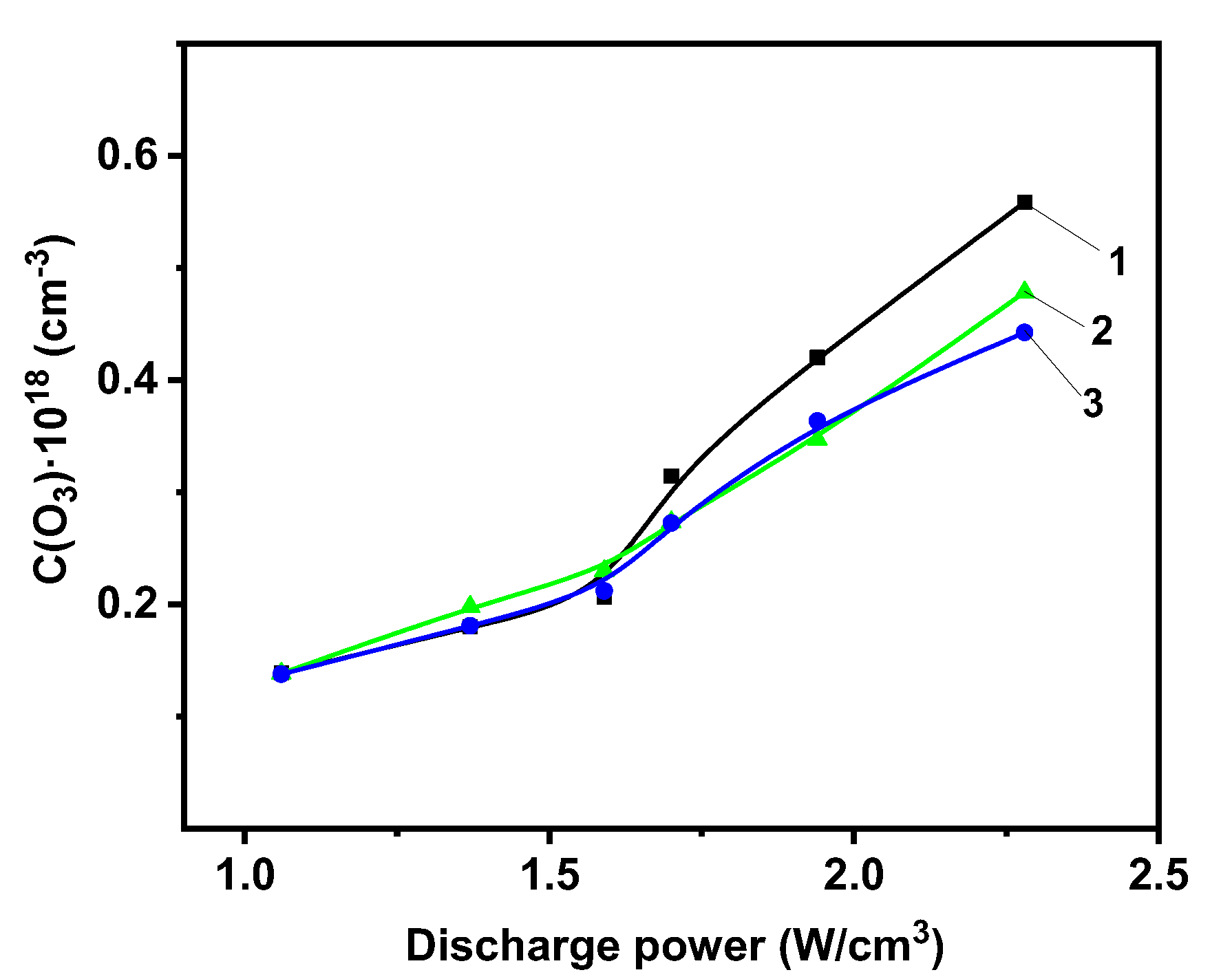
2.2. Photolytic and Plasmolytic Characterization
3. Materials and Methods
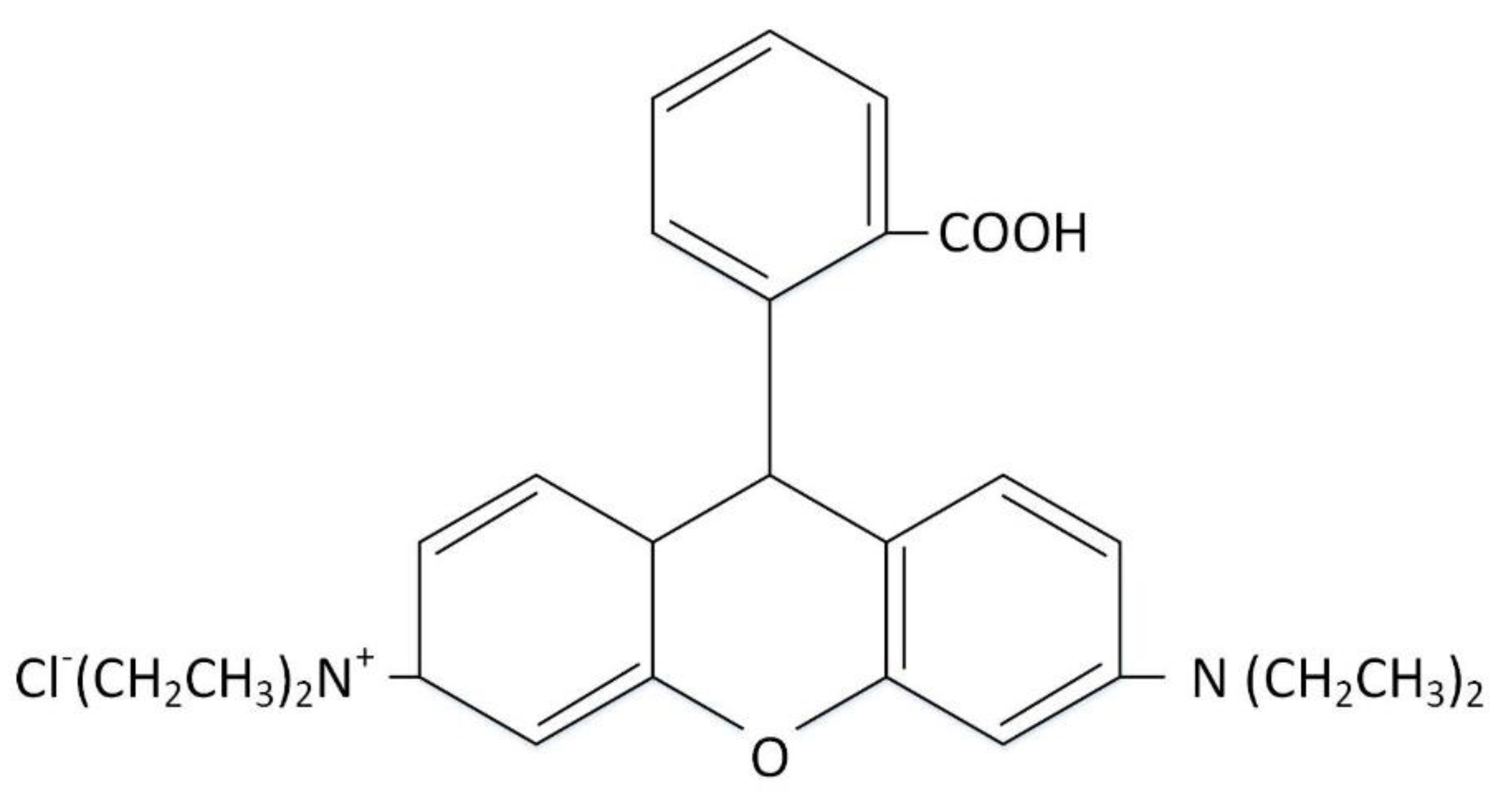
3.1. Materials
3.2. Methods
3.2.1. Preparation of Photocatalysts Based on TiO2-Pillared Montmorillonite
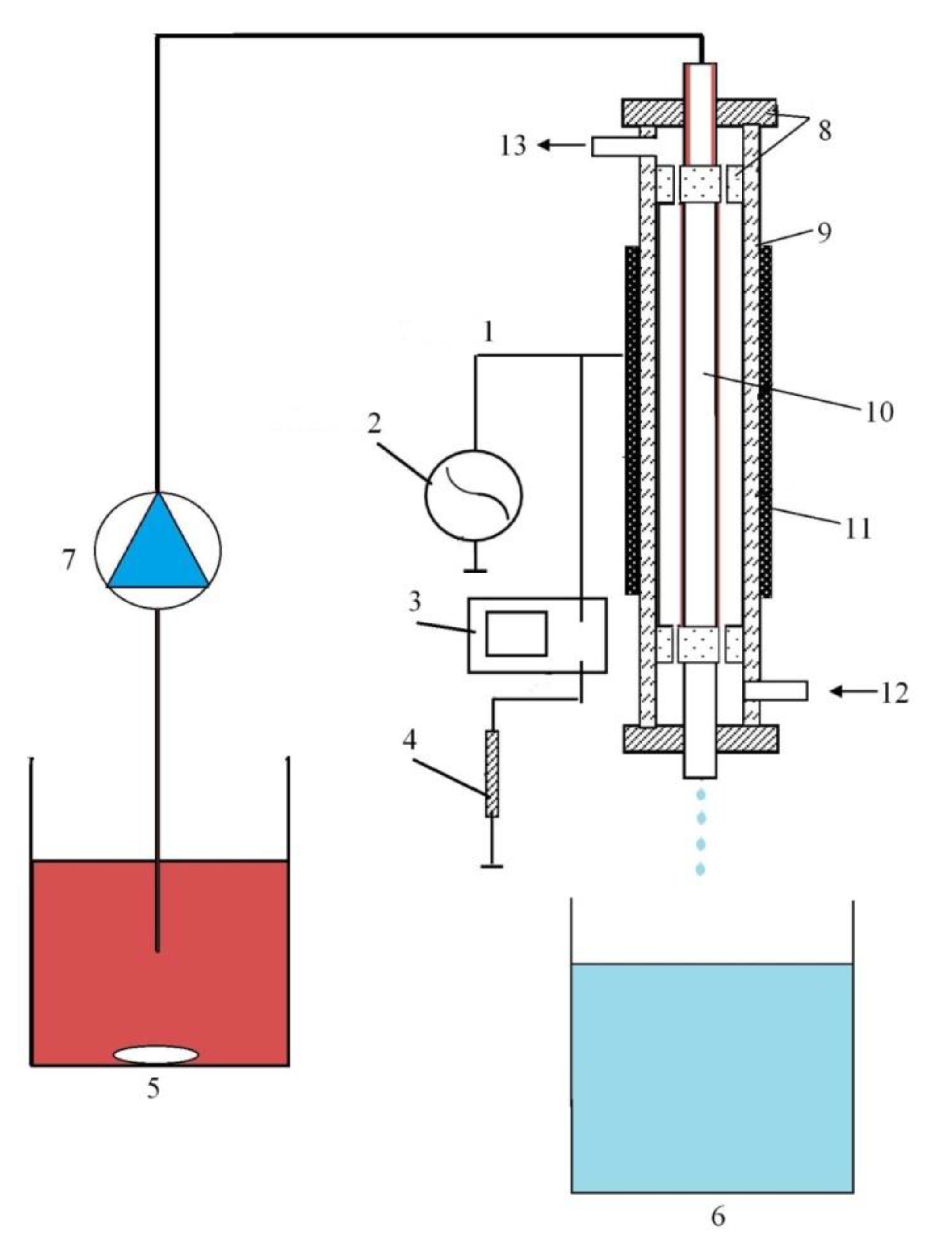
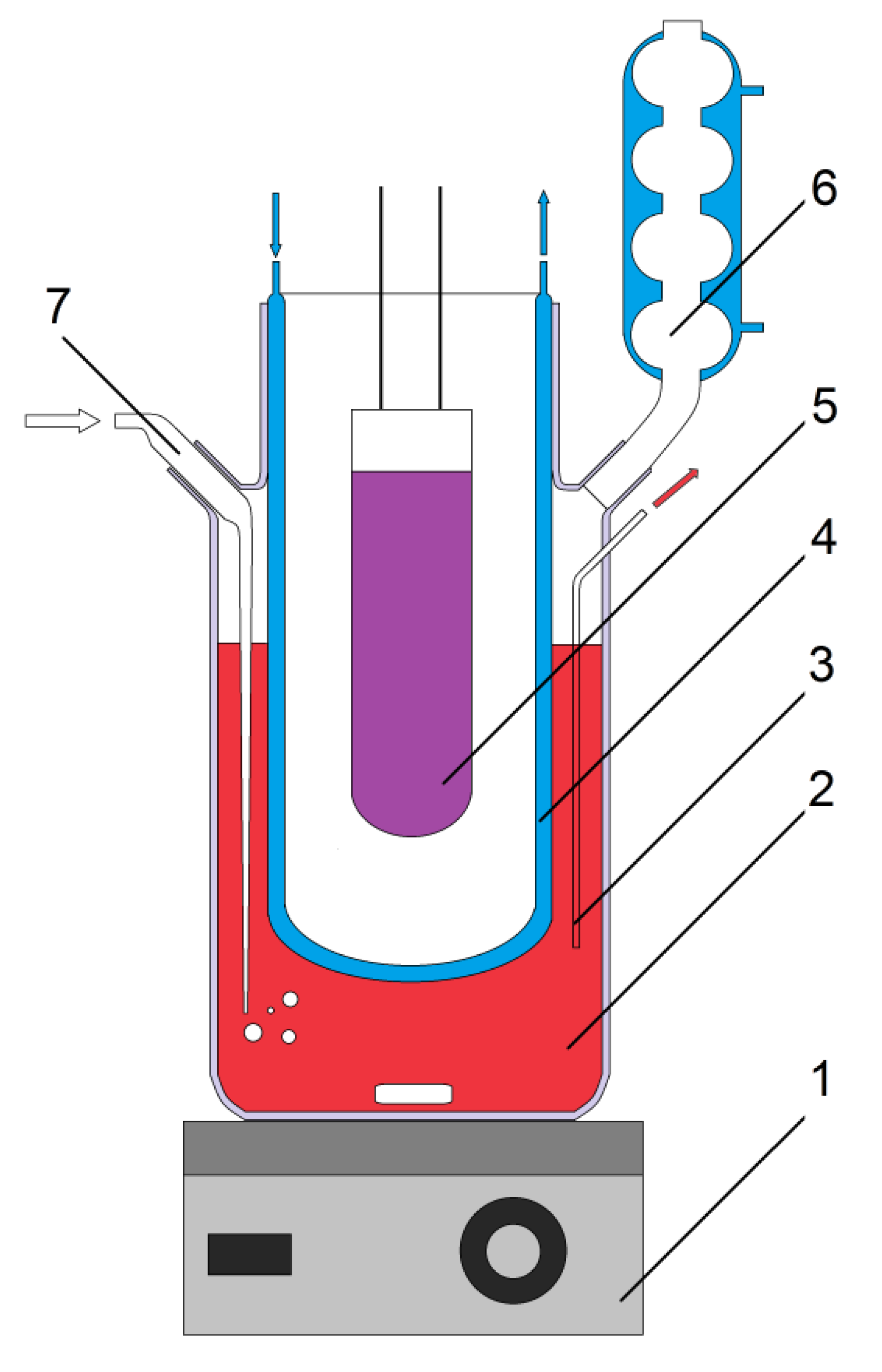
3.2.2. Description of Experimental Installations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thao, N.T.; Nga, H.T.P.; Vo, N.Q.; Nguyen, H.D.K. Advanced oxidation of rhodamine B with hydrogen peroxide over Zn–Cr layered double hydroxide catalysts. J. Sci. Adv. Mater. Devices 2017, 2, 317–325. [Google Scholar] [CrossRef]
- Khandare, R.V.; Govindwar, S.P. Phytoremediation of textile dyes and effluents: Current scenario and future prospects. Biotechnol. Adv. 2015, 33, 1697–1714. [Google Scholar] [CrossRef]
- Lai, C.-L.; Lin, K.-S. Sludge conditioning characteristics of copper chemical mechanical polishing wastewaters treated by electrocoagulation. J. Hazard. Mater. 2006, 136, 183–187. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Samanta, S.K. Microwave-enhanced advanced oxidation processes for the degradation of dyes in water. Environ. Chem. Lett. 2018, 16, 969–1007. [Google Scholar] [CrossRef]
- Dhanalakshmi, J.; Padiyan, D.P. Photocatalytic degradation of methyl orange and bromophenol blue dyes in water using sol-gel synthesized TiO2 nanoparticles. Mater. Res. Express 2017, 095020. [Google Scholar] [CrossRef]
- Hou, X.; Shen, W.; Huang, X.; Ai, Z.; Zhang, L. Ascorbic acid enhanced activation of oxygen by ferrous iron: A case of aerobic degradation of rhodamine B. J. Hazard. Mater. 2016, 308, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kurade, M.B.; Waghmode, T.R.; Khandare, R.V.; Jeon, B.-H.; Govindwar, S.P. Biodegradation and detoxification of textile dye Disperse Red 54 by Brevibacillus laterosporus and determination of its metabolic fate. J. Biosci. Bioeng. 2016, 121, 442–449. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Shanker, U.; Rani, M.; Jassal, V. Degradation of hazardous organic dyes in water by nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
- Bilińska, L.; Gmurek, M.; Ledakowicz, S. Comparison between industrial and simulated textile wastewater treatment by AOPs—Biodegradability, toxicity and cost assessment. Chem. Eng. J. 2016, 306, 550–559. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Neves, T.M.; Frantz, T.S.; do Schenque, E.C.C.; Gelesky, M.A.; Mortola, V.B. An investigation into an alternative photocatalyst based on CeO2/Al2O3 in dye degradation. Environ. Technol. Innov. 2017, 8, 349–359. [Google Scholar] [CrossRef]
- Gusev, G.I.; Gushchin, A.A.; Grinevich, V.I.; Osti, A.A.; Izvekova, T.V.; Kvitkova, E.Y. Regeneration of natural sorbents contaminated with oil products in dielectric barrier discharge plasma. Izv. Vyss. Uchebnykh Zaved. Seriya Khimiya Khimicheskaya Tekhnol. 2017, 60, 72–76. [Google Scholar] [CrossRef]
- Gushchin, A.A.; Grinevich, V.I.; Gusev, G.I.; Kvitkova, E.Y.; Rybkin, V.V. Removal of Oil Products from Water Using a Combined Process of Sorption and Plasma Exposure to DBD. Plasma Chem. Plasma Process. 2018, 38, 1021–1033. [Google Scholar] [CrossRef]
- Gushchin, A.A.; Grinevich, V.I.; Shulyk, V.Y.; Kvitkova, E.Y.; Rybkin, V.V. Destruction Kinetics of 2,4 Dichlorophenol Aqueous Solutions in an Atmospheric Pressure Dielectric Barrier Discharge in Oxygen. Plasma Chem. Plasma Process. 2018, 38, 123–134. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mahyar, A.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Application of a planar falling film reactor for decomposition and mineralization of methylene blue in the aqueous media via ozonation, Fenton, photocatalysis and non-thermal plasma: A comparative study. Process Saf. Environ. Prot. 2018, 113, 319–329. [Google Scholar] [CrossRef]
- Lu, N.; Hui, Y.; Shang, K.; Jiang, N.; Li, J.; Wu, Y. Diagnostics of Plasma Behavior and TiO2 Properties Based on DBD/TiO2 Hybrid System. Plasma Chem. Plasma Process. 2018, 38, 1239–1258. [Google Scholar] [CrossRef]
- Ochiai, T.; Nakata, K.; Murakami, T.; Morito, Y.; Hosokawa, S.; Fujishima, A. Development of an air-purification unit using a photocatalysis-plasma hybrid reactor. Electrochemistry 2011, 79, 838–841. [Google Scholar] [CrossRef]
- Tichonovas, M.; Krugly, E.; Racys, V.; Hippler, R.; Kauneliene, V.; Stasiulaitiene, I.; Martuzevicius, D. Degradation of various textile dyes as wastewater pollutants under dielectric barrier discharge plasma treatment. Chem. Eng. J. 2013, 229, 9–19. [Google Scholar] [CrossRef]
- Palau, J.; Assadi, A.A.; Penya-Roja, J.M.; Bouzaza, A.; Wolbert, D.; Martínez-Soria, V. Isovaleraldehyde degradation using UV photocatalytic and dielectric barrier discharge reactors, and their combinations. J. Photochem. Photobiol. A Chem. 2015, 299, 110–117. [Google Scholar] [CrossRef]
- Wang, T.C.; Lu, N.; Li, J.; Wu, Y. Plasma-TiO2 catalytic method for high-efficiency remediation of p-nitrophenol contaminated soil in pulsed discharge. Environ. Sci. Technol. 2011, 45, 9301–9307. [Google Scholar] [CrossRef] [PubMed]
- Neyts, E.C. Plasma-Surface Interactions in Plasma Catalysis. Plasma Chem. Plasma Process. 2016, 36, 185–212. [Google Scholar] [CrossRef]
- Attri, P.; Tochikubo, F.; Park, J.H.; Choi, E.H.; Koga, K.; Shiratani, M. Impact of Gamma rays and DBD plasma treatments on wastewater treatment. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.A.; Bouzaza, A.; Vallet, C.; Wolbert, D. Use of DBD plasma, photocatalysis, and combined DBD plasma/photocatalysis in a continuous annular reactor for isovaleraldehyde elimination—Synergetic effect and byproducts identification. Chem. Eng. J. 2014, 254, 124–132. [Google Scholar] [CrossRef]
- Guaitella, O.; Thevenet, F.; Puzenat, E.; Guillard, C.; Rousseau, A. C2H2 oxidation by plasma/TiO2 combination: Influence of the porosity, and photocatalytic mechanisms under plasma exposure. Appl. Catal. B Environ. 2008, 80, 296–305. [Google Scholar] [CrossRef]
- Yamashita, H.; Li, H. Nanostructured Photocatalysts: Advanced Functional Materials; Springer: Berlin, Germany, 2016. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Vicente, M.A.; Gil, A.; Bergaya, F. Pillared Clays and Clay Minerals, 2nd ed.; Developments in Clay Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Zhao, B.X.; Dang, L.P.; Zhang, X.L.; Yang, N.; Sun, Y.Y. Preparation of TiO2-Pillared Montmorillonite as Photocatalyst and Photocatalytic Degradation of Methyl Orange. Appl. Mech. Mater. 2012, 190–191, 534–538. [Google Scholar] [CrossRef]
- Butman, M.F.; Ovchinnikov, N.L.; Karasev, N.S.; Kochkina, N.E.; Agafonov, A.V.; Vinogradov, A.V. Photocatalytic and adsorption properties of TiO2-pillared montmorillonite obtained by hydrothermally activated intercalation of titanium polyhydroxo complexes. Beilstein J. Nanotechnol. 2018, 9, 364–378. [Google Scholar] [CrossRef]
- Ovchinnikov, N.L.; Arbuznikov, V.V.; Kapinos, A.P.; Belozerov, A.G.; Butman, M.F. Effect of mechanical activation of montmorillonite on the intercalation efficiency of polyhydroxyaluminum cations in the formation of pillar structure. Nanotechnol. Russ. 2015, 10, 254–260. [Google Scholar] [CrossRef]
- Mandlimath, T.R.; Moliya, A.; Sathiyanarayanan, K.I. General Synthesis of Bi2Mo3O12 and Bi2−xRExMo3O12 nanorods (RE = Eu3+ and Pr3+ and x = 0.07–0.3): Improved photocatalytic activity towards the degradation of Rhodamine B dye under visible light. Appl. Catal. A Gen. 2016, 519, 34–47. [Google Scholar] [CrossRef]
- Al-Shamiri, H.A.; Kana, M.A. Laser performance and photostability of Rhodamin B in solid host matrices. Appl. Phys. B 2010, 101, 129–135. [Google Scholar] [CrossRef]
- Asano, M.; Doi, M.; Baba, K.; Taniguchi, M.; Shibano, M.; Tanaka, S.; Sakaguchi, M.; Takaoka, M.; Hirata, M.; Yanagihara, R.; et al. Bio-imaging of hydroxyl radicals in plant cells using the fluorescent molecular probe rhodamine B hydrazide, without any pretreatment. J. Biosci. Bioeng. 2014, 118, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.J.; Zhang, X.B.; Mao, G.J.; Su, L.; Meng, H.M.; Tan, W.; Feng, S.; Zhang, G. A unique approach toward near-infrared fluorescent probes for bioimaging with remarkably enhanced contrast. Chem. Sci. 2016, 7, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.; Wei, X.; Tao, H.; Pan, H. Molecularly imprinted electrochemical luminescence sensor based on signal amplification for selective determination of trace gibberellin A3. Anal. Chem. 2012, 84, 9951–9955. [Google Scholar] [CrossRef] [PubMed]
- Baviskar, P.K.; Zhang, J.B.; Gupta, V.; Chand, S.; Sankapal, B.R. Nanobeads of zinc oxide with rhodamine B dye as a sensitizer for dye sensitized solar cell application. J. Alloys Compd. 2012, 510, 33–37. [Google Scholar] [CrossRef]
- Dire, D.J.; Wilkinson, J.A. Acute exposure to rhodamine B. J. Toxicol. Clin. Toxicol. 1987, 25, 603–607. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Physical and biophysical chemistry division commission on colloid and surface chemistry including catalysis. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Ninness, B.J.; Bousfield, D.W.; Tripp, C.P. Formation of a thin TiO2 layer on the surfaces of silica and kaolin pigments through atomic layer deposition. Colloids Surf. A 2003, 214, 195–204. [Google Scholar] [CrossRef]
- Son, H.-J.; Wang, X.; Prasittichai, C.; Jeong, N.C.; Aaltonen, T.; Gordon, R.G.; Hupp, J.T. Glass-encapsulated light harvesters: More efficient dye-sensitized solar cells by deposition of self-aligned, conformal, and self-limited silica layers. J. Am. Chem. Soc. 2012, 134, 9537–9540. [Google Scholar] [CrossRef] [PubMed]
- Damardji, B.; Khalaf, H.; Duclaux, L.; David, B. Preparation of TiO2-pillared montmorillonite as photocatalyst Part II. Photocatalytic degradation of a textile azo dye. Appl. Clay Sci. 2009, 45, 98–104. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Sherwood, T.K. The Properties of Gases and Liquids, 4th ed.; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar] [CrossRef]
- Jain, R.; Mathur, M.; Sikarwar, S.; Mittal, A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Y.; Jiang, H.; Feng, J. Removal of caffeine from water by combining dielectric barrier discharge (DBD) plasma with goethite. J. Saudi Chem. Soc. 2017, 21, 545–557. [Google Scholar] [CrossRef]
- Cuiping, B.; Xianfeng, X.; Wenqi, G.; Dexin, F.; Mo, X.; Zhongxue, G.; Nian, X. Removal of rhodamine B by ozone-based advanced oxidation process. Desalination 2011, 278, 84–90. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Eliasson, B.; Hirth, M.; Kogelschatz, U. Ozone synthesis from oxygen in dielectric barrier discharges. J. Phys. D Appl. Phys. 1987, 20, 1421–1437. [Google Scholar] [CrossRef]
- Gordon, P.F.; Gregory, P. Organic Chemistry in Colour; Springer: Berlin, Germany, 2012. [Google Scholar] [CrossRef]
- Singh, S.; Parveen, N.; Gupta, H. Adsorptive decontamination of rhodamine-B from water using banana peel powder: A biosorbent. Environ. Technol. Innov. 2018, 12, 189–195. [Google Scholar] [CrossRef]
- Nasedkin, V.V.; Shiriizade, N.A. Dash-Salakhlinskoe mestorozhdenie bentonita. In Stanovlenie i Perspektivy Razvitiia; GEOS: Moscow, Russia, 2008. [Google Scholar]
- Sterte, J. Synthesis and properties of titanium oxide cross-linked montmorillonite. Clays Clay Miner. 1986, 34, 658–664. [Google Scholar] [CrossRef]
- Ooka, C.; Akita, S.; Ohashi, Y.; Horiuchi, T.; Suzuki, K.; Komai, S.-I.; Yoshida, H.; Hattori, T. Crystallization of hydrothermally treated TiO2 pillars in pillared montmorillonite for improvement of the photocatalytic activity. J. Mater. Chem. 1999, 9, 2943–2952. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley and Sons Inc.: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Chen, X.; Xue, Z.; Yao, Y.; Wang, W.; Zhu, F.; Hong, C. Oxidation degradation of rhodamine B in aqueous by UV/S2O82- treatment system. Int. J. Photoenergy 2012. [Google Scholar] [CrossRef]
- Parkinson, W.H.; Yoshino, K.; Freeman, D.E.; Observatory, A. Absolute Absorption Cross-Section Measurements of Ozone and the Temperature Dependence at Four Reference Wavelengths Leading to Renormalization of the Cross-Section between 240 and 350 nm; Smithsonian Astrophysical Observatory: Cambridge, MA, USA, 1988. [Google Scholar]
- UOP 603-88: Trace CO and CO2 in Hydrogen and Light Gaseous Hydrocarbons by GC standard by UOP LLC; A Honeywell Company: Charlotte, NC, USA, 1988.
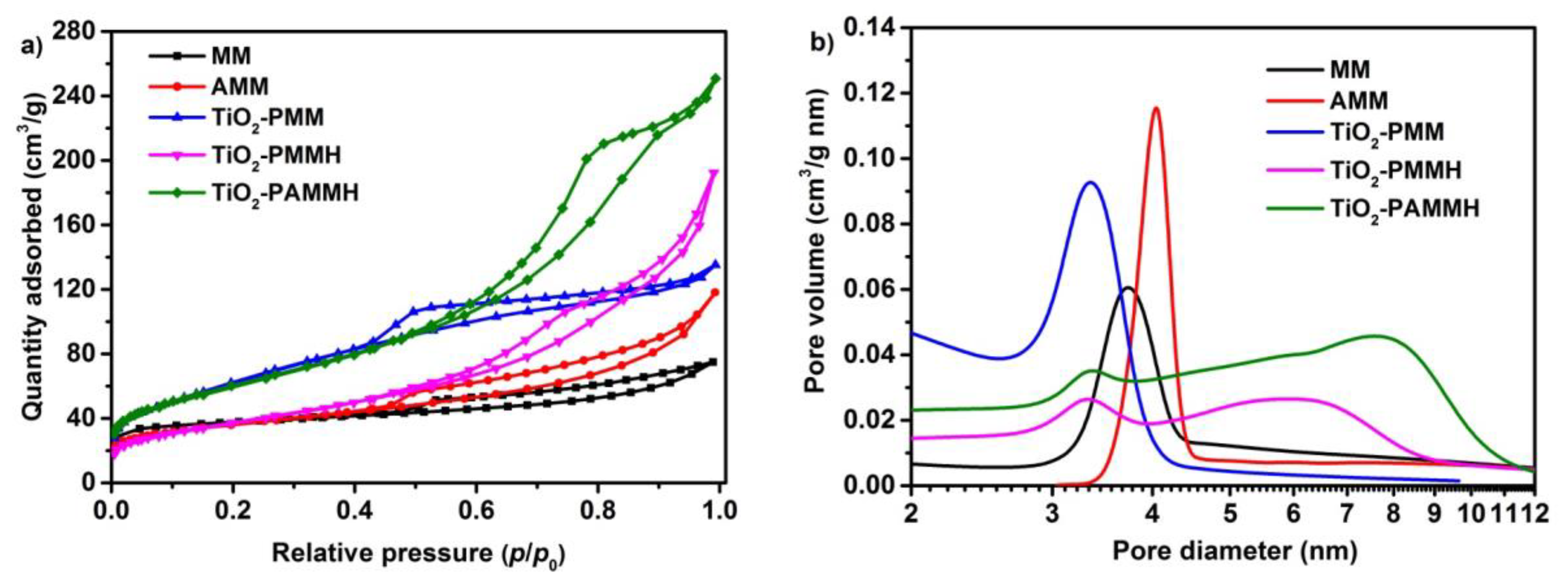

| Sample | SBET (m2/g) | ∑Vpore (cm3/g) | Dav (nm) |
|---|---|---|---|
| MM | 96.0 | 0.170 | 7.00 |
| AMM | 118.0 | 0.180 | 6.20 |
| TiO2-PMM | 228.2 | 0.209 | 3.98 |
| TiO2-PMMH | 135.1 | 0.303 | 8.34 |
| TiO2-PAMMH | 216.0 | 0.393 | 6.42 |
| Purification Method | K, s−1 | Y50%, g/(kW·h) |
|---|---|---|
| Photocatalysis in the presence of TiO2-PMM | 5.2 × 10−5 | -** |
| Photocatalysis in the presence of TiO2-PMMH | 6.9 × 10−5 | 0.09 |
| Photocatalysis in the presence of TiO2-PAMMH | 2.3 × 10−4 | 0.24 |
| DBD | 0.1893 | 1.21 |
| DBD in the presence of TiO2-PMM | 0.2849 | 1.89 |
| DBD in the presence of TiO2-PMMH | 0.4592 | 1.96 |
| DBD in the presence of TiO2-PAMMH | 0.6910 | 2.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butman, M.F.; Gushchin, A.A.; Ovchinnikov, N.L.; Gusev, G.I.; Zinenko, N.V.; Karamysheva, S.P.; Krämer, K.W. Synergistic Effect of Dielectric Barrier Discharge Plasma and TiO2-Pillared Montmorillonite on the Degradation of Rhodamine B in an Aqueous Solution. Catalysts 2020, 10, 359. https://doi.org/10.3390/catal10040359
Butman MF, Gushchin AA, Ovchinnikov NL, Gusev GI, Zinenko NV, Karamysheva SP, Krämer KW. Synergistic Effect of Dielectric Barrier Discharge Plasma and TiO2-Pillared Montmorillonite on the Degradation of Rhodamine B in an Aqueous Solution. Catalysts. 2020; 10(4):359. https://doi.org/10.3390/catal10040359
Chicago/Turabian StyleButman, Mikhail F., Andrey A. Gushchin, Nikolay L. Ovchinnikov, Grigoriy I. Gusev, Nikolay V. Zinenko, Sofia P. Karamysheva, and Karl W. Krämer. 2020. "Synergistic Effect of Dielectric Barrier Discharge Plasma and TiO2-Pillared Montmorillonite on the Degradation of Rhodamine B in an Aqueous Solution" Catalysts 10, no. 4: 359. https://doi.org/10.3390/catal10040359
APA StyleButman, M. F., Gushchin, A. A., Ovchinnikov, N. L., Gusev, G. I., Zinenko, N. V., Karamysheva, S. P., & Krämer, K. W. (2020). Synergistic Effect of Dielectric Barrier Discharge Plasma and TiO2-Pillared Montmorillonite on the Degradation of Rhodamine B in an Aqueous Solution. Catalysts, 10(4), 359. https://doi.org/10.3390/catal10040359







