Effects of Lanthanide Doping on the Catalytic Activity and Hydrothermal Stability of Cu-SAPO-18 for the Catalytic Removal of NOx (NH3-SCR) from Diesel Engines
Abstract
1. Introduction
2. Results
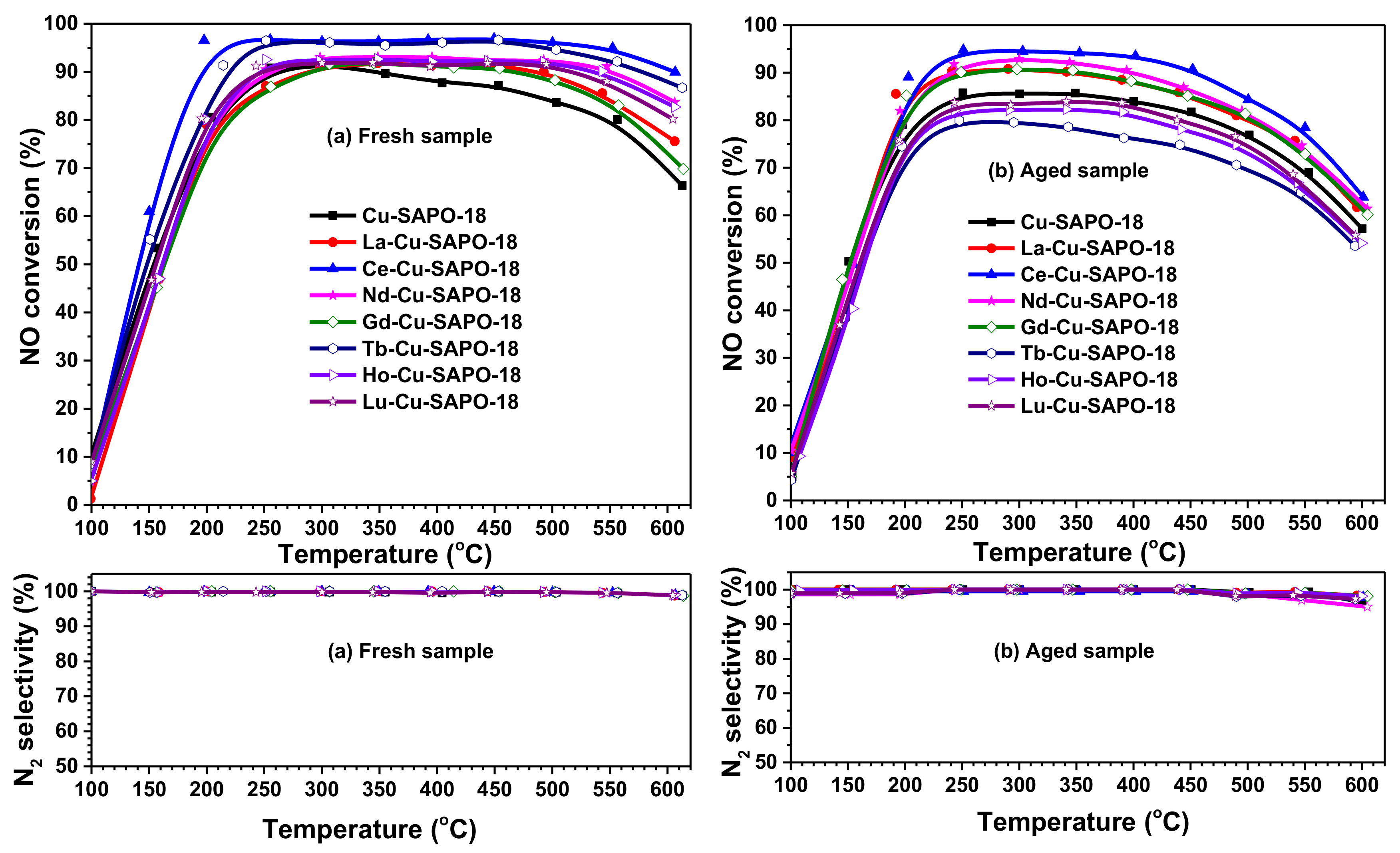
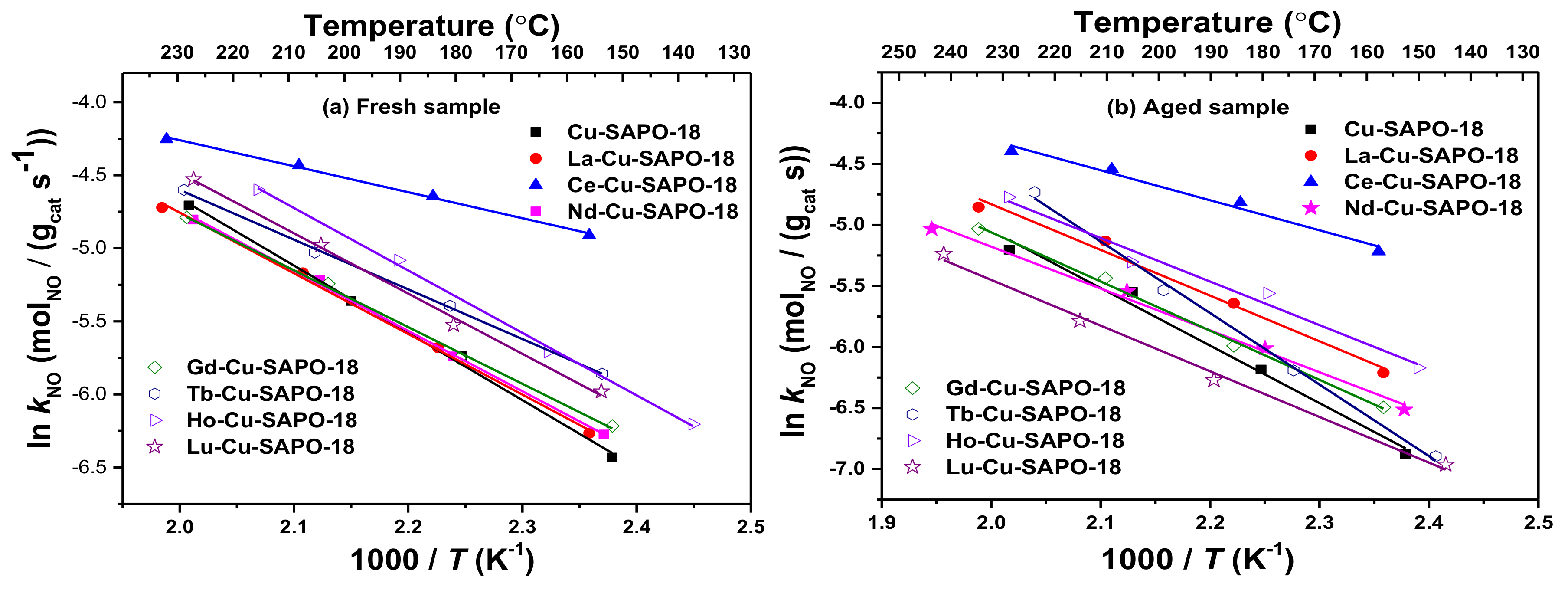
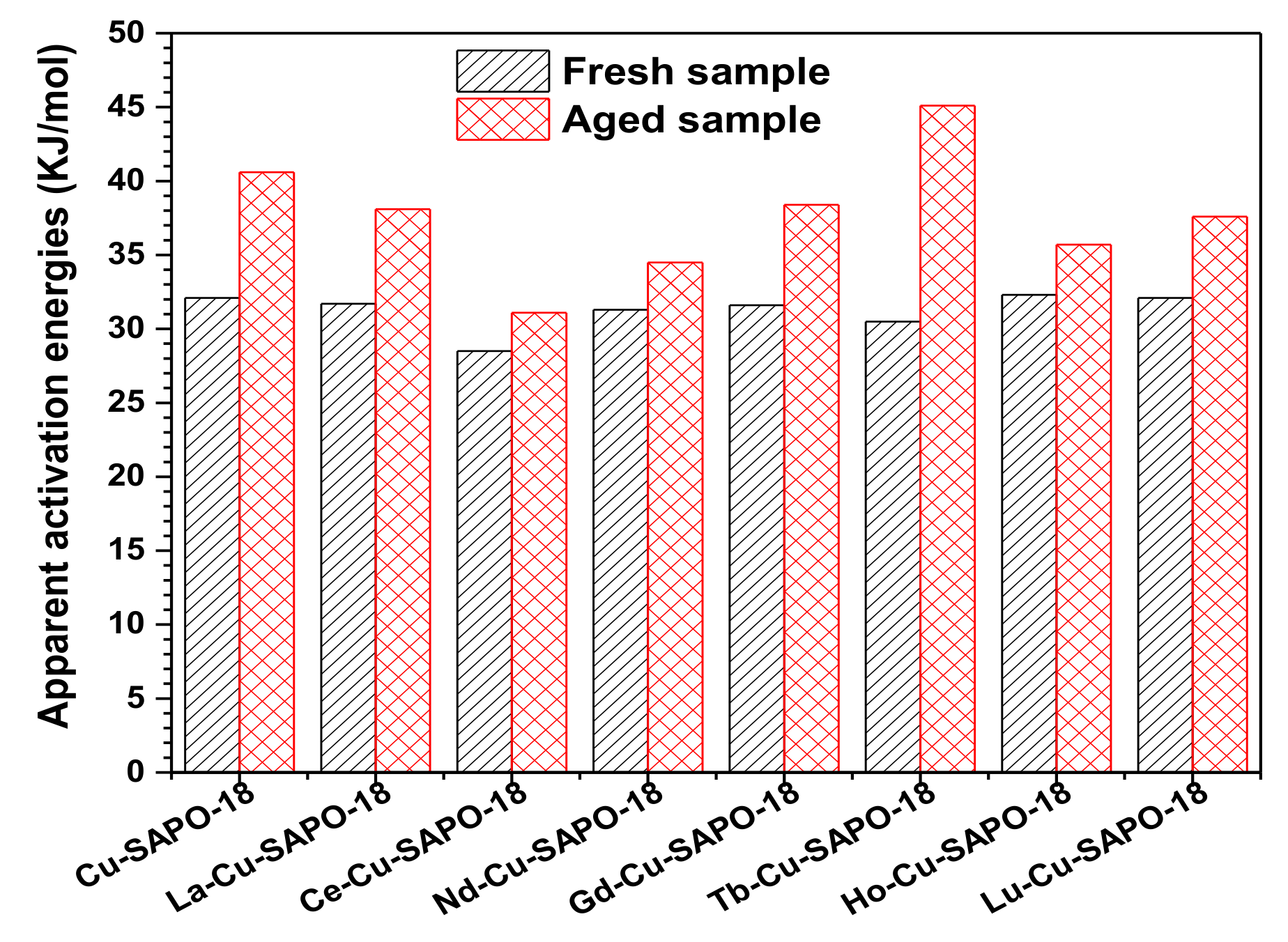
2.1. NH3-SCR Performance and Hydrothermal Stability
2.2. NH3 Oxidation
2.3. Characterization of the Fresh and Aged Cu-SAPO-18 and M-Cu-SAPO-18 Catalysts
2.3.1. XRD and BET Results
2.3.2. XPS Results
2.3.3. H2-TPR Results
2.3.4. NMR Results
2.3.5. NH3-TPD Results
2.3.6. Reactivity of NH3 Adsorption
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matsumoto, K.; Tominaga, S.; Igawa, M. Measurements of atmospheric aerosols with diameters greater than 10 μm and their contribution to fixed nitrogen deposition in coastal urban environment. Atmos. Environ. 2011, 45, 6433–6438. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, R.J.; Kim, J. Urban air quality modeling with full O3–NOx–VOC chemistry: Implications for O3 and PM air quality in a street canyon. Atmos. Environ. 2012, 47, 330–340. [Google Scholar] [CrossRef]
- Shen, M.; Wen, H.; Hao, T.; Yu, T.; Fan, D.; Wang, J.; Li, W.; Wang, J. Deactivation mechanism of SO2 on Cu/SAPO-34 NH3-SCR catalysts: structure and active Cu2+. Catal. Sci. Technol. 2015, 5, 1741–1749. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Praliaud, H.; Mikhailenko, S.; Chajar, Z.; Primet, M. Surface and bulk properties of Cu–ZSM-5 and Cu/Al2O3 solids during redox treatments. Correlation with the selective reduction of nitric oxide by hydrocarbons. Appl. Catal. B Environ. 1998, 16, 359–374. [Google Scholar] [CrossRef]
- Bates, S.A.; Verma, A.A.; Paolucci, C.; Parekh, A.A.; Anggara, T.; Yezerets, A.; Schneider, W.F.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Identification of the active Cu site in standard selective catalytic reduction with ammonia on Cu-SSZ-13. J. Catal. 2014, 312, 87–97. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Choo, S.T.; Yim, S.D.; Nam, I.; Ham, S.; Lee, J. Effect of promoters including WO3 and BaO on the activity and durability of V2O5/sulfated TiO2 catalyst for NO reduction by NH3. Appl. Catal. B Environ. 2003, 44, 237–252. [Google Scholar] [CrossRef]
- Fickel, D.W.; Addio, E.D.; Lauterbach, J.A.; Lobo, R.F. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B Environ. 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Sjövall, H.; Blint, R.J.; Olsson, L. Detailed kinetic modeling of NH3 SCR over Cu-ZSM-5. Appl. Catal. B Environ. 2009, 92, 138–153. [Google Scholar] [CrossRef]
- Mihai, O.; Widyastuti, C.R.; Andonova, S.; Kamasamudram, K.; Li, J.; Joshi, S.Y.; Currier, N.W.; Yezerets, A.; Olsson, L. The effect of Cu-loading on different reactions involved in NH3-SCR over Cu-BEA catalysts. J. Catal. 2014, 311, 170–181. [Google Scholar] [CrossRef]
- Iwamoto, M.; Furukawa, H.; Mine, Y.; Uemura, F.; Mikuriya, S.; Kagawa, S. Copper(II) ion-exchanged ZSM-5 zeolites as highly active catalysts for direct and continuous decomposition of nitrogen monoxide. J. Chem. Soc. Chem. Commun. 1986, 1272–1273. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Schmieg, S.J.; Oh, S.H.; Kim, C.H.; Brown, D.B.; Lee, J.H.; Peden, C.H.F.; Kim, D.H. Thermal durability of Cu-CHA NH3-SCR catalysts for diesel NOx reduction. Catal. Today 2012, 184, 252–261. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, L.; Yang, R.T. Activity, propene poisoning resistance and hydrothermal stability of copper exchanged chabazite-like zeolite catalysts for SCR of NO with ammonia in comparison to Cu/ZSM-5. Appl. Catal. A. Gen. 2012, 427–428, 24–34. [Google Scholar] [CrossRef]
- Martínez-Franco, R.; Moliner, M.; Corma, A. Direct synthesis design of Cu-SAPO-18, a very efficient catalyst for the SCR of NOx. J. Catal. 2014, 319, 36–43. [Google Scholar] [CrossRef]
- Bin, F.; Wei, X.; Li, B.; Hui, K.S. Self-sustained combustion of carbon monoxide promoted by the Cu-Ce/ZSM-5 catalyst in CO/O2/N2 atmosphere. Appl. Catal. B Envivron. 2015, 162, 282–288. [Google Scholar] [CrossRef]
- Samojeden, B.; Teresa, G.; Kowal, J.; Szymaszek, A.; Jabłońska, M.; Gläser, R.; Motak, M. The influence of holmium on catalytic properties of Fe or Cu-modified vermiculites. Physicochem. Probl. Mi. 2019, 55, 1484–1495. [Google Scholar]
- Chen, J.; Li, J.; Yuan, C.; Xu, S.; Wei, Y. Elucidating the olefin formation mechanism in the methanol to olefin reaction over AlPO-18 and SAPO-18. Catal. Sci. Technol. 2014, 4, 3268–3277. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Feng, G.; Chang, L.; Bao, W. In situ synthesis of CuSAPO-34/cordierite and its selective catalytic reduction of nitrogen oxides in vehicle exhaust: The effect of HF. Fuel 2013, 109, 101–109. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Vesaghi, M.A.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS study of the Cu@Cu2O core-shell nanoparticles. Appl. Surf. Sci. 2008, 255, 2730–2734. [Google Scholar] [CrossRef]
- Han, S.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effect of the hydrothermal aging temperature and Cu/Al ratio on the hydrothermal stability of CuSSZ-13 catalysts for NH3-SCR. Catal. Sci. Technol. 2017, 7, 703–717. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Z.; Qiao, H.; Yu, H.; Hu, Y.; Chang, L.; Bao, W. Cerium-Stabilized Cu-SSZ-13 Catalyst for the Catalytic Removal of NOx by NH3. Ind. Eng. Chem. Res. 2016, 55, 1174–1182. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.K.; Min, K.M.; Hong, S.B.; Nam, I.; Cho, B.K. Hydrothermal stability of CuSSZ13 for reducing NOx by NH3. J. Catal. 2014, 311, 447–457. [Google Scholar] [CrossRef]
- Xue, J.; Wang, X.; Qi, G.; Wang, J.; Shen, M.; Li, W. Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia: Relationships between active Cu sites and de-NOx performance at low temperature. J. Catal. 2013, 297, 56–64. [Google Scholar] [CrossRef]
- Adachi, G.; Imanaka, N. The Binary Rare Earth Oxides. Chem. Rev. 1998, 98, 1479–1514. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. The role of ceria on the activity and SO2 resistance of catalysts for the selective catalytic reduction of NOx by NH3. Appl. Catal. B Environ. 2015, 166–167, 37–44. [Google Scholar] [CrossRef]
- Cao, Y.; Lan, L.; Feng, X.; Yang, Z.; Zou, S. Cerium promotion on the hydrocarbon resistance of a Cu-SAPO-34 NH3-SCR monolith catalyst. Catal. Sci. Technol. 2015, 5, 4511–4521. [Google Scholar] [CrossRef]
- Han, S.; Cheng, J.; Zheng, C.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effect of Si/Al ratio on catalytic performance of hydrothermally aged Cu-SSZ-13 for the NH3-SCR of NO in simulated diesel exhaust. Appl. Surf. Sci. 2017, 419, 382–392. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Li, J. Design and synthesis of core-shell structured meso-Cu-SSZ-13@mesoporous aluminosilicate catalyst for SCR of NOx with NH3: Enhancement of activity, hydrothermal stability and propene poisoning resistance. Appl. Catal. B Environ. 2016, 195, 48–58. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollár, M.; Szanyi, J.; Peden, C.H.F. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. Catal. Today 2014, 231, 64–74. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Li, J.; Kamasamudram, K.; Epling, W.S. NH3-SCR over Cu/SAPO-34–Zeolite acidity and Cu structure changes as a function of Cu loading. Catal. Today 2014, 231, 64–74. [Google Scholar] [CrossRef]
- Szanyi, J.; Kwak, J.H.; Zhu, H.; Peden, C.H. Characterization of Cu-SSZ-13 NH3 SCR catalysts: an in situ FTIR study. Phys. Chem. Chem. Phys. 2013, 15, 2368–2380. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, T.; Wang, X.; Qi, G.; Xue, J.; Shen, M.; Li, W. The influence of silicon on the catalytic properties of Cu/SAPO-34 for NOx reduction by ammonia-SCR. Appl. Catal. B Environ. 2012, 127, 137–147. [Google Scholar] [CrossRef]
- Onida, B.; Gabelica, Z.; Lourenco, J.; Garrone, E. Spectroscopic characterization of hydroxyl groups in SAPO-40. 1. Study of the template-free samples and their interaction with ammonia. J. Phys. Chem. C 1996, 100, 11072–11079. [Google Scholar] [CrossRef]
- Zhu, H.; Kwak, J.H.; Peden, C.H.F.; Szanyi, J. In situ DRIFTS-MS studies on the oxidation of adsorbed NH3 by NOx over a Cu-SSZ-13 zeolite. Catal. Today 2013, 205, 16–23. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Qi, G.; Wen, D. Location and nature of Cu species in Cu/SAPO-34 for selective catalytic reduction of NO with NH3. J. Catal. 2012, 289, 21–29. [Google Scholar] [CrossRef]
- Sommer, L.; Mores, D.; Svelle, S.; Stöcker, M.; Weckhuysen, B.M.; Olsbye, U. Mesopore formation in zeolite H-SSZ-13 by desilication with NaOH. Microporous Mesoporous Mater. 2010, 132, 384–394. [Google Scholar] [CrossRef]
- Li, Y.; Deng, J.; Song, W.; Liu, J.; Zhao, Z.; Gao, M.; Wei, Y.; Zhao, L. Nature of Cu species in Cu-SAPO-18 catalyst for NH3-SCR:combination of experiments and DFT calculations. J. Phys. Chem. C 2016, 120, 14669–14680. [Google Scholar] [CrossRef]
- Hun, K.; Zhu, H.; Lee, J.; Peden, C.; Szanyi, J. Two different cationic positions in Cu-SSZ-13? Chem. Commun. 2012, 48, 4758–4760. [Google Scholar]

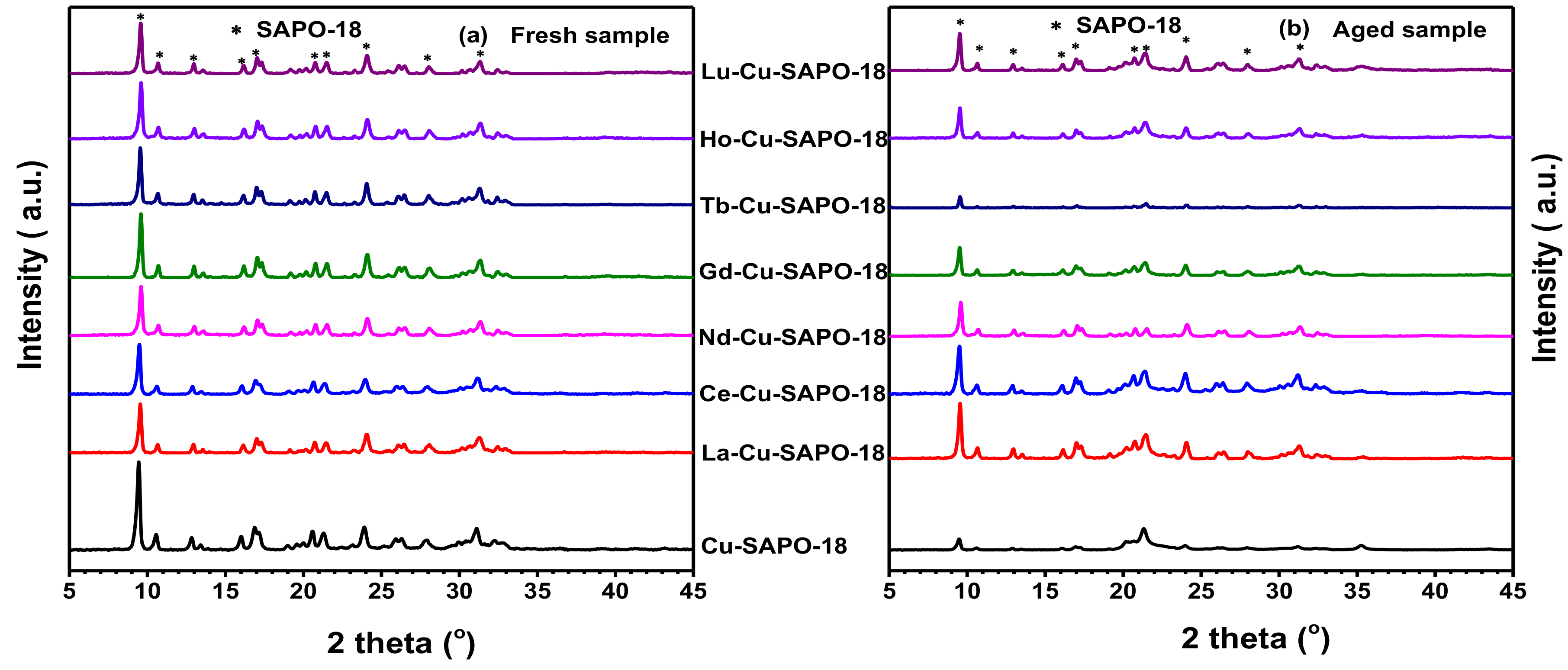
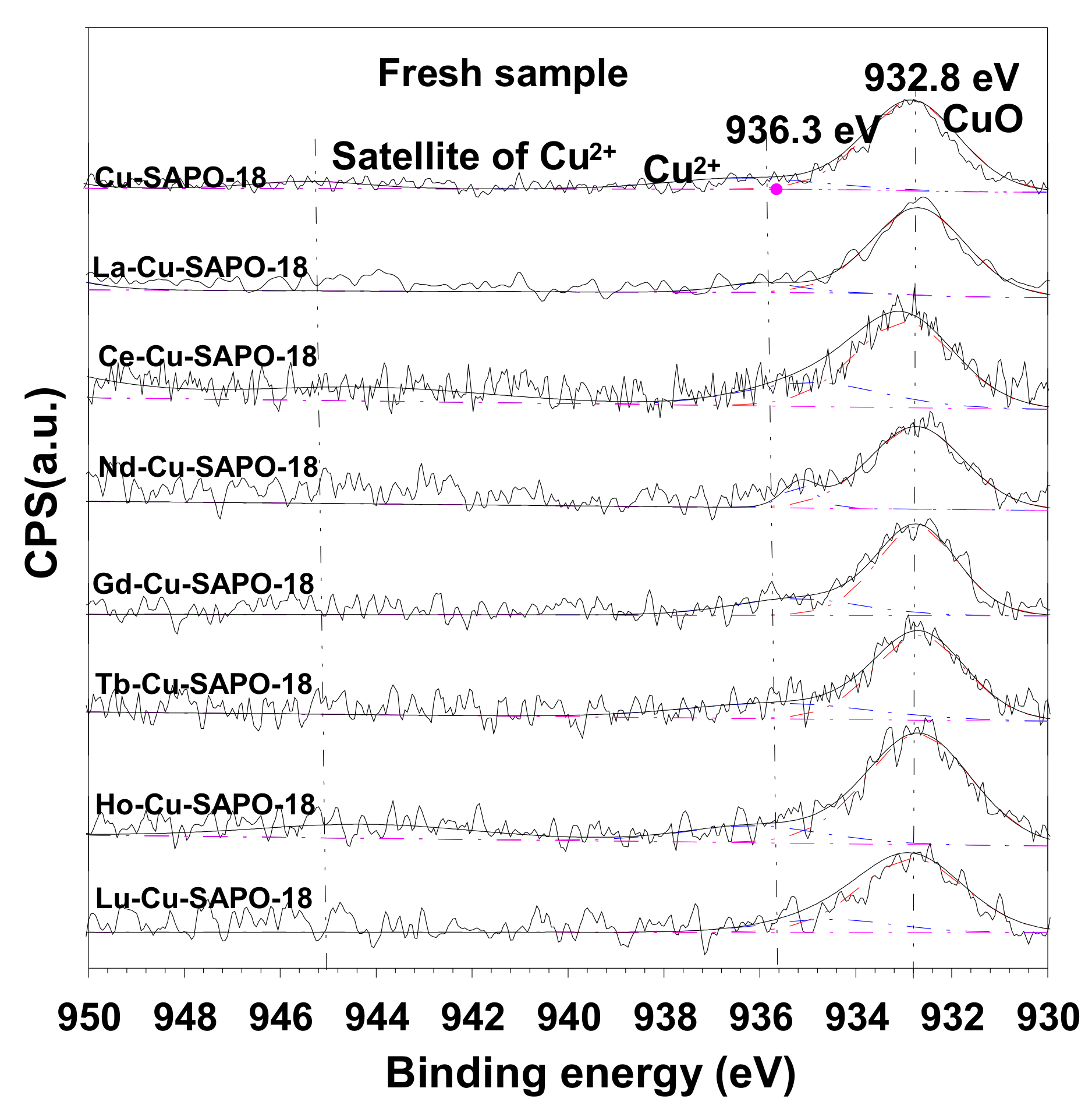
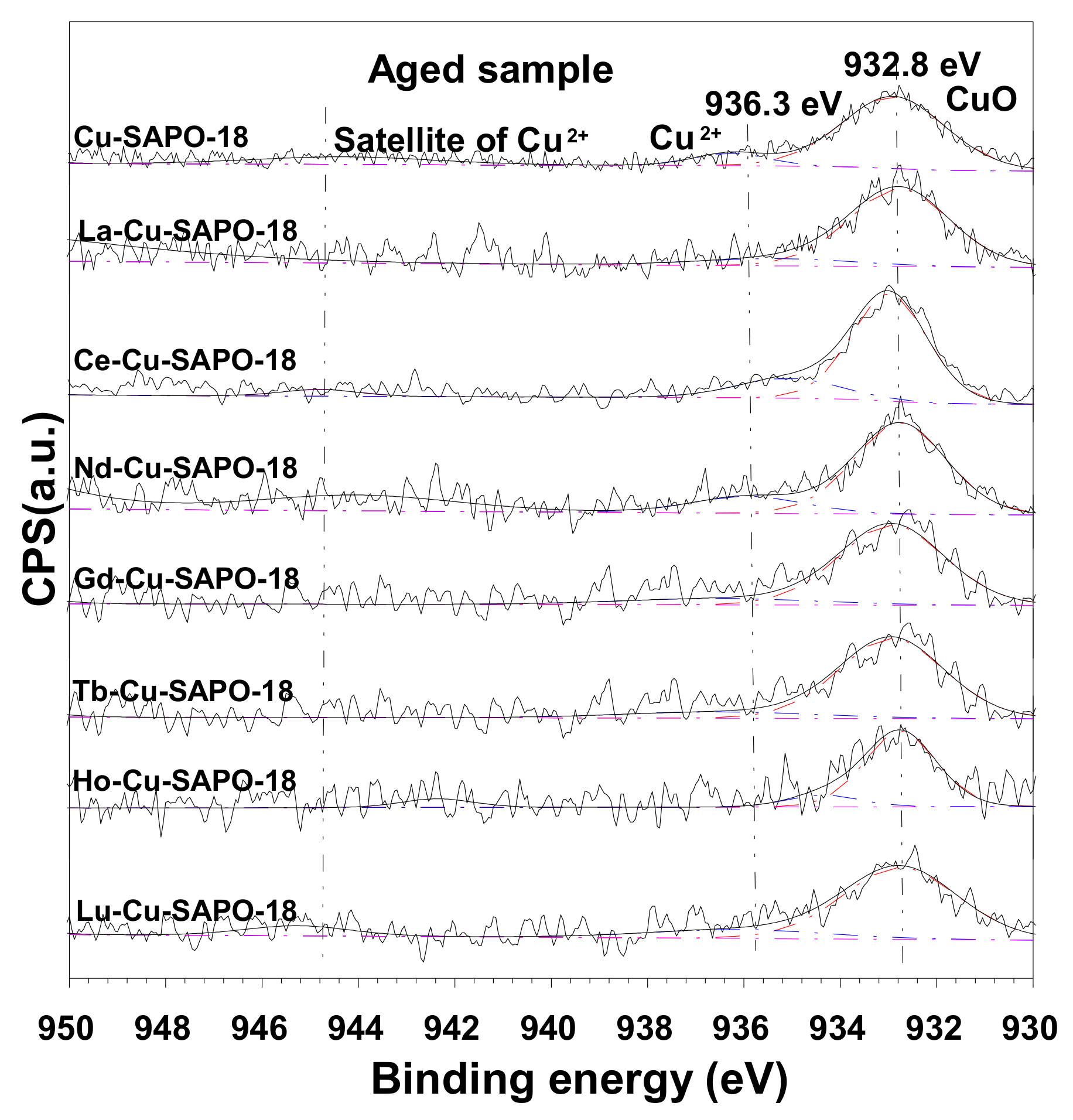
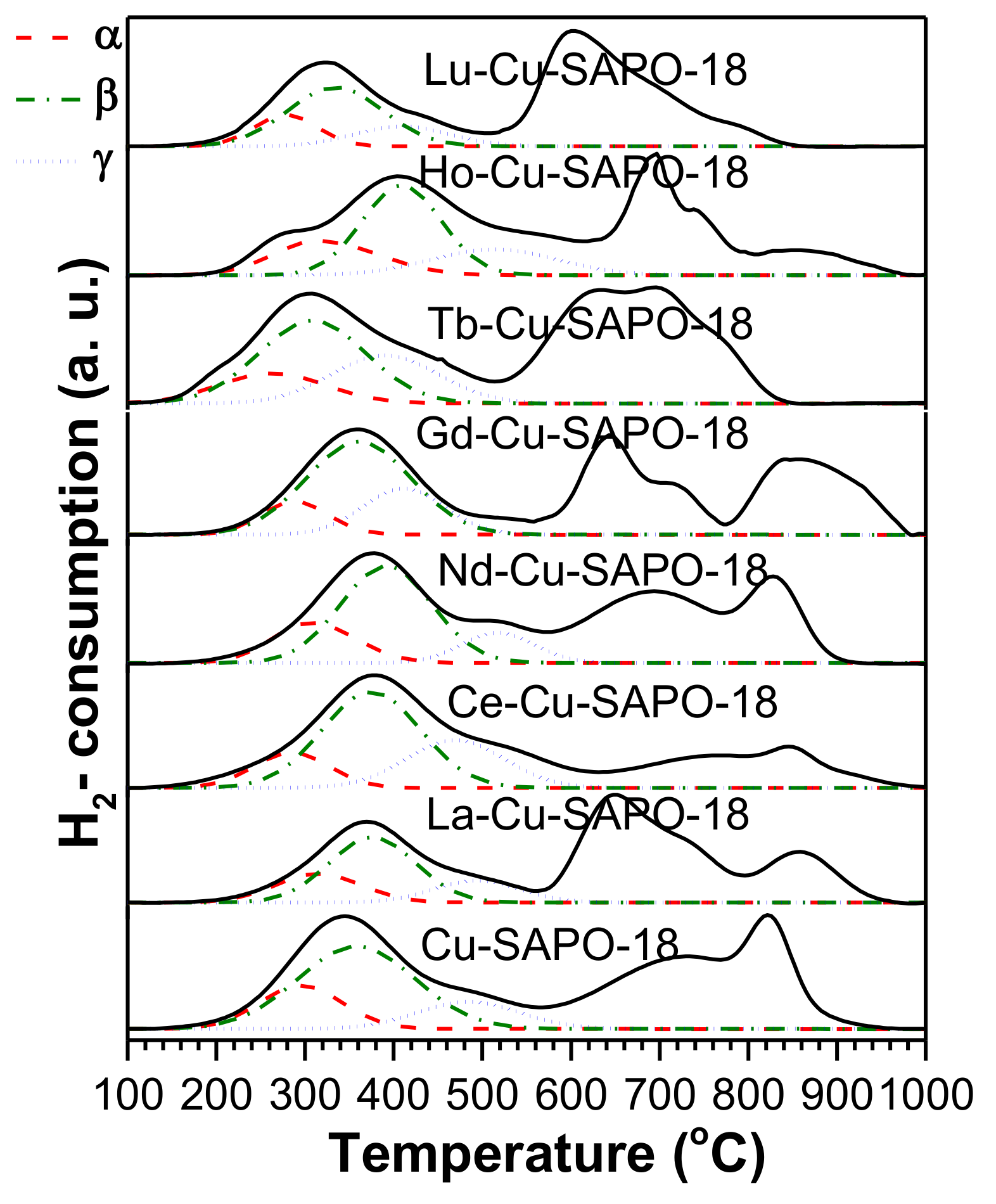

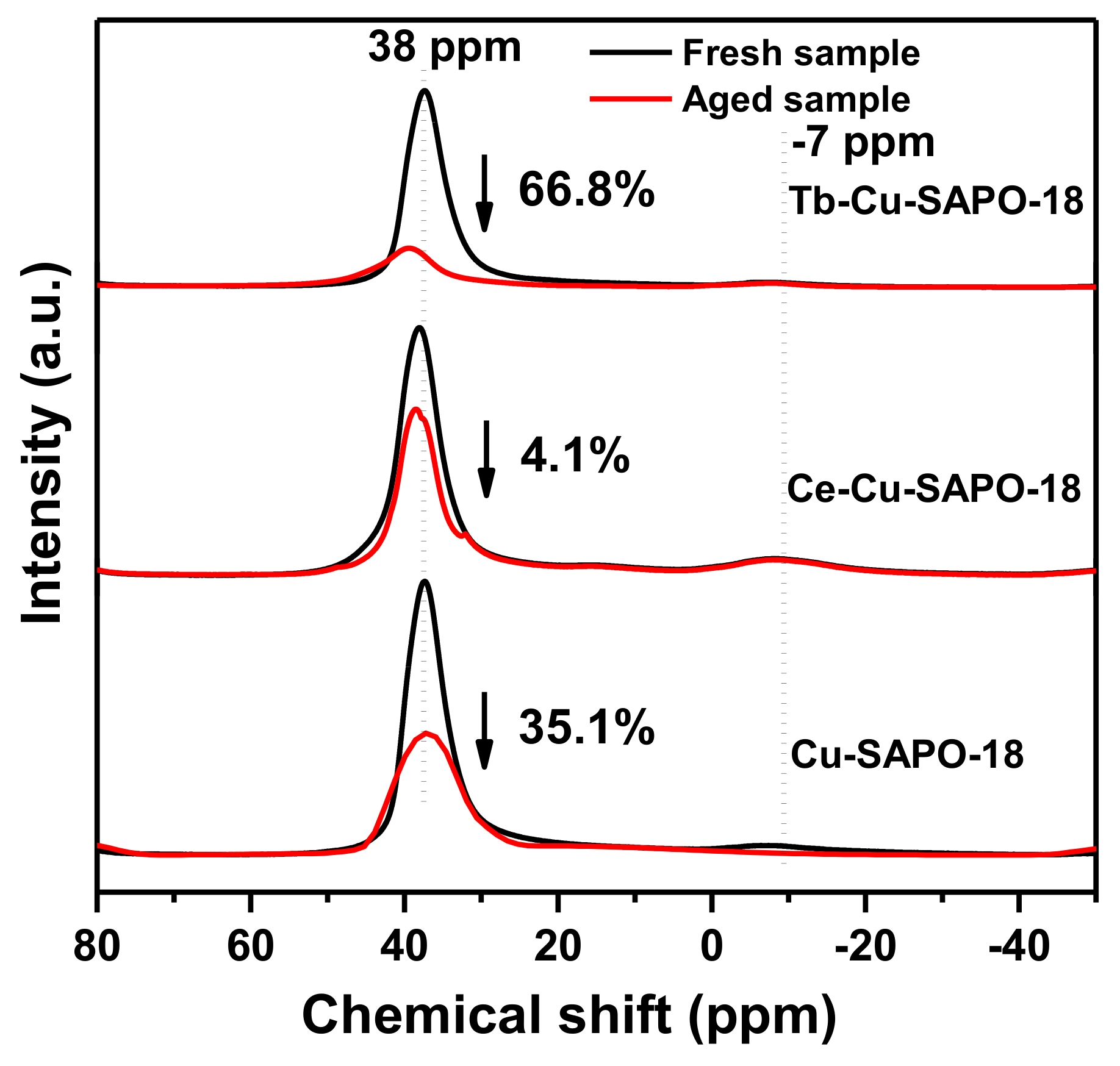
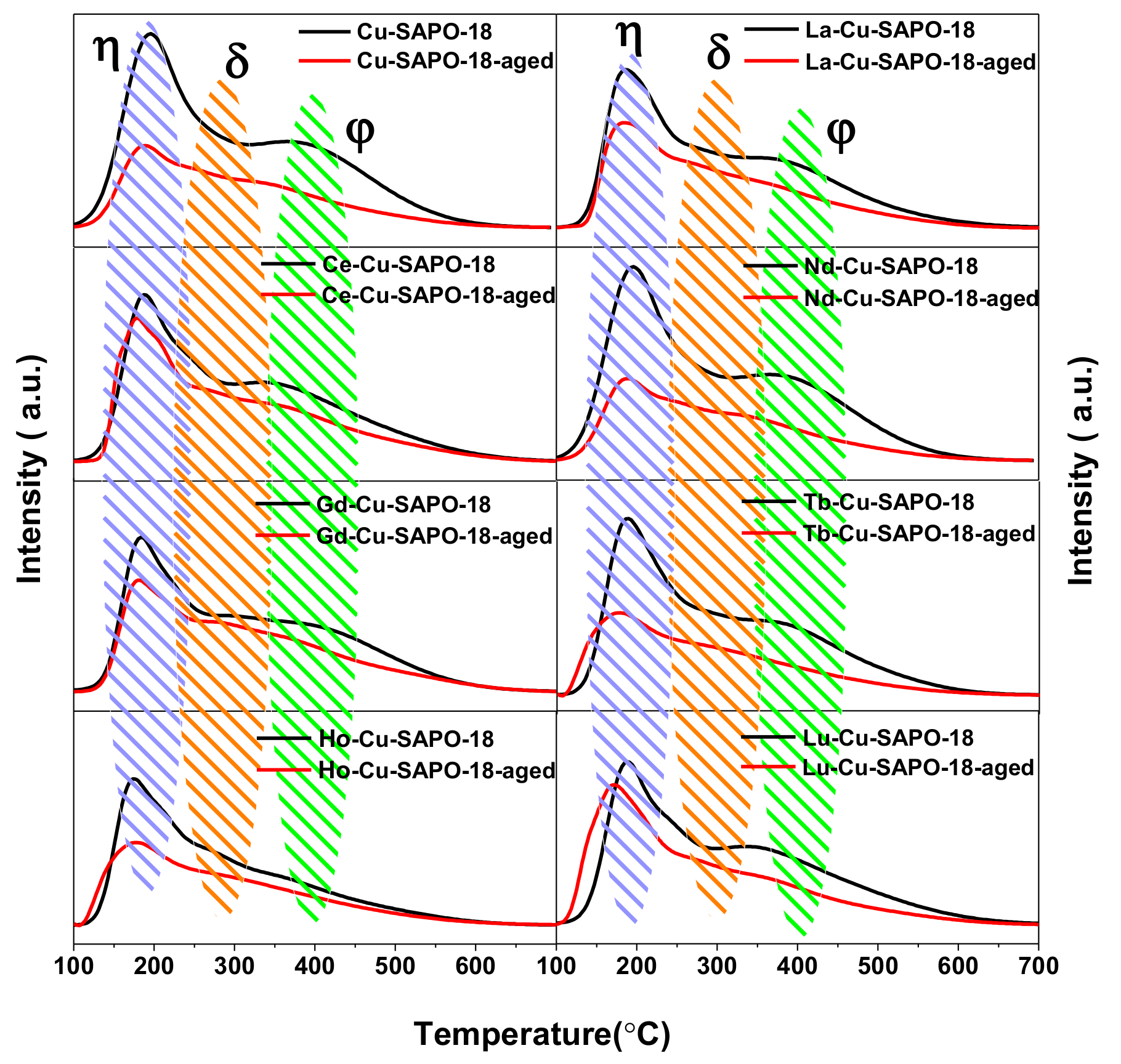
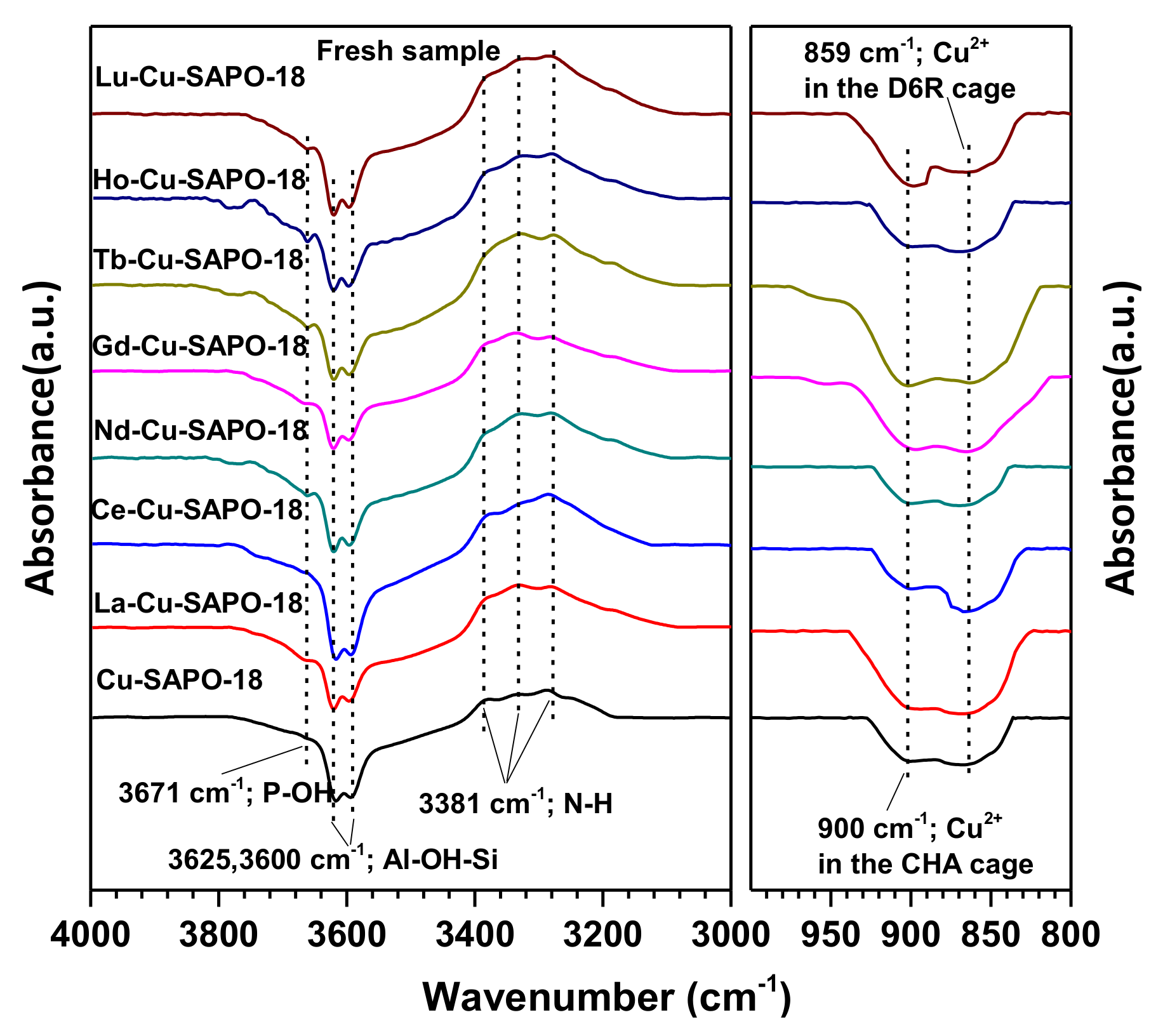

| Sample | Cu (wt %) | Lanthanide (wt %) | Al (wt %) | P (wt %) | Si (wt %) | SBET (m2/g) | Micropore Volume (cm3/g) |
|---|---|---|---|---|---|---|---|
| Cu-SAPO-18 | 1.76 | - | 31.57 | 53.19 | 10.63 | 597 | 0.234 |
| Cu-SAPO-18-aged | 1.76 | - | 31.57 | 53.19 | 10.63 | 304 | 0.129 |
| La-Cu-SAPO-18 | 1.69 | 1.27 | 30.80 | 53.62 | 8.95 | 563 | 0.229 |
| La-Cu-SAPO-18-aged | 1.69 | 1.27 | 30.80 | 53.62 | 8.95 | 428 | 0.178 |
| Ce-Cu-SAPO-18 | 1.71 | 1.24 | 29.62 | 53.94 | 10.56 | 579 | 0.233 |
| Ce-Cu-SAPO-18-aged | 1.71 | 1.24 | 29.62 | 53.94 | 10.56 | 510 | 0.198 |
| Nd-Cu-SAPO-18 | 1.68 | 1.35 | 30.05 | 52.80 | 8.93 | 559 | 0.228 |
| Nd-Cu-SAPO-18-aged | 1.68 | 1.35 | 30.05 | 52.80 | 8.93 | 421 | 0.177 |
| Gd-Cu-SAPO-18 | 1.57 | 1.36 | 29.76 | 51.56 | 9.29 | 561 | 0.229 |
| Gd-Cu-SAPO-18-aged | 1.57 | 1.36 | 29.76 | 51.56 | 9.29 | 366 | 0.144 |
| Tb-Cu-SAPO-18 | 1.65 | 1.18 | 29.52 | 52.16 | 8.80 | 569 | 0.230 |
| Tb-Cu-SAPO-18-aged | 1.65 | 1.18 | 29.52 | 52.16 | 8.80 | 358 | 0.142 |
| Ho-Cu-SAPO-18 | 1.55 | 1.18 | 29.92 | 51.58 | 8.42 | 556 | 0.228 |
| Ho-Cu-SAPO-18-aged | 1.55 | 1.31 | 29.92 | 51.58 | 8.42 | 410 | 0.174 |
| Lu-Cu-SAPO-18 | 1.69 | 1.28 | 27.81 | 48.47 | 9.03 | 543 | 0.224 |
| Lu-Cu-SAPO-18-aged | 1.69 | 1.28 | 27.81 | 48.47 | 9.03 | 415 | 0.175 |
| Sample | Isolated Cu2+ (mmol/g) | CuO (mmol/g) | Cusurf (mmol/g) | Cu2+/Cusurf Molar Ratio |
|---|---|---|---|---|
| Cu-SAPO-18 | 0.0248 | 0.0900 | 0.1044 | 0.238 |
| Cu-SAPO-18-aged | 0.0131 | 0.0670 | 0.0812 | 0.161 |
| La-Cu-SAPO-18 | 0.0456 | 0.1360 | 0.1651 | 0.276 |
| La-Cu-SAPO-18-aged | 0.0390 | 0.1950 | 0.2230 | 0.175 |
| Ce-Cu-SAPO-18 | 0.0841 | 0.1685 | 0.2525 | 0.333 |
| Ce-Cu-SAPO-18-aged | 0.0674 | 0.1778 | 0.2452 | 0.275 |
| Nd-Cu-SAPO-18 | 0.0460 | 0.1740 | 0.2000 | 0.230 |
| Nd-Cu-SAPO-18-aged | 0.0325 | 0.1489 | 0.1828 | 0.178 |
| Gd-Cu-SAPO-18 | 0.0520 | 0.1444 | 0.1870 | 0.278 |
| Gd-Cu-SAPO-18-aged | 0.0401 | 0.1892 | 0.2169 | 0.185 |
| Tb-Cu-SAPO-18 | 0.0638 | 0.1564 | 0.2142 | 0.298 |
| Tb-Cu-SAPO-18-aged | 0.0273 | 0.1880 | 0.2167 | 0.126 |
| Ho-Cu-SAPO-18 | 0.0563 | 0.1994 | 0.2394 | 0.235 |
| Ho-Cu-SAPO-18-aged | 0.0228 | 0.1540 | 0.1783 | 0.128 |
| Lu-Cu-SAPO-18 | 0.0414 | 0.1690 | 0.1840 | 0.225 |
| Lu-Cu-SAPO-18-aged | 0.0249 | 0.1586 | 0.1890 | 0.132 |
| Sample | Isolated Cu2+ (μmol/g) | Total Cu2+ (μmol/g) | CuO (μmol/g) | |
|---|---|---|---|---|
| 8MRs | D6R | |||
| Cu-SAPO-18 | 51.1 | 47.2 | 98.3 | 116.3 |
| Cu-SAPO-18-aged | 33.9 | 36.7 | 70.6 | 156.9 |
| La-Cu-SAPO-18 | 46.8 | 53.1 | 99.9 | 112.4 |
| La-Cu-SAPO-18-aged | 39.4 | 42.2 | 81.6 | 140.8 |
| Ce-Cu-SAPO-18 | 41.1 | 67.6 | 108.7 | 93.0 |
| Ce-Cu-SAPO-18-aged | 38.0 | 65.5 | 103.5 | 100.5 |
| Nd-Cu-SAPO-18 | 46.2 | 50.4 | 96.6 | 115.1 |
| Nd-Cu-SAPO-18-aged | 41.7 | 39.4 | 81.1 | 136.1 |
| Gd-Cu-SAPO-18 | 34.5 | 66.1 | 100.6 | 105.1 |
| Gd-Cu-SAPO-18-aged | 33.0 | 52.7 | 85.7 | 140.0 |
| Tb-Cu-SAPO-18 | 60.3 | 42.2 | 102.5 | 103.8 |
| Tb-Cu-SAPO-18-aged | 26.4 | 23.5 | 49.9 | 176.4 |
| Ho-Cu-SAPO-18 | 55.0 | 43.0 | 98 | 106.5 |
| Ho-Cu-SAPO-18-aged | 32.7 | 35.5 | 68.2 | 166.7 |
| Lu-Cu-SAPO-18 | 47.8 | 33.6 | 81.4 | 126.9 |
| Lu-Cu-SAPO-18-aged | 28.2 | 19.9 | 48.1 | 148.1 |
| Sample | Acidity (mmol/g) | Amount (mmol/g) | ||
|---|---|---|---|---|
| Weak | Moderate | Strong | ||
| Cu-SAPO-18 | 0.204 | 0.101 | 0.329 | 0.634 |
| Cu-SAPO-18-aged | 0.071 | 0.086 | 0.224 | 0.381 |
| La-Cu-SAPO-18 | 0.082 | 0.085 | 0.322 | 0.489 |
| La-Cu-SAPO-18-aged | 0.070 | 0.074 | 0.289 | 0.443 |
| Ce-Cu-SAPO-18 | 0.107 | 0.159 | 0.318 | 0.584 |
| Ce-Cu-SAPO-18-aged | 0.143 | 0.091 | 0.301 | 0.535 |
| Nd-Cu-SAPO-18 | 0.097 | 0.090 | 0.280 | 0.467 |
| Nd-Cu-SAPO-18-aged | 0.079 | 0.065 | 0.242 | 0.386 |
| Gd-Cu-SAPO-18 | 0.084 | 0.089 | 0.326 | 0.499 |
| Gd-Cu-SAPO-18-aged | 0.073 | 0.086 | 0.299 | 0.458 |
| Tb-Cu-SAPO-18 | 0.104 | 0.103 | 0.301 | 0.508 |
| Tb-Cu-SAPO-18-aged | 0.081 | 0.074 | 0.199 | 0.354 |
| Ho-Cu-SAPO-18 | 0.091 | 0.092 | 0.293 | 0.476 |
| Ho-Cu-SAPO-18-aged | 0.080 | 0.084 | 0.211 | 0.375 |
| Lu-Cu-SAPO-18 | 0.078 | 0.085 | 0.310 | 0.473 |
| Lu-Cu-SAPO-18-aged | 0.075 | 0.081 | 0.192 | 0.348 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Han, S.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effects of Lanthanide Doping on the Catalytic Activity and Hydrothermal Stability of Cu-SAPO-18 for the Catalytic Removal of NOx (NH3-SCR) from Diesel Engines. Catalysts 2020, 10, 336. https://doi.org/10.3390/catal10030336
Gao Q, Han S, Ye Q, Cheng S, Kang T, Dai H. Effects of Lanthanide Doping on the Catalytic Activity and Hydrothermal Stability of Cu-SAPO-18 for the Catalytic Removal of NOx (NH3-SCR) from Diesel Engines. Catalysts. 2020; 10(3):336. https://doi.org/10.3390/catal10030336
Chicago/Turabian StyleGao, Qi, Shuai Han, Qing Ye, Shuiyuan Cheng, Tianfang Kang, and Hongxing Dai. 2020. "Effects of Lanthanide Doping on the Catalytic Activity and Hydrothermal Stability of Cu-SAPO-18 for the Catalytic Removal of NOx (NH3-SCR) from Diesel Engines" Catalysts 10, no. 3: 336. https://doi.org/10.3390/catal10030336
APA StyleGao, Q., Han, S., Ye, Q., Cheng, S., Kang, T., & Dai, H. (2020). Effects of Lanthanide Doping on the Catalytic Activity and Hydrothermal Stability of Cu-SAPO-18 for the Catalytic Removal of NOx (NH3-SCR) from Diesel Engines. Catalysts, 10(3), 336. https://doi.org/10.3390/catal10030336