Copper-Iron Bimetal Ion-Exchanged SAPO-34 for NH3-SCR of NOx
Abstract
:1. Introduction
- The catalyst’s properties of synthesized samples to clarify the influence of Cu, Fe, and co-doping Cu-Fe supported on SAPO-34.
- Additionally, the catalytic performance of the NH3-SCR reaction, as well as the hydrothermal durability of the catalysts, was tested in this study.
2. Results and Discussion
2.1. Structure and Texture of Catalysts
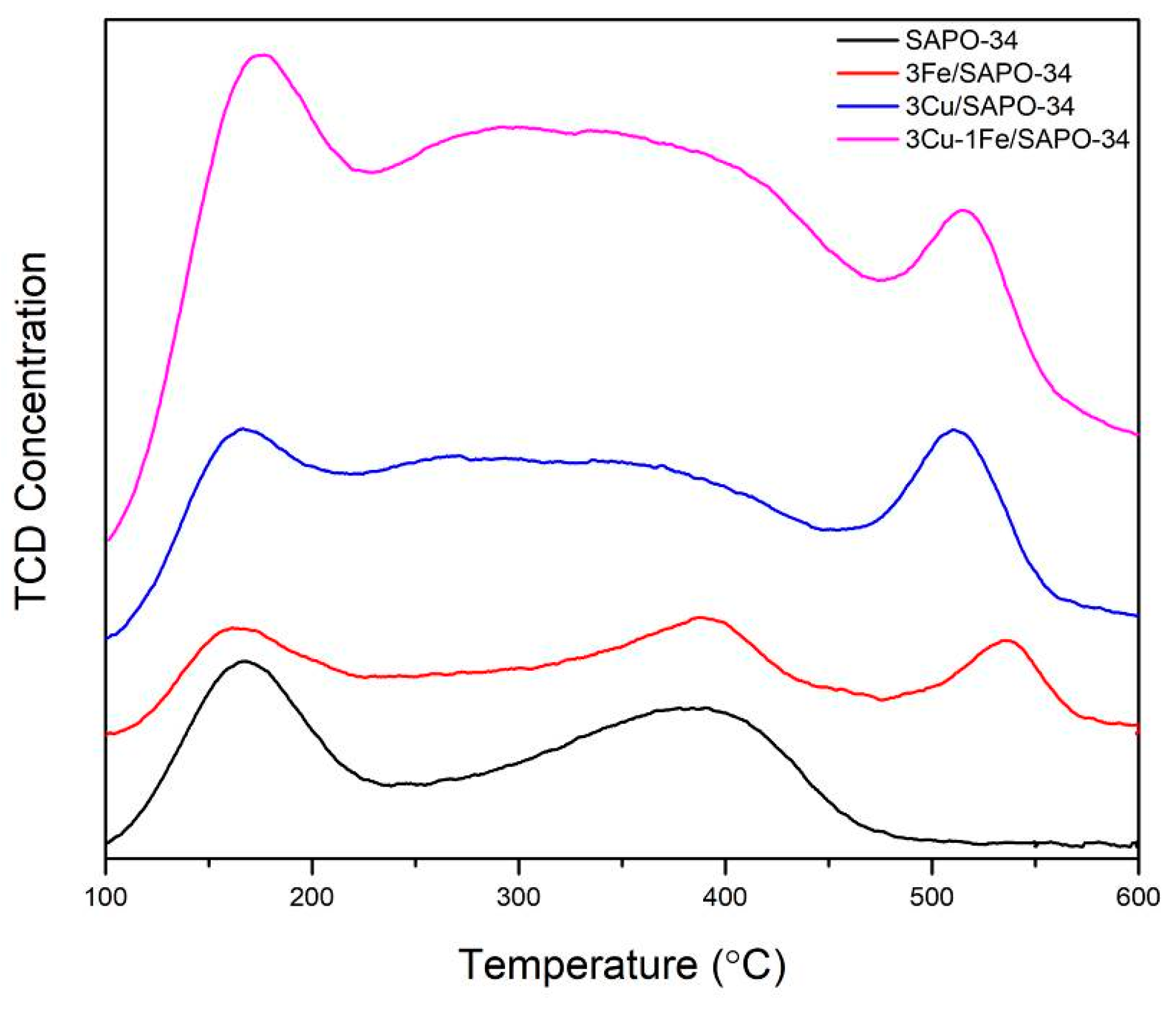
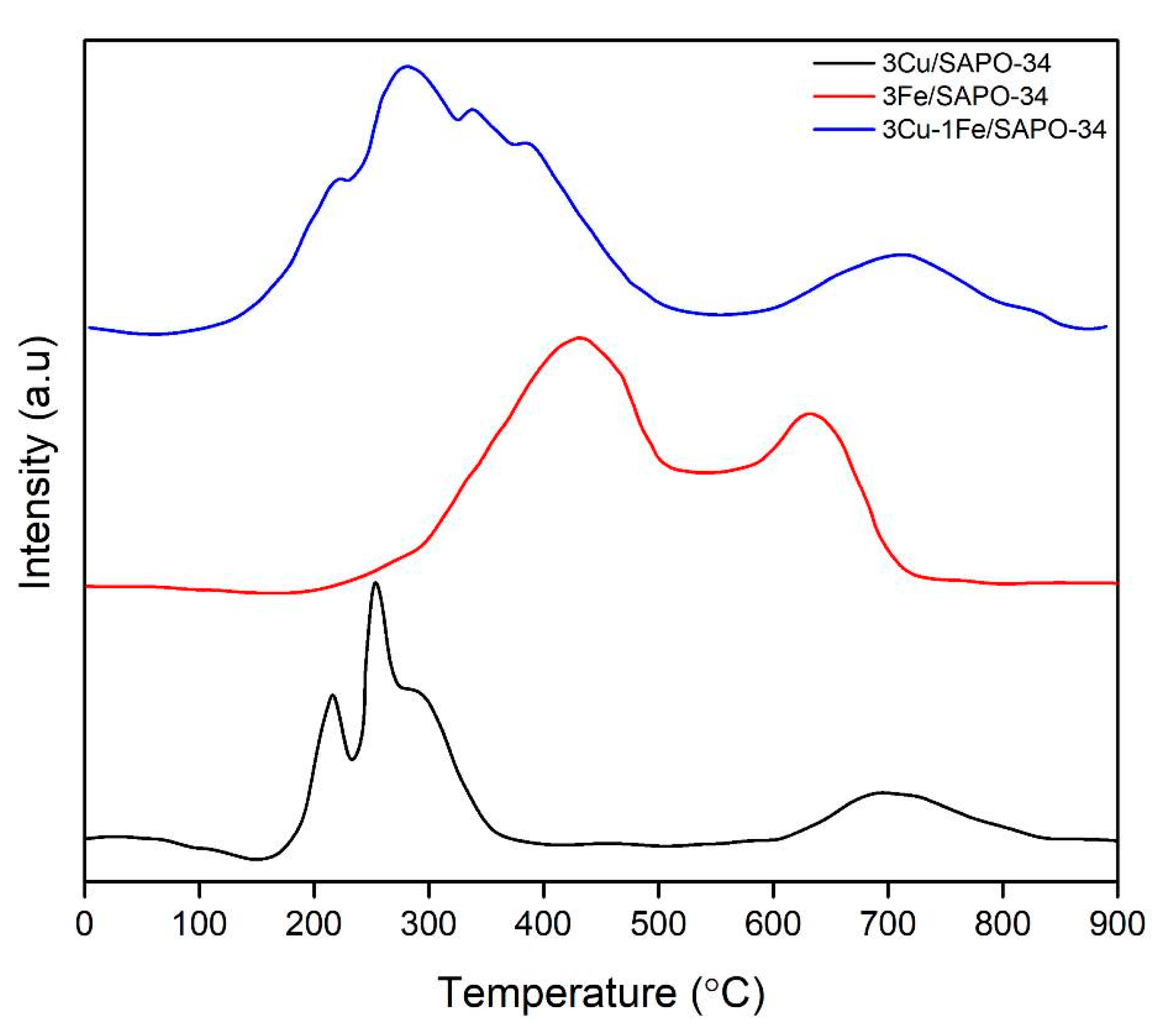
2.2. Chemisorption Results
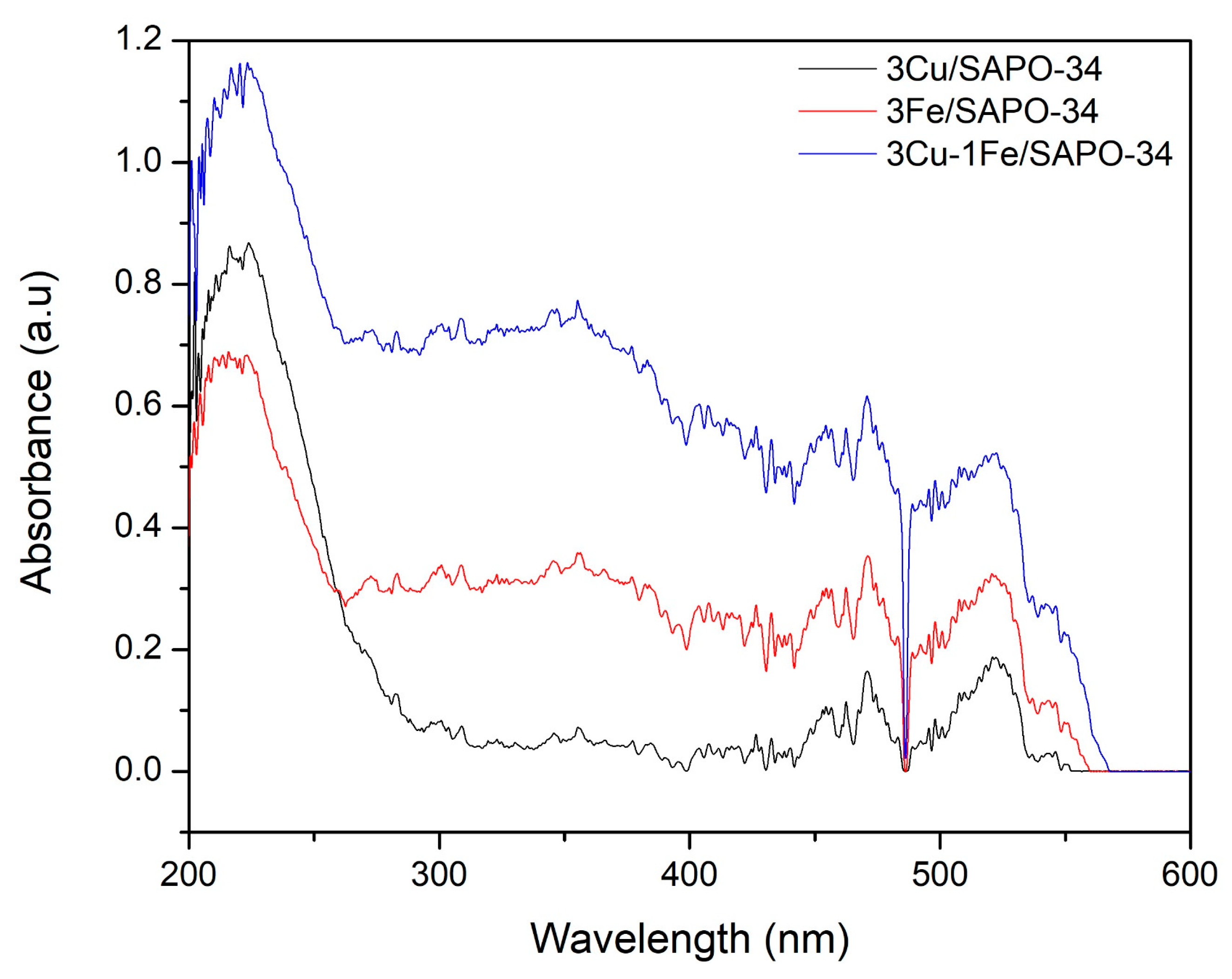
2.3. Cu and Fe Species onto SAPO-34
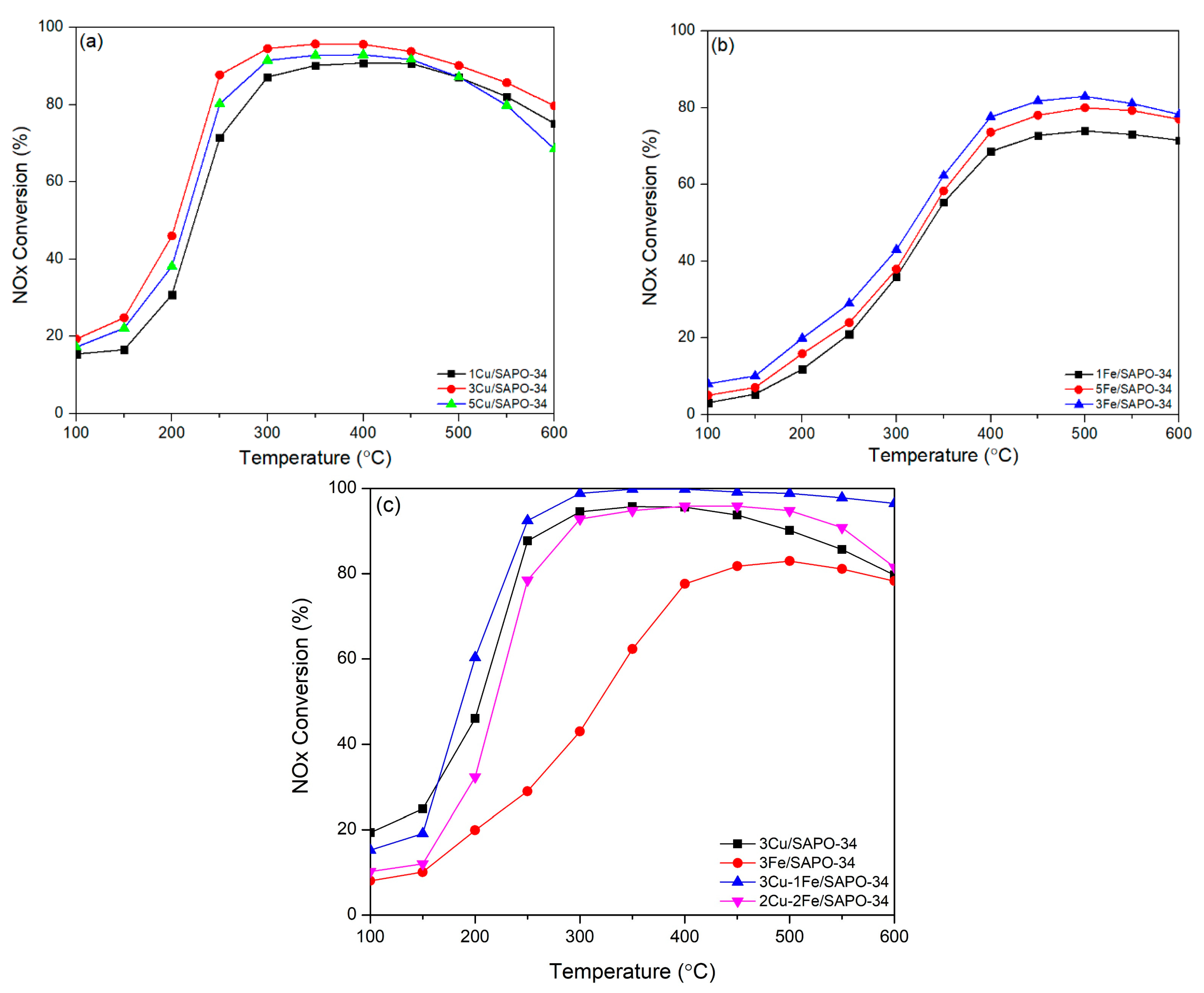
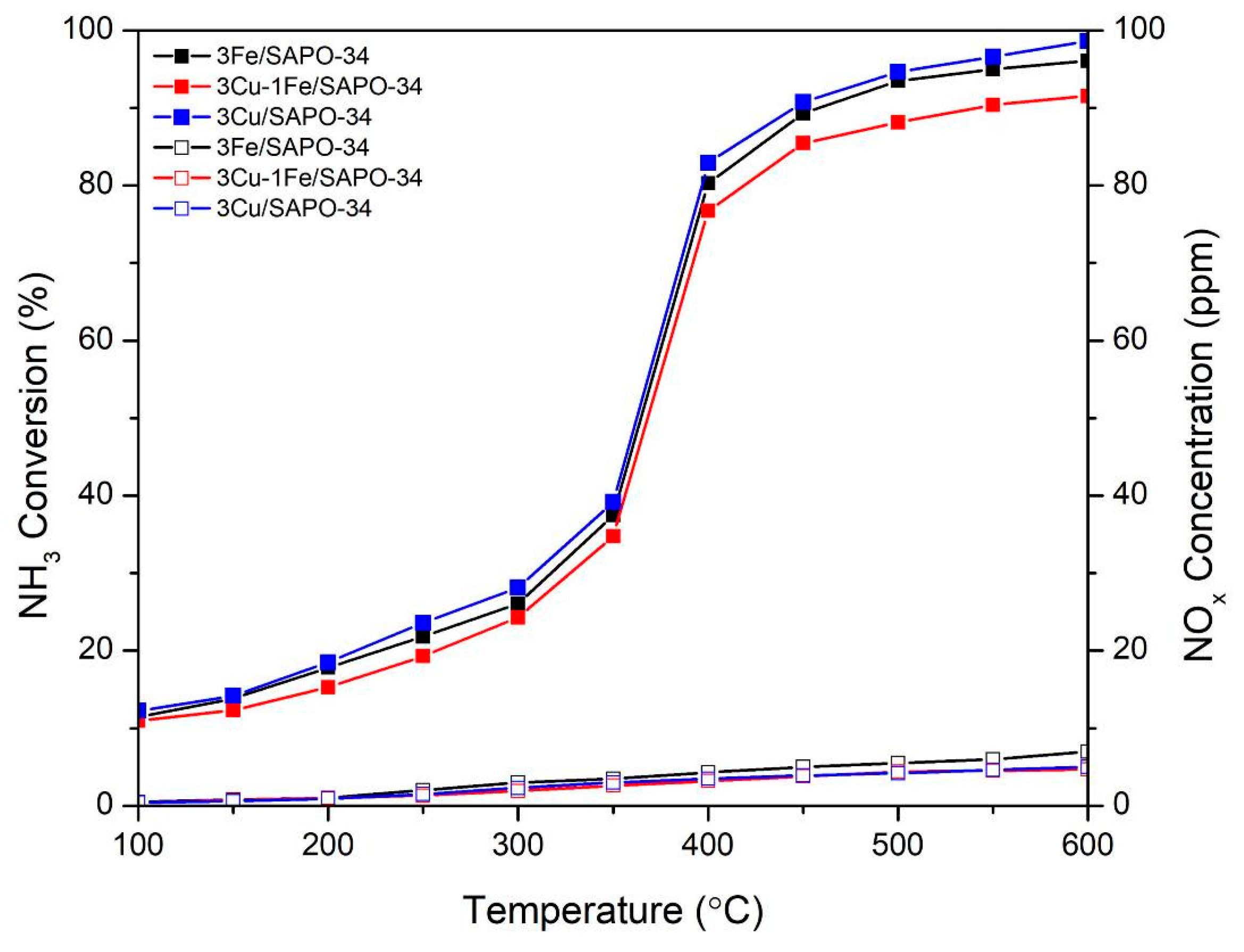
2.4. Catalyst Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Chen, H.; Hu, Z.; Yao, M.; Li, Y. A Review on the Pd-Based Three-Way Catalyst. Catal. Rev. 2015, 57, 79–144. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Sher, E. Handbook of Air Pollution from Internal Combustion Engines: Pollutant Formation and Control; Academic Press: Cambridge, MA, USA, 1998; ISBN 0-12-6398554-0. [Google Scholar]
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar] [CrossRef]
- Wang, T.J.; Baek, S.W.; Kwon, H.J.; Kim, Y.J.; Nam, I.-S.; Cha, M.-S.; Yeo, G.K. Kinetic Parameter Estimation of a Commercial Fe-Zeolite SCR. Ind. Eng. Chem. Res. 2011, 50, 2850–2864. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx Abatement Systems for Mobile Sources: From Three-Way to Lean Burn after-Treatment Technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Zhao, H.; Li, Y. The promotion effect of CeOx on Cu-SAPO-34 catalyst for selective catalytic reduction of NOx with ammonia. Catal. Today 2015, 258, 28–34. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.L.; Kollar, M.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. A comparative kinetics study between Cu/SSZ-13 and Fe/SSZ-13 SCR catalysts. Catal. Today 2015, 258, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Zones, S.I. Zeolite SSZ-13 and Its Method of Preparation. U.S. Patent 4544538, 1 October 1985. [Google Scholar]
- Lok, B.M.M.; Patton, C.A.; Gajek, R.L.; Cannan, R.T.; Flanigen, T.R.; Flanigen, E.M. Crystalline Silicoaluminophosphates. U.S. Patent 4440871, 3 April 1984. [Google Scholar]
- Andersen, P.J.; Bailie, J.E.; Casci, J.L.; Chen, H.Y.; Fedeyko, J.M.; Foo, R.K.S.; Rajaram, R.R. Transition Metal/Zeolite SCR Catalysts. International Patent WO/2008/132452, 6 November 2008. [Google Scholar]
- Bull, I.; Xue, W.M.; Burk, P.; Boorse, R.S.; Jaglowski, W.M.; Koermer, G.S.; Moini, A.; Patchett, A.; Dettling, J.C.; Caudle, M.T. Copper CHA Zeolite Catalysts. U.S. Patent US7601662B2 B2, 13 October 2009. [Google Scholar]
- Schill, L.; Putluru, S.S.R.; Jensen, A.D.; Fehrmann, R. MnFe/Al2O3 Catalyst Synthesized by Deposition Precipitation for Low-Temperature Selective Catalytic Reduction of NO with NH3. Catal. Lett. 2015, 145, 1724–1732. [Google Scholar] [CrossRef]
- Qi, K.; Xie, J.L.; Fang, D.; Li, F.X.; He, F. Performance enhancement mechanism of Mn-based catalysts prepared under N2 for NOx removal: Evidence of the poor crystallization and oxidation of MnOx. Chin. J. Catal. 2017, 38, 845–852. [Google Scholar] [CrossRef]
- Andonova, S.; Tamm, S.; Montreuil, C.; Lambert, C.; Olsson, L. The effect of iron loading and hydrothermal aging on one-pot synthesized Fe/SAPO-34 for ammonia SCR. Appl. Catal. B Environ. 2016, 180, 775–787. [Google Scholar] [CrossRef]
- Park, J.H.; Park, H.J.; Baik, J.H.; Nam, I.-S.; Shin, C.H.; Lee, J.-H.; Cho, B.K.; Oh, S.H. Hydrothermal stability of CuZSM5 catalyst in reducing NO by NH3 for the urea selective catalytic reduction process. J. Catal. 2006, 240, 47–57. [Google Scholar] [CrossRef]
- Fan, F.; Sun, K.; Feng, Z.; Xia, H.; Han, B.; Lian, Y.; Ying, P.; Li, C. From Molecular Fragments to Crystals: A UV Raman Spectroscopic Study on the Mechanism of Fe-ZSM-5 Synthesis. Chem. Eur. J. 2009, 15, 3268–3276. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, F.; Shan, W.; He, H. Chin Hydrothermal Deactivation of Fe-ZSM-5 Prepared by Different Methods for the Selective Catalytic Reduction of NOx with NH3. J. Catal. 2012, 33, 454–464. [Google Scholar]
- Metkar, P.S.; Harold, M.P.; Balakotaiah, V. Experimental and kinetic modeling study of NH3-SCR of NOx on Fe-ZSM-5, Cu-chabazite and combined Fe- and Cu-zeolite monolithic catalysts. Chem. Eng. Sci. 2013, 87, 51–66. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Liu, J.; Wang, D.; Zhao, Z.; Cheng, K.; Li, J. High activity and wide temperature window of Fe-Cu-SSZ-13 in the selective catalytic reduction of NO with ammonia. AIChE J. 2015, 61, 3825–3837. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Z.; Moses-Debusk, M.; Mullins, D.R.; Mahurin, S.M.; Geiger, R.A.; Kidder, M.; Narula, C.K. Heterometal Incorporation in Metal-Exchanged Zeolites Enables Low-Temperature Catalytic Activity of NOx Reduction. J. Phys. Chem. C 2012, 116, 23322–23331. [Google Scholar] [CrossRef]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, L.F.; Yang, R.T. Activity, propene poisoning resistance and hydrothermal stability of copper exchanged chabazite-like zeolite catalysts for SCR of NO with ammonia in comparison to Cu/ZSM-5. Appl. Catal. A 2012, 428, 24–34. [Google Scholar] [CrossRef]
- Wang, D.; Jangjou, Y.; Liu, Y.; Sharma, M.K.; Luo, J.; Li, J.; Kamasamudram, K.; Epling, W.S. A comparison of hydrothermal aging effects on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B Environ. 2015, 165, 438–445. [Google Scholar] [CrossRef]
- Bordiga, S.; Regli, L.; Lamberti, C.; Zecchina, A.; Bjørgen, M.; Lillerud, K.P. FTIR Adsorption Studies of H2O and CH3OH in the Isostructural H-SSZ-13 and H-SAPO-34: Formation of H-Bonded Adducts and Protonated Clusters. J. Phys. Chem. B 2005, 109, 7724–7732. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. Synthesis and evaluation of Cu/SAPO-34 catalysts for NH3-SCR 2: Solid-state ion exchange and one-pot synthesis. Appl. Catal. B Environ. 2015, 162, 501–514. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Wang, J.; Huang, Y.; Shen, M.; Li, W.; Wang, J. NH3 Oxidation Mechanism over Cu/SAPO-34 Catalysts Prepared by Different Methods. ChemCatChem 2014, 6, 2074–2083. [Google Scholar] [CrossRef]
- Doan, T.; Nguyen, K.; Dam, P.; Vuong, T.H.; Le, M.T.; Thanh, H.P. Synthesis of SAPO-34 Using Different Combinations of Organic Structure-Directing Agents. J. Chem. 2019, 2019, 6197527. [Google Scholar] [CrossRef] [Green Version]
- Lok, B.M.; Messina, C.A.; Patton, R.L.; Gajek, R.T.; Cannan, T.R.; Flanigen, E.M. Silicoaluminophosphate molecular sieves: Another new class of microporous crystalline inorganic solids. J. Amer. Chem. Soc. 1984, 106, 6092–6093. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Qi, G.; Wang, D. Location and nature of Cu species in Cu/SAPO-34 for selective catalytic reduction of NO with NH3. J. Catal. 2012, 289, 21–29. [Google Scholar] [CrossRef]
- Yu, T.; Hao, T.; Fan, D.Q.; Wang, J.; Shen, M.Q.; Li, W. Recent NH3-SCR Mechanism Research over Cu/SAPO-34 Catalyst. J. Phys. Chem. C. 2014, 118, 6565–6575. [Google Scholar] [CrossRef]
- Zhang, N.; Xin, Y.; Li, Q.; Ma, X.; Qi, Y.; Zheng, L.; Zhang, Z. Ion Exchange of One-Pot Synthesized Cu-SAPO-44 with NH4NO3 to Promote Cu Dispersion and Activity for Selective Catalytic Reduction of NOx with NH3. Catalysts 2019, 9, 882. [Google Scholar] [CrossRef] [Green Version]
- Parlitz, B.; Schreier, E.; Zubowa, H.L.; Eckelt, R.; Lieske, E.; Lischke, G.; Fricke, R. Isomerization of n-Heptane over Pd-Loaded Silico-Alumino-Phosphate Molecular Sieves. J. Catal. 1995, 155, 1–11. [Google Scholar] [CrossRef]
- Niwa, M.; Katada, N.; Sawa, M.; Murakami, Y. Temperature-Programmed Desorption of Ammonia with Readsorption Based on the Derived Theoretical Equation. J. Phys. Chem. 1995, 99, 8812–8816. [Google Scholar] [CrossRef]
- Wang, J.; Yu, T.; Wang, X.Q.; Qi, G.S.; Xue, J.J.; Shen, M.Q. The influence of silicon on the catalytic properties of Cu/SAPO-34 for NOx reduction by ammonia-SCR. Appl. Catal. B. Environ. 2012, 127, 137–147. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. In situ DRIFTS and temperature-programmed technology study on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B. Environ. 2014, 156, 428–437. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, H.J.; Heo, I.; Nam, I.-S.; Cho, B.K.; Choung, J.W.; Cha, M.-S.; Yeo, G.K. Mn–Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl. Catal. B Environ. 2012, 126, 9–21. [Google Scholar] [CrossRef]
- Zhao, H.W.; Li, H.S.; Li, X.H.; Liu, M.K.; Li, Y.D. The promotion effect of Fe to Cu-SAPO-34 for selective catalytic reduction of NOx with NH3. Catal. Today 2017, 297, 84–91. [Google Scholar] [CrossRef]
- Sultana, A.; Sasaki, M.; Suzuki, K.; Hamada, H. Tuning the NOx conversion of Cu-Fe/ZSM-5 catalyst in NH3-SCR. Catal. Commun. 2013, 41, 21–25. [Google Scholar] [CrossRef]
- Yuan, E.; Wu, G.; Dai, W.; Guan, N.; Li, L. One-pot construction of Fe/ZSM-5 zeolites for the selective catalytic reduction of nitrogen oxides by ammonia. Catal. Sci. Technol. 2017, 7, 3036–3044. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Structure–activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Weng, D.; Si, Z.; Ran, R. Migration, reactivity, and sulfur tolerance of copper species in SAPO-34 zeolite toward NOx reduction with ammonia. RSC Adv. 2017, 7, 37787–37796. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Si, Z.C.; Weng, D.; Wu, X.D.; Ma, Y. Potassium poisoning on Cu-SAPO-34 catalyst for selective catalytic reduction of NOx with ammonia. Chem. Eng. J. 2015, 267, 191–200. [Google Scholar] [CrossRef]
- Xue, J.; Wang, X.; Qi, G.; Wang, J.; Shen, M.; Li, W. Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia: Relationships between active Cu sites and de-NOx performance at low temperature. J. Catal. 2013, 297, 56–64. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Ren, L.; Shi, X.; Xiao, F.S.; He, H. Excellent Performance of One-Pot Synthesized Cu-SSZ-13 Catalyst for the Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2014, 48, 566–572. [Google Scholar] [CrossRef]
- Zhang, R.R.; Li, Y.H.; Zhen, T.L. Ammonia selective catalytic reduction of NO over Fe/Cu-SSZ-13. RSC Adv. 2014, 4, 52130–52139. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.; Wang, D.; Zhao, Z.; Wei, Y.; Cheng, K.; Jiang, G.; Duan, A. Selective catalytic reduction of NO with NH3 over HZSM-5-supported Fe–Cu nanocomposite catalysts: The Fe–Cu bimetallic effect. Appl. Catal. B 2014, 148, 520–531. [Google Scholar] [CrossRef]
- Kumara, M.S.; Schwidder, M.; Grunert, W.; Bentrup, U.; Bruckner, A. Selective reduction of NO with Fe-ZSM-5 catalysts of low Fe content: Part II. Assessing the function of different Fe sites by spectroscopic in situ studies. J. Catal. 2006, 239, 173–186. [Google Scholar]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef]
- Fahami, A.R.; Günter, T.; Doronkin, D.E.; Casapu, M.; Zengel, D.; Vuong, T.H.; Grunwaldt, J. The dynamic nature of Cu sites in Cu-SSZ-13 and the origin of the seagull NOx conversion profile during NH3-SCR. React. Chem. Eng. 2019, 4, 1000–1018. [Google Scholar] [CrossRef] [Green Version]
- Hoj, M.; Beier, M.J.; Grunwaldt, J.D.; Dahl, S. The role of monomeric iron during the selective catalytic reduction of NOx by NH3 over Fe-BEA zeolite catalysts. Appl. Catal. B 2009, 93, 166–176. [Google Scholar] [CrossRef]
- Schwidder, M.; Kumar, M.S.; Klementiev, K.; Pohl, M.M.; Brückner, A.; Grünert, W. Selective reduction of NO with Fe-ZSM-5 catalysts of low Fe content: I. Relations between active site structure and catalytic performance. J. Catal. 2005, 231, 314–330. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. Synthesis and evaluation of Cu-SAPO-34 catalysts for ammonia selective catalytic reduction. 1. Aqueous solution ion exchange. ACS Catal. 2013, 9, 2083–2093. [Google Scholar] [CrossRef]
- Niu, C.; Shi, X.; Liu, F.; Liu, K.; Xie, L.; You, Y.; He, H. High hydrothermal stability of Cu–SAPO-34 catalysts for the NH3-SCR of NOx. Chem. Eng. 2016, 294, 254–263. [Google Scholar] [CrossRef]

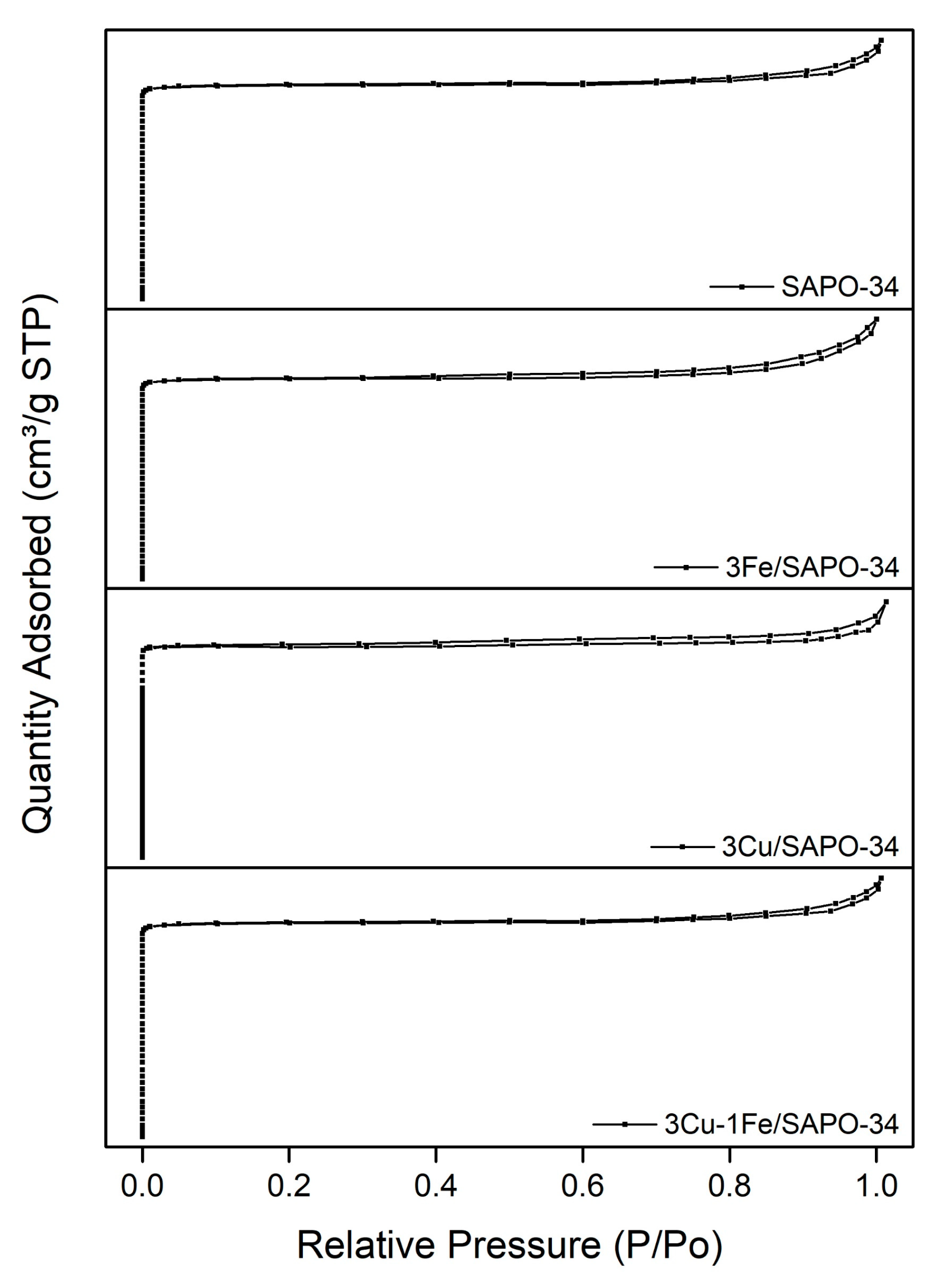
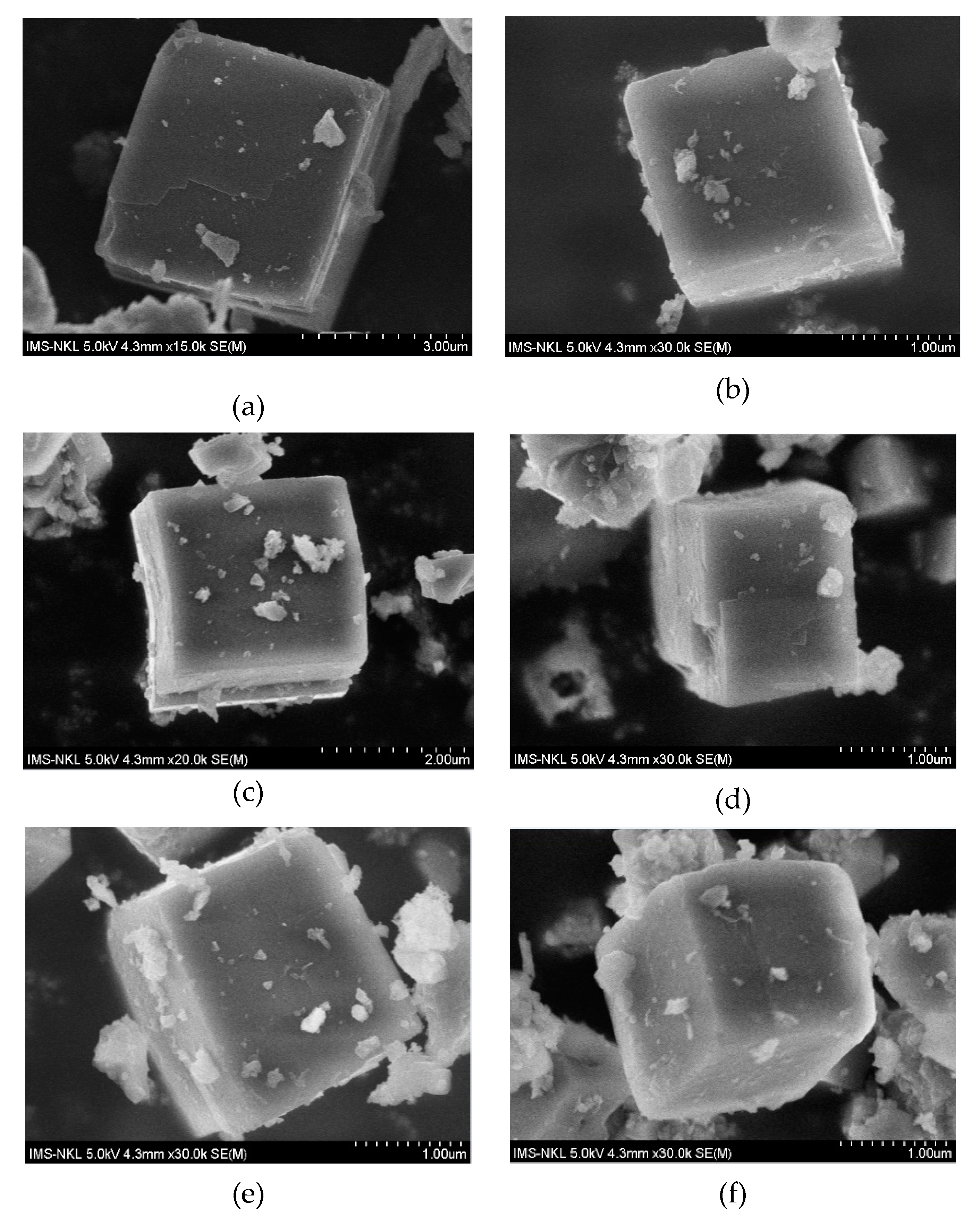
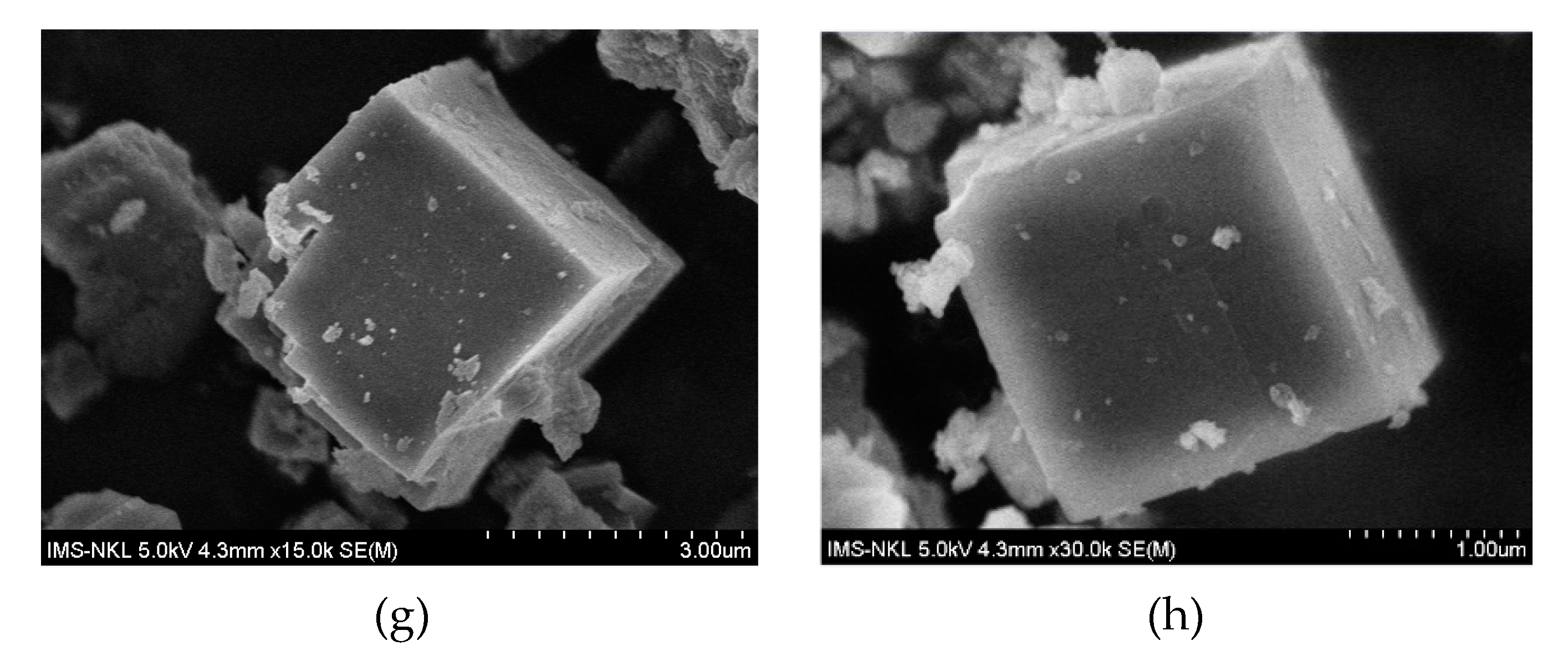

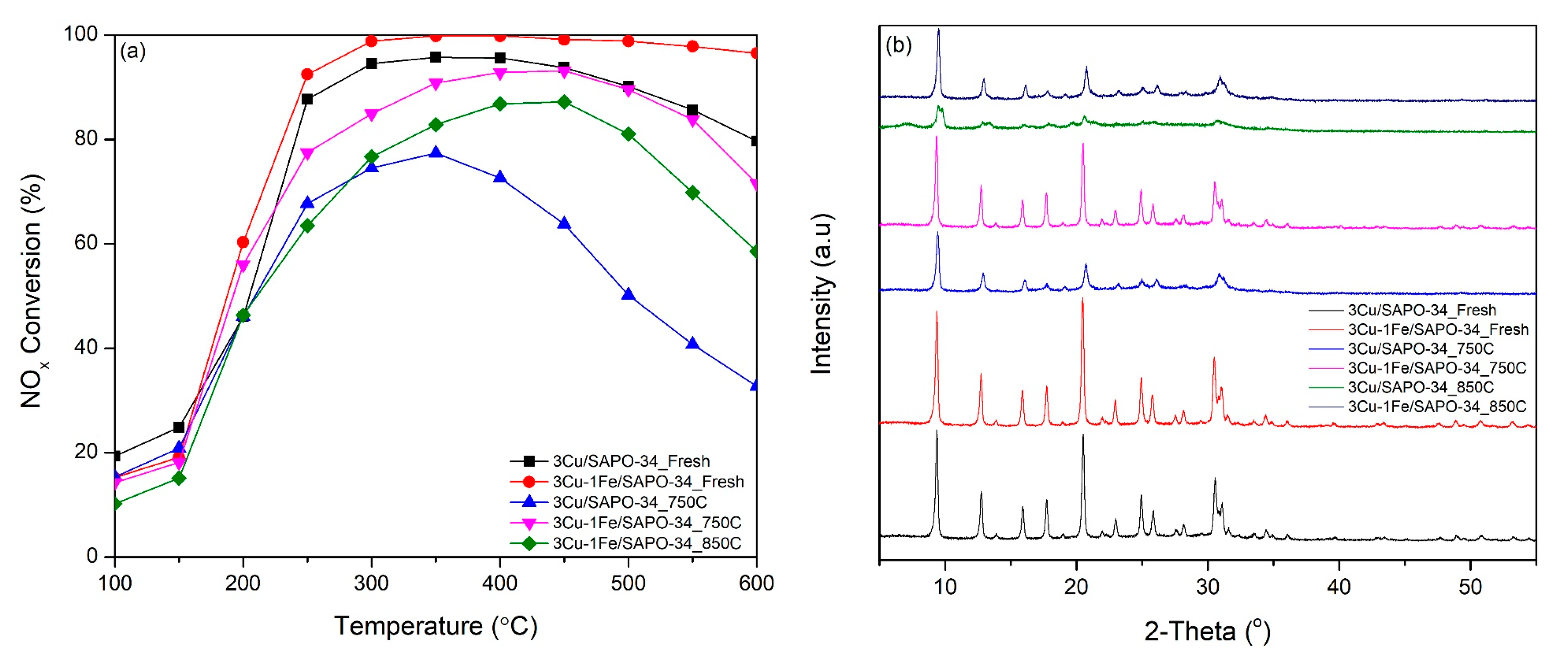
| Catalyst | Metal Atomic Concentration (wt.%) a | BET Surface (m2/g) b | Average Pore Diameter (nm) b | Mean Crystallite Size (nm) c | Crystallinity (%) |
|---|---|---|---|---|---|
| SAPO-34 | - | 697 | 0.75 | 34.29 | 77.48 |
| 1Cu/SAPO-34 | 1.07 | 583 | 0.69 | 34.77 | 75.06 |
| 3Cu/SAPO-34 | 3.12 | 501 | 0.60 | 35.64 | 72.29 |
| 5Cu/SAPO-34 | 4.89 | 357 | 0.47 | 36.76 | 67.68 |
| 1Fe/SAPO-34 | 0.99 | 555 | 0.65 | 34.60 | 74.04 |
| 3Fe/SAPO-34 | 2.91 | 487 | 0.60 | 38.30 | 71.94 |
| 5Fe/SAPO-34 | 4.78 | 332 | 0.44 | 39.43 | 63.67 |
| 2Cu-2Fe/SAPO-34 | 2.02 Cu–1.82 Fe | 479 | 0.53 | 38.31 | 72.82 |
| 3Cu-1Fe/SAPO-34 | 3.03 Cu–0.91 Fe | 499 | 0.56 | 34.71 | 73.87 |
| Samples | Peak (mmol/g) | Total (mmol/g) | ||
|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | ||
| SAPO-34 | 1.22 | 0.98 | - | 2.2 |
| 1Cu/SAPO-34 | 1.35 | 2.97 | 1.32 | 5.64 |
| 3Cu/SAPO-34 | 1.82 | 4.45 | 2.41 | 8.68 |
| 5Cu/SAPO-34 | 3.22 | 6.02 | 2.93 | 12.17 |
| 1Fe/SAPO-34 | 1.05 | 2.11 | 1.36 | 4.52 |
| 3Fe/SAPO-34 | 1.69 | 2.81 | 1.79 | 6.29 |
| 5Fe/SAPO-34 | 1.97 | 3.18 | 1.96 | 7.11 |
| 2Cu-2Fe/SAPO-34 | 1.91 | 3.02 | 1.89 | 6.82 |
| 3Cu-1Fe/SAPO-34 | 2.92 | 5.34 | 2.47 | 10.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doan, T.; Dam, P.; Nguyen, K.; Vuong, T.H.; Le, M.T.; Pham, T.H. Copper-Iron Bimetal Ion-Exchanged SAPO-34 for NH3-SCR of NOx. Catalysts 2020, 10, 321. https://doi.org/10.3390/catal10030321
Doan T, Dam P, Nguyen K, Vuong TH, Le MT, Pham TH. Copper-Iron Bimetal Ion-Exchanged SAPO-34 for NH3-SCR of NOx. Catalysts. 2020; 10(3):321. https://doi.org/10.3390/catal10030321
Chicago/Turabian StyleDoan, Tuan, Phong Dam, Khang Nguyen, Thanh Huyen Vuong, Minh Thang Le, and Thanh Huyen Pham. 2020. "Copper-Iron Bimetal Ion-Exchanged SAPO-34 for NH3-SCR of NOx" Catalysts 10, no. 3: 321. https://doi.org/10.3390/catal10030321
APA StyleDoan, T., Dam, P., Nguyen, K., Vuong, T. H., Le, M. T., & Pham, T. H. (2020). Copper-Iron Bimetal Ion-Exchanged SAPO-34 for NH3-SCR of NOx. Catalysts, 10(3), 321. https://doi.org/10.3390/catal10030321






