Selective Oxidation of Methane over Fe-Zeolites by In Situ Generated H2O2
Abstract
1. Introduction
2. Results
2.1. Catalyst Characterization
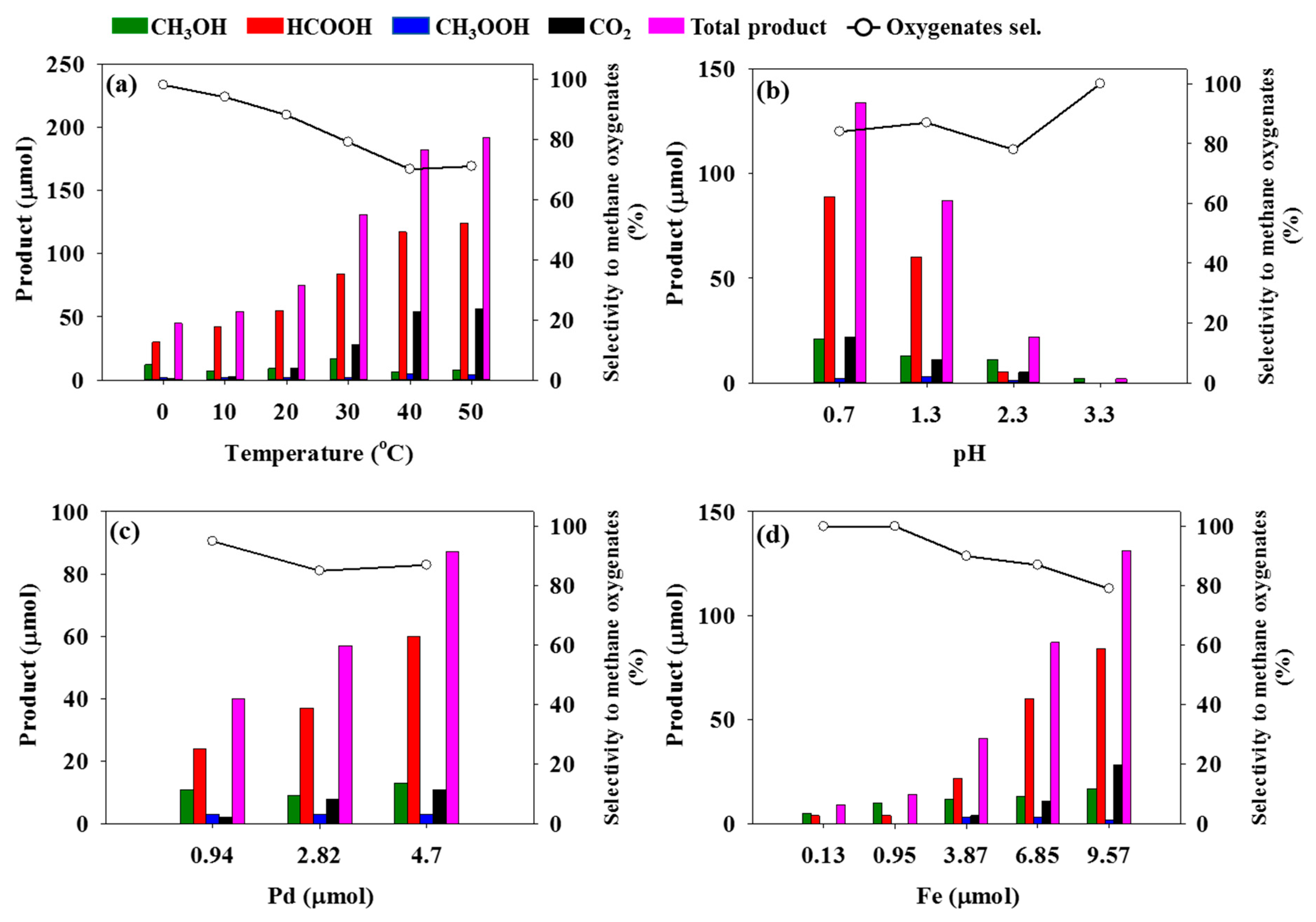
2.2. Catalytic Performance
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalytic Activity Test
4.3. Analytical Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McFarland, E. Unconventional Chemistry for Unconventional Natural Gas. Science 2012, 338, 340–342. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Ellison, G.B. Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Significant Advances in C1 Catalysis: Highly Efficient Catalysts and Catalytic Reactions. ACS Catal. 2019, 9, 3026–3053. [Google Scholar] [CrossRef]
- Park, M.B.; Park, E.D.; Ahn, W. Recent Progress in Direct Conversion of Methane to Methanol Over Copper-Exchanged Zeolites. Front. Chem. 2019, 7, 514. [Google Scholar] [CrossRef] [PubMed]
- Park, M.B.; Ahn, S.H.; Mansouri, A.; Ranocchiari, M.; Bokhoven, J.A. Comparative Study of Diverse Copper Zeolites for the Conversion of Methane into Methanol. ChemCatChem 2017, 9, 3705–3713. [Google Scholar] [CrossRef]
- Wang, X.; Martin, N.M.; Nilsson, J.; Carlson, s.; Gustafson, J.; Skoglundh, M.; Carlsson, P. Copper-Modified Zeolites and Silica for Conversion of Methane to Methanol. Catalysts 2018, 8, 545. [Google Scholar] [CrossRef]
- Tomkins, P.; Ranocchiari, M.; Bokhoven, J.A. Direct conversion of methane to methanol under mild conditions over Cu-zeolites and beyond. Acc. Chem. Res. 2017, 50, 418–425. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, T.Y.; Lee, H.; Yi, J. Distinct activation of Cu-MOR for direct oxidation of methane to methanol. Chem. Commun. 2017, 53, 4116–4119. [Google Scholar] [CrossRef]
- Wood, b.J.; Reimer, J.A.; Bell, A.T.; Janicke, M.T.; Ott, K.C. Methanol formation on Fe/Al-MFI via the oxidation of methane by nitrous oxide. J. Catal. 2004, 225, 300–306. [Google Scholar] [CrossRef]
- Starokon, E.V.; Parfenov, M.V.; Arzumanov, S.S.; Pirutko, L.V.; Stepanov, A.G.; Panov, G.I. Oxidation of methane to methanol on the surface of FeZSM-5 zeolite. J. Catal. 2013, 300, 47–54. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Palagin, D.; Ranocchiari, M.; van Bokhoven, J.A. Selective Anaerobic Oxidation of Methane Enables Direct Synthesis of Methanol. Science 2017, 356, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, J.K.; Park, E.D. Continuous methanol synthesis directly from methane and steam over Cu(II)-exchanged mordenite. Korean J. Chem. Eng. 2018, 35, 2145–2149. [Google Scholar] [CrossRef]
- Periana, R.A.; Taube, D.J.; Gamble, S.; Taube, H.; Satoh, T.; Fujii, H. Platinum Catalysts for the High-Yield Oxidation of Methane to a Methanol Derivative. Science 1998, 280, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Soorholtz, M.; White, R.J.; Zimmermann, T.; Titirici, M.M.; Antonietti, M.; Palkovits, R.; Schüth, F. Direct methane oxidation over Pt-modified nitrogen-doped carbons. Chem. Commun. 2013, 49, 240–242. [Google Scholar] [CrossRef]
- Zimmermann, T.; Bilke, M.; Soorholtz, M.; Schüth, F. Influence of Catalyst Concentration on Activity and Selectivity in Selective Methane Oxidation with Platinum Compounds in Sulfuric Acid and Oleum. ACS Catal. 2018, 8, 9262–9268. [Google Scholar] [CrossRef]
- Dang, H.T.; Lee, H.W.; Lee, J.; Choo, H.; Hong, S.H.; Cheong, M.; Lee, H. Enhanced Catalytic Activity of (DMSO)2PtCl2 for the Methane Oxidation in the SO3–H2SO4 System. ACS Catal. 2018, 8, 11854–11862. [Google Scholar] [CrossRef]
- Periana, R.A.; Mironov, O.; Taube, D.; Bhalla, G.; Jones, C.J. Catalytic, Oxidative Condensation of CH4 to CH3COOH in One Step via CH Activation. Science 2003, 301, 814–818. [Google Scholar] [CrossRef]
- An, Z.; Pan, X.; Liu, X.; Han, X.; Bao, X. Combined redox couples for catalytic oxidation of methane by dioxygen at low temperatures. J. Am. Chem. Soc. 2006, 128, 16028–16029. [Google Scholar] [CrossRef]
- Lin, M.; Hogan, T.E.; Sen, A. Catalytic Carbon−Carbon and Carbon−Hydrogen Bond Cleavage in Lower Alkanes. Low-Temperature Hydroxylations and Hydroxycarbonylations with Dioxygen as the Oxidant. J. Am. Chem. Soc. 1996, 118, 4574–4580. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, T.Y.; Kwon, G.; Yi, J.; Lee, H. Selective Activation of Methane on Single-Atom Catalyst of Rhodium Dispersed on Zirconia for Direct Conversion. J. Am. Chem. Soc. 2017, 139, 17694–17699. [Google Scholar] [CrossRef]
- Yuan, Q.; Deng, W.; Zhang, Q.; Wang, Y. Osmium-catalyzed selective oxidations of methane and ethane with hydrogen peroxide in aqueous medium. Adv. Synth. Catal. 2007, 349, 1199–1209. [Google Scholar] [CrossRef]
- Periana, R.A.; Taube, D.J.; Evitt, E.R.; Loffler, D.G.; Wentrcek, P.R.; Voss, G.; Masuda, T. A Mercury-Catalyzed, High-Yield System for the Oxidation of Methane to Methanol. Science 1993, 259, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Park, E.D.; Hwang, Y.; Lee, C.W.; Lee, J.S. Copper- and vanadium-catalyzed methane oxidation into oxygenates with in situ generated H2O2 over Pd/C. Appl. Catal. A: General 2003, 247, 269–284. [Google Scholar] [CrossRef]
- Seki, Y.; Mizuno, N.; Misono, M. High-yield liquid-phase oxygenation of methane with hydrogen peroxide catalyzed by 12-molybdovanadophosphoric acid catalyst precursor. Appl. Catal. A: General 1997, 158, L47–L51. [Google Scholar] [CrossRef]
- Hammond, C.; Forde, M.M.; Ab Rahim, M.H.; Thetford, A.; He, Q.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Dummer, N.F.; Murphy, D.M.; et al. Direct catalytic conversion of methane to methanol in an aqueous medium by using copper-promoted Fe-ZSM-5. Angew. Chem. Int. Ed. 2012, 51, 5129–5133. [Google Scholar] [CrossRef]
- Cui, X.; Li, H.; Cui, X.; Li, H.; Wang, Y.; Hu, Y.; Hua, L.; Li, H.; Han, X. Room-Temperature Methane Conversion by Graphene-Confined Single Iron Atoms Room-Temperature Methane Conversion by Graphene-Confined Single Iron Atoms. CHEM 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Yamanaka, I.; Soma, M.; Otsuka, K. Oxidation of Methane to Methanol with Oxygen Catalysed by Europium Trichloride at Room Temeprature. J. Chem. Soc. Chem. Commun. 1995, 21, 2235–2236. [Google Scholar] [CrossRef]
- Vargaftik, M.N.; Stolarov, I.P.; Moiseev, I.I. Highly Selective Partial Oxidation of Methane to Methyl Trifluoroacetate. J. Chem. Soc. Chem. Commun. 1990, 15, 1049–1050. [Google Scholar] [CrossRef]
- Strassner, T.; Ahrens, S.; Muehlhofer, M.; Munz, D.; Zeller, A. Cobalt-Catalyzed Oxidation of Methane to Methyl Trifluoroacetate by Dioxygen. Eur. J. Inorg. Chem. 2013, 21, 3659–3663. [Google Scholar] [CrossRef]
- Ravi, M.; van Bokhoven, J.A. Homogeneous Copper-Catalyzed Conversion of Methane to Methyl Trifluoroacetate in High Yield at Low Pressure. ChemCatChem 2018, 10, 2383–2386. [Google Scholar] [CrossRef]
- Ab Rahim, M.H.; Armstrong, R.D.; Hammond, C.; Dimitratos, N.; Freakley, S.J.; Forde, M.M.; Morgan, D.J.; Lalev, G.; Jenkins, R.L.; Lopez-Sanchez, J.A.; et al. Low temperature selective oxidation of methane to methanol using titania supported gold palladium copper catalysts. Catal. Sci. Technol. 2016, 6, 3410–3418. [Google Scholar] [CrossRef]
- Lewis, R.J.; Bara-Estaun, A.; Agarwal, N.; Freakley, S.J.; Morgan, D.J.; Hutchings, G.J. The Direct Synthesis of H2O2 and Selective Oxidation of Methane to Methanol Using HZSM-5 Supported AuPd Catalysts. Catal. Lett. 2019, 149, 3066–3075. [Google Scholar] [CrossRef]
- He, Y.; Luan, C.; Fang, Y.; Feng, X.; Peng, X.; Yang, G.; Tsubaki, N. Low-temperature direct conversion of methane to methanol over carbon materials supported Pd-Au nanoparticles. Catal. Today 2020, 339, 48–53. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 2020, 367, 193–197. [Google Scholar] [PubMed]
- Kang, J.; Park, E.D. Aqueous-Phase Selective Oxidation of Methane with Oxygen over Iron Salts and Pd/C in the Presence of Hydrogen. ChemCatChem 2019, 11, 4247–4251. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Pino, F.; Arrigo, R.; Giordano, G.; Katovic, A.; Pedulà, V. Performances of Fe-[Al, B]MFI catalysts in benzene hydroxylation with N2O: The role of zeolite defects as host sites for highly active iron species. Catal. Today 2005, 110, 211–220. [Google Scholar] [CrossRef]
- Hammond, C.; Dimitratos, N.; Lopez-Sanchez, J.A.; Jenkins, R.L.; Whiting, G.; Kondrat, S.A.; ab Rahim, M.H.; Forde, M.M.; Thetford, A.; Hagen, H.; et al. Aqueous-Phase Methane Oxidation over Fe-MFI Zeolites; Promotion through Isomorphous Framework Substitution. ACS Catal. 2013, 3, 1835–1844. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Samanta, C. Role of chloride or bromide anions and protons for promoting the selective oxidation of H2 by O2 to H2O2 over supported Pd catalysts in an aqueous medium. J. Catal. 2006, 238, 28–38. [Google Scholar] [CrossRef]


| Fe Catalyst | [H2O2]fin (mM) | Product (μmol) | Selectivity to Methane Oxygenates b (%) | TON c | ||||
|---|---|---|---|---|---|---|---|---|
| CH3OH | HCOOH | CH3OOH | CO2 | Total Product | ||||
| Fe-ZSM-5 | 9 | 22 | 52 | 0 | 16 | 90 | 82 | 11 |
| Fe-mordenite | 7 | 14 | 57 | 0 | 22 | 93 | 76 | 7.0 |
| Fe-β | 2 | 15 | 144 | 1 | 131 | 291 | 55 | 28 |
| Fe-Y | 7 | 10 | 139 | 5 | 167 | 321 | 48 | 51 |
| Fe-ferrierite | 4 | 5 | 4 | 0 | 0 | 9 | 100 | 4.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Park, E.D. Selective Oxidation of Methane over Fe-Zeolites by In Situ Generated H2O2. Catalysts 2020, 10, 299. https://doi.org/10.3390/catal10030299
Kang J, Park ED. Selective Oxidation of Methane over Fe-Zeolites by In Situ Generated H2O2. Catalysts. 2020; 10(3):299. https://doi.org/10.3390/catal10030299
Chicago/Turabian StyleKang, Jongkyu, and Eun Duck Park. 2020. "Selective Oxidation of Methane over Fe-Zeolites by In Situ Generated H2O2" Catalysts 10, no. 3: 299. https://doi.org/10.3390/catal10030299
APA StyleKang, J., & Park, E. D. (2020). Selective Oxidation of Methane over Fe-Zeolites by In Situ Generated H2O2. Catalysts, 10(3), 299. https://doi.org/10.3390/catal10030299







