Biofuels of Green Diesel–Kerosene–Gasoline Production from Palm Oil: Effect of Palladium Cooperated with Second Metal on Hydrocracking Reaction
Abstract
1. Introduction
2. Results and Discussion
2.1. Composition of Palm Oil
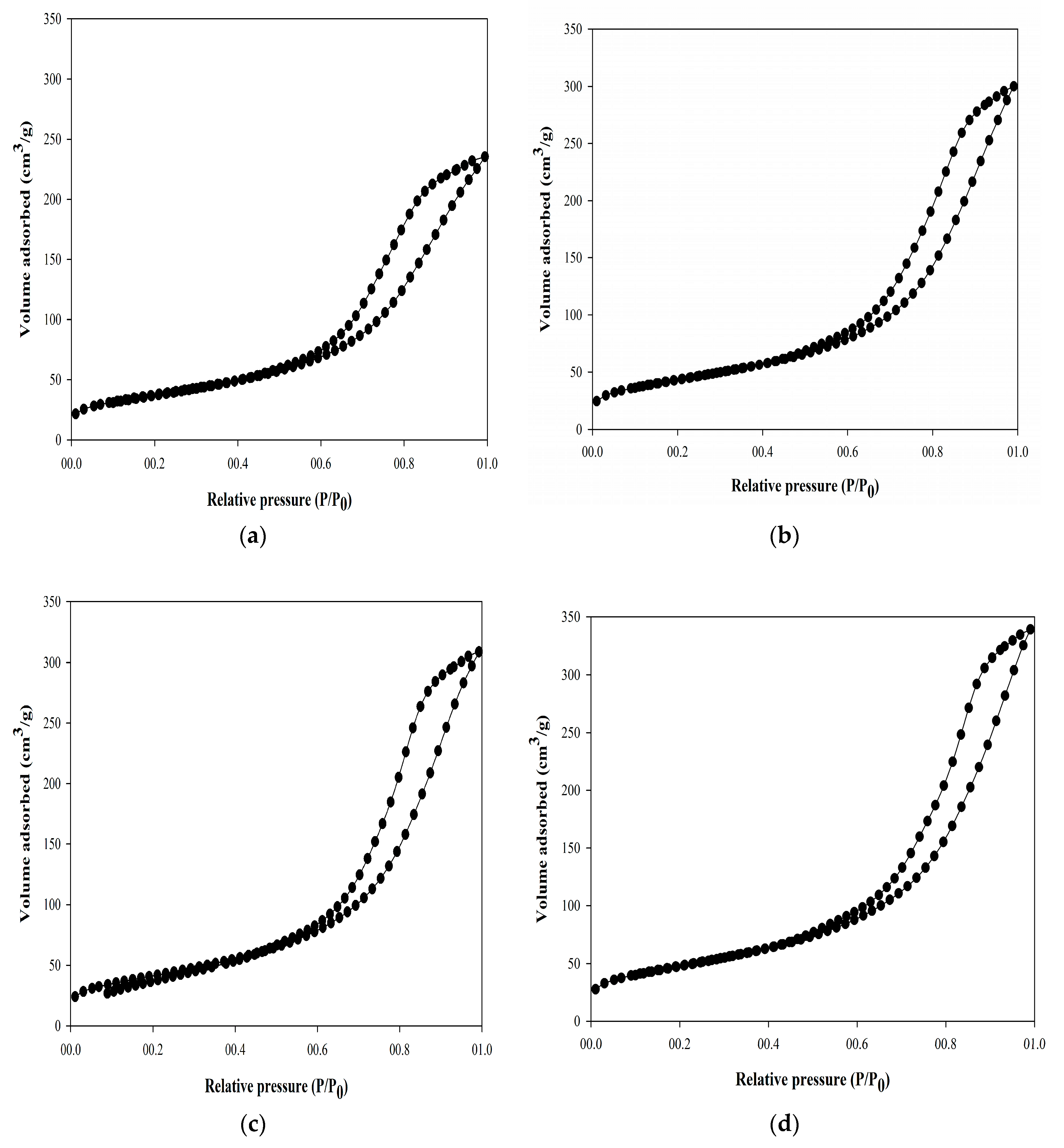
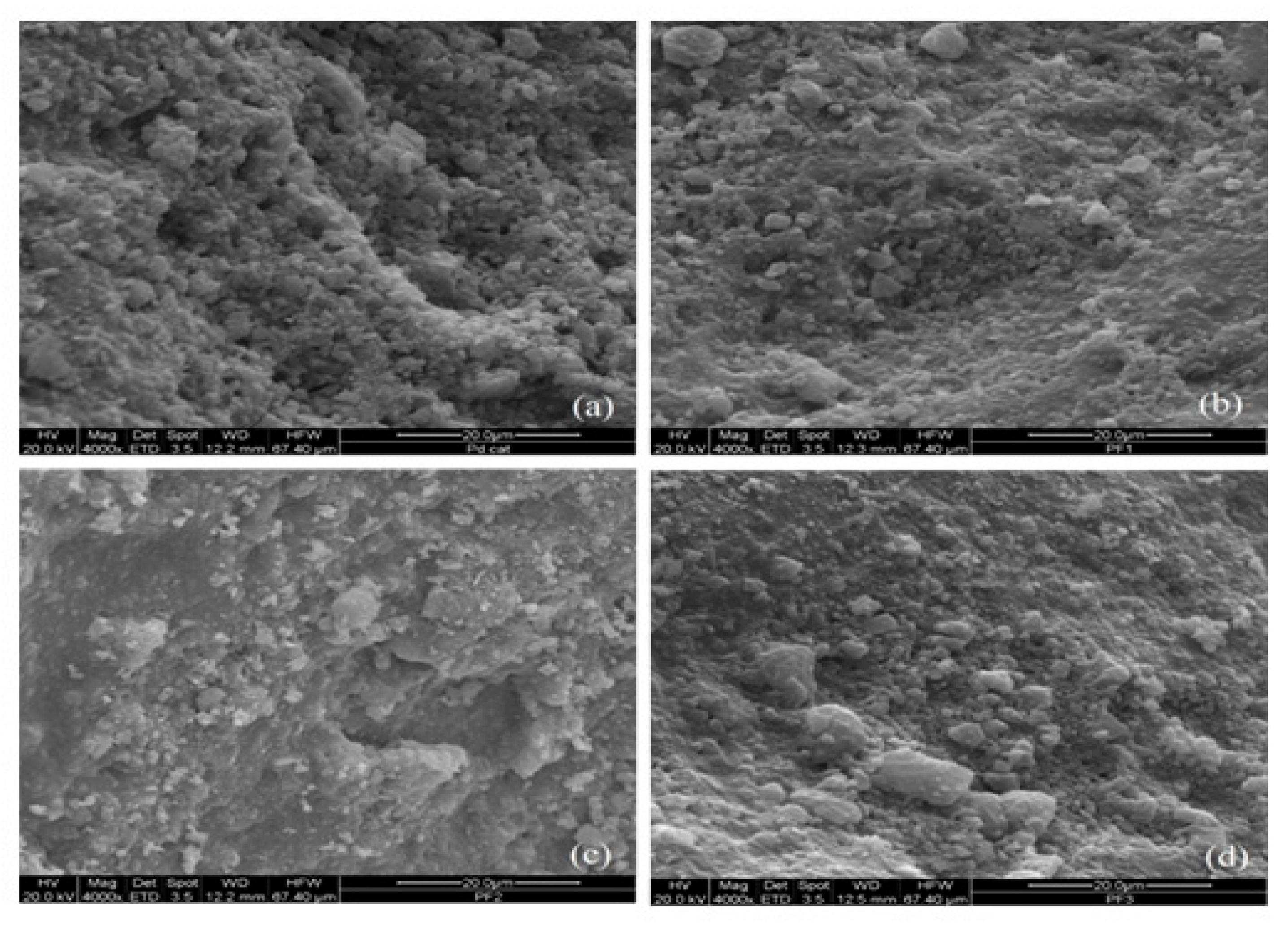
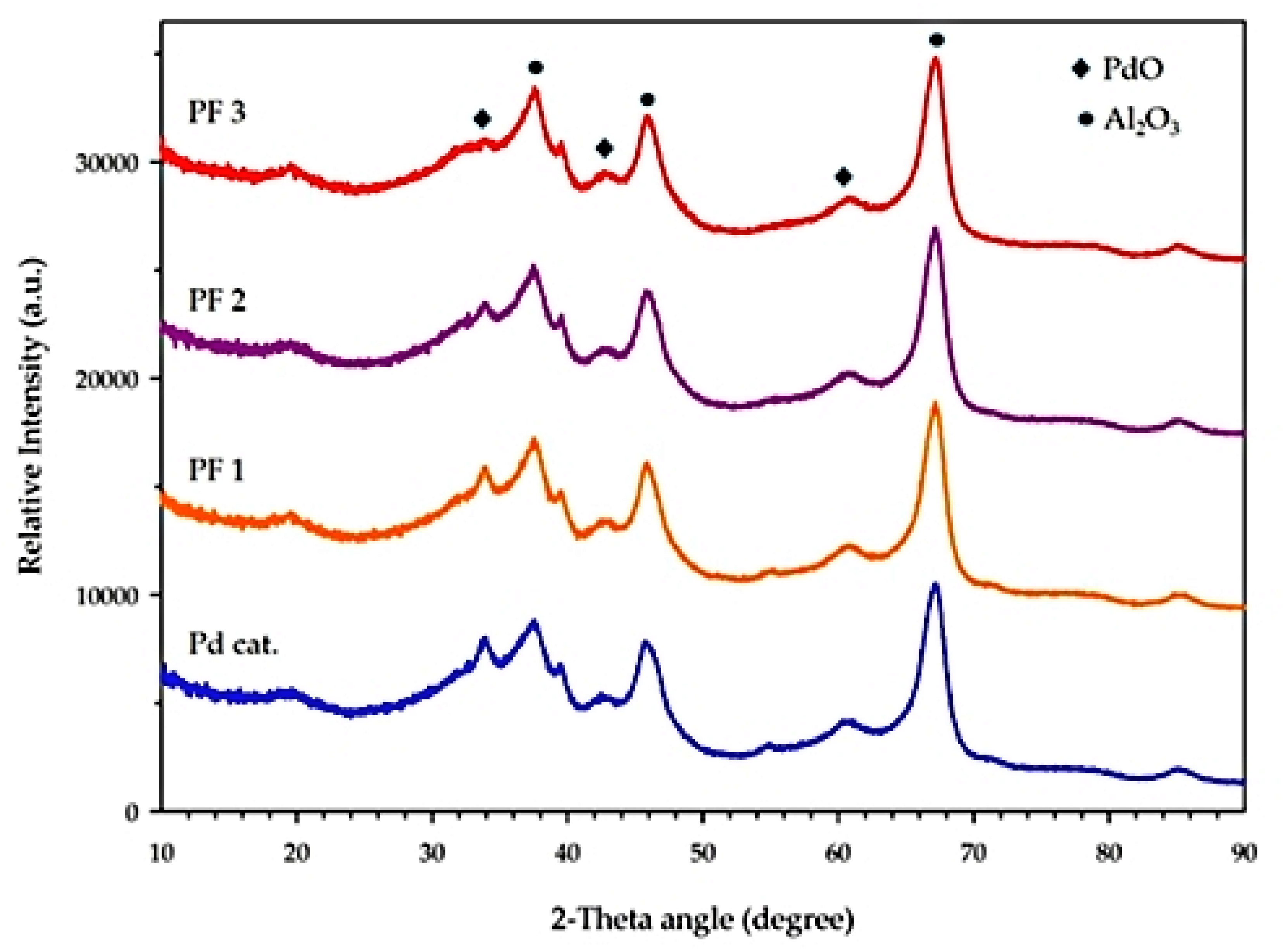
2.2. Catalyst Characterisation
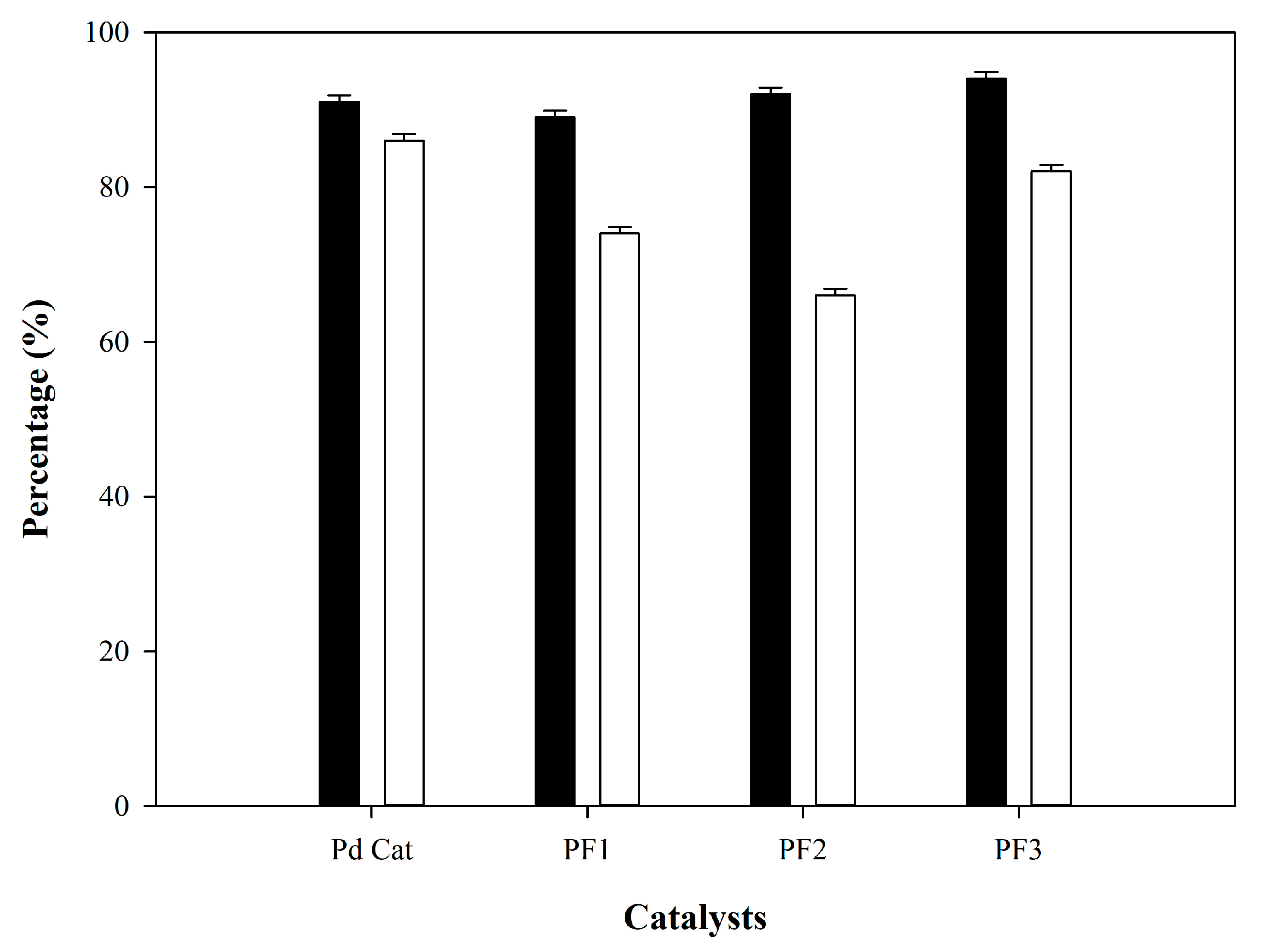
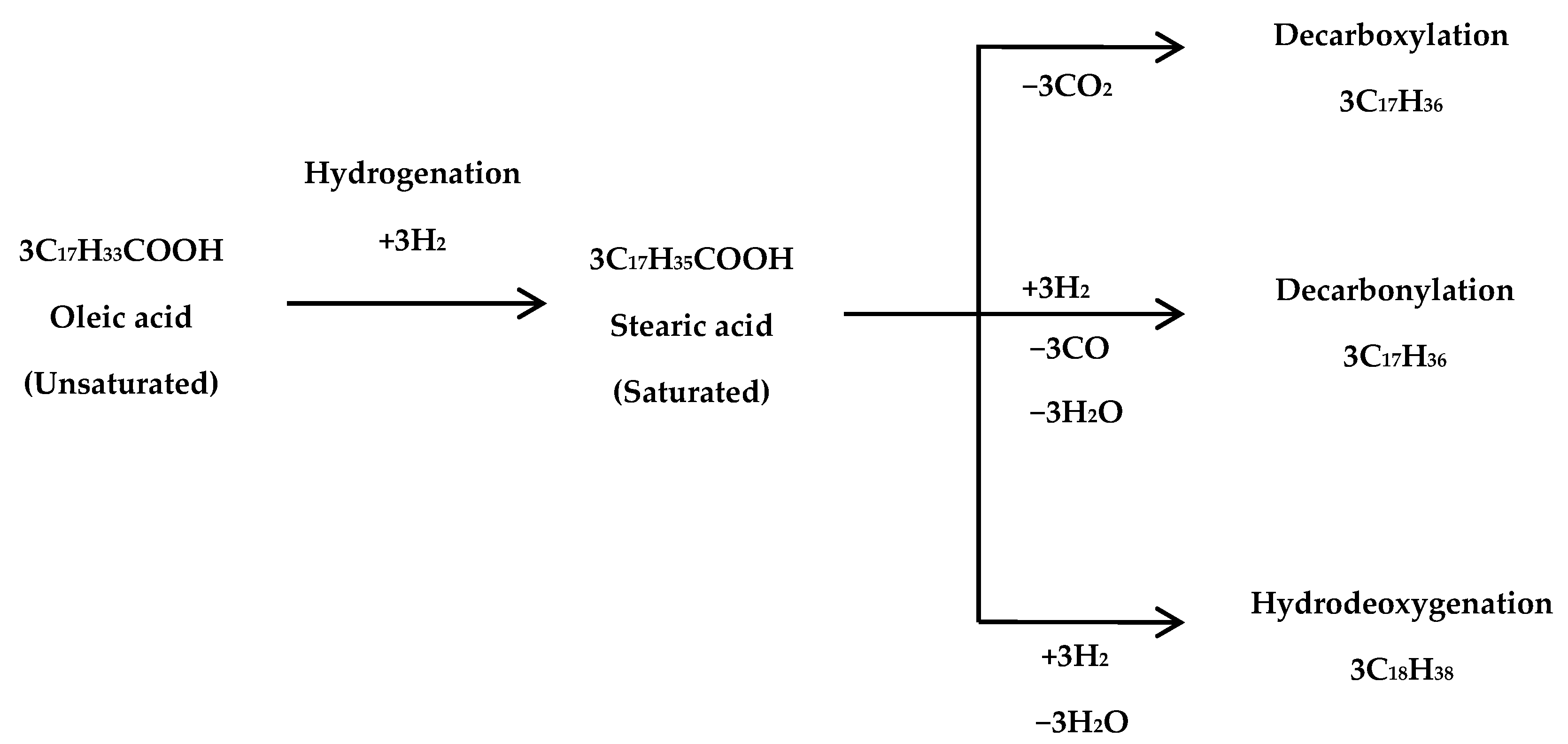
2.3. Catalytic Hydrocracking Reaction Test
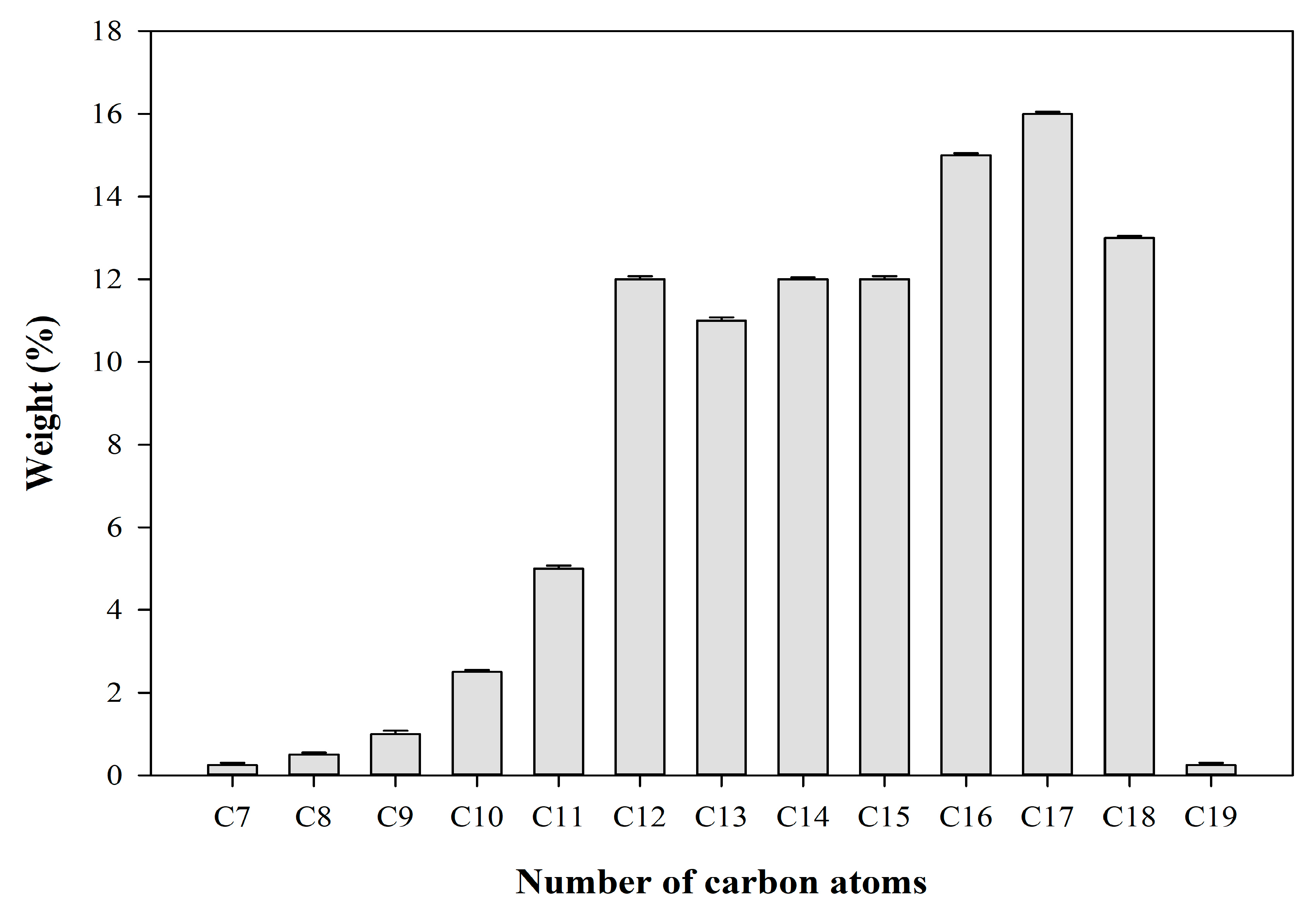
2.4. Characterisation of Refined Biofuel Obtained from Hydrocracking Reaction
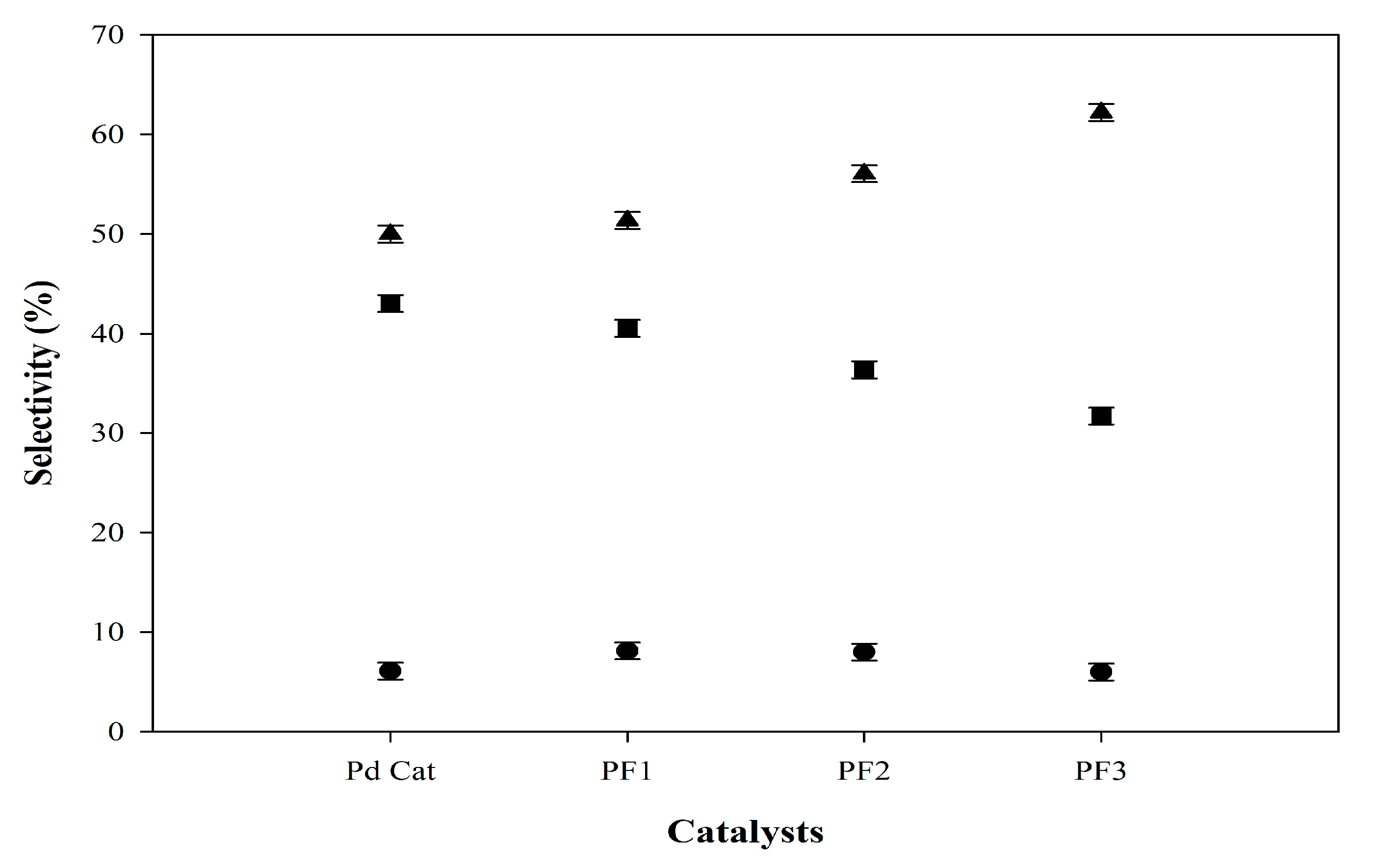
2.5. Comparative Green Kerosene and Green Diesel Yields via Catalytic Cracking Reactions with Different Catalysts and Operating Conditions
3. Materials and Methods
3.1. Chemical Reagents
3.2. Characterisation of Palm Oil
3.3. Preparation of Supporting Material
3.4. Monometallic Catalyst
3.5. Bimetallic Catalyst
3.6. Catalyst Characterisation
3.7. Hydrocracking Catalytic Reaction
3.8. Characterisation of Refine -Biofuel Obtained from Hydrocracking Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M. Process optimization for biodiesel production from waste cooking oil using multi-enzyme systems through response surface methodology. Renew. Energy 2017, 105, 465–472. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Kaewkannetra, P. Production of bio-hydrogenated kerosene by catalytic hydrocracking from refined bleached deodorised palm/ palm kernel oils. Renew. Energy 2020, 147, 464–472. [Google Scholar] [CrossRef]
- Muanruksa, P.; Kaewkannetra, P. Combination of fatty acids extraction and enzymatic esterification for biodiesel production using sludge palm oil as a low-cost substrate. Renew. Energy 2020, 146, 901–906. [Google Scholar] [CrossRef]
- Jeong, H.; Shin, M.; Jeong, B.; Jang, J.H.; Han, G.B.; Suh, Y.W. Comparison of activity and stability of supported Ni2P and Pt catalysts in the hydro-processing of palm oil into normal paraffins. J. Ind. Eng. Chem. 2020, 83, 189–199. [Google Scholar] [CrossRef]
- Anahas, A.M.P.; Muralitharan, G. Characterization of heterocystous cyanobacterial strains for biodiesel production based on fatty acid content analysis and hydrocarbon production. Energy Convers. Manag. 2018, 157, 423–437. [Google Scholar] [CrossRef]
- Yang, L.; Carreon, M.A. Effect of reaction parameters on the decarboxylation of oleic acid over Pt/ZIF-67membrane/zeolite 5A bead catalysts. J. Chem. Technol. Biotechnol. 2017, 92, 52–58. [Google Scholar] [CrossRef]
- Srifa, A.; Faungnawakij, K.; Itthibenchapong, V.; Viriya-empikul, N.; Charinpanitkul, T.; Assabumrungrat, S. Production of bio-hydrogenated diesel by catalytic hydrotreating of palm oil over NiMoS2/γ-Al2O3 catalyst. Bioresour. Technol. 2014, 158, 81–90. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Neramittagapong, A.; Kaewkannetra, P. Optimization of Bio-Hydrogenated Kerosene from Refined Palm Oil by Catalytic Hydrocracking. Energies 2019, 12, 3196. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Neramittagapong, A.; Kaewkannetra, P. H2-Assisted Chemical Reaction for Green-Kerosene Production. Defect Diffus. Forum 2015, 364, 104–111. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Phillips, C.B. Renewable fuels via catalytic hydrodeoxygenation. Appl. Catal. A Gen. 2011, 397, 1–12. [Google Scholar] [CrossRef]
- Kiatkittipong, W.; Phimsen, S.; Kiatkittipong, K.; Wongsakulphasatch, S.; Laosiripojana, N.; Assabumrungrat, S. Diesel-like hydrocarbon production from hydro-processing of relevant refining palm oil. Fuel Process. Technol. 2013, 116, 16–26. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Hu, X. A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 2016, 7, 12324. [Google Scholar] [CrossRef]
- Hwang, S.M.; Zhang, C.; Han, S.J.; Park, H.G.; Kima, Y.T.; Yang, S.; Jun, K.W.; Kim, S.K. Mesoporous carbon as an effective support for Fe catalyst for CO2 hydrogenation to liquid hydrocarbons. J. CO2 Util. 2020, 37, 65–73. [Google Scholar] [CrossRef]
- Wang, X.; Gorte, R.J. The effect of Fe and other promoters on the activity of Pd/ceria for the water-gas shift reaction. Appl. Catal. A Gen. 2003, 247, 157–162. [Google Scholar] [CrossRef]
- Metin, Ö.; Mendoza-Garcia, A.; Dalmizrak, D.; Gültekin, M.S.; Sun, S. FePd alloy nanoparticles assembled on reduced graphene oxide as a catalyst for selective transfer hydrogenation of nitroarenes to anilines using ammonia borane as a hydrogen source. Catal. Sci. Technol. 2016, 6, 6137–6143. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Li, K.; Zhang, C.; He, Y.; Du, R.; Wang, J.; Chen, L. Coupling membrane and Fe–Pd bimetallic nanoparticles for trichloroethene removing from water. J. Ind. Eng. Chem. 2019, 78, 198–209. [Google Scholar] [CrossRef]
- Cheng, Y.; Pham, H.; Huo, J.; Johnson, R.; Datye, A.K.; Shanks, B. High activity Pd-Fe bimetallic catalysts for aqueous phase hydrogenations. Mol. Catal. 2019, 477, 110546. [Google Scholar] [CrossRef]
- Cen, Y.; Yue, Y.; Wang, S.; Lu, J.; Wang, B.; Jin, C.; Guo, L.; Hu, Z.T.; Zhao, J. Adsorption Behavior and Electron Structure Engineering of Pd-Based Catalysts for Acetylene Hydrochlorination. Catalysts 2020, 10, 24. [Google Scholar] [CrossRef]
- Espro, C.; Gumina, B.; Paone, E.; Mauriello, F. Upgrading lignocellulosic biomasses: Hydrogenolysis of platform derived molecules promoted by heterogeneous Pd-Fe catalysts. Catalysts 2017, 7, 78. [Google Scholar] [CrossRef]
- Malara, A.; Paone, E.; Bonaccorsi, L.; Mauriello, F.; Macario, A.; Frontera, P. Nanofibers for the Catalytic Conversion of Lignin-Derived Benzyl Phenyl Ether under Transfer Hydrogenolysis Conditions. Catalysts 2020, 10, 20. [Google Scholar] [CrossRef]
- Arun, N.; Sharma, R.V.; Dalai, A.K. Green diesel synthesis by hydrodeoxygenation of bio-based feedstocks: Strategies for catalyst design and development. Renew. Sustain. Energy Rev. 2015, 48, 240–255. [Google Scholar] [CrossRef]
- Long, Y.; Liang, K.; Niu, J.; Tong, X.; Yuan, B.; Ma, J. Agglomeration of Pd0 nanoparticles causing different catalytic activities of Suzuki carbonylative cross-coupling reactions catalyzed by PdII and Pd0 immobilized on dopamine-functionalized magnetite nanoparticles. New J. Chem. 2015, 39, 2988–2996. [Google Scholar] [CrossRef]
- Lyu, D.; Mollamahale, Y.B.; Huang, S.; Zhu, P.; Zhang, X.; Du, Y.; Wang, S.; Qing, M.; Tian, Z.Q.; Shen, P.K. Ultra-high surface area graphitic Fe-N-C nanospheres with single-atom iron sites as highly efficient non-precious metal bifunctional catalysts towards oxygen redox reactions. J. Catal. 2018, 368, 279–290. [Google Scholar] [CrossRef]
- Ooi, X.Y.; Oi, L.E.; Choo, M.Y.; Ong, H.C.; Lee, H.V.; Show, P.L.; Lin, Y.C.; Juan, J.C. Efficient deoxygenation of triglycerides to hydrocarbon-biofuel over mesoporous Al2O3-TiO2 catalyst. Fuel Process. Technol. 2019, 194, 106120. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Rabnawaz, M. Upgrading pyrolysis bio-oil through hydro-deoxygenation (HDO) using non-sulfided Fe-Co/SiO2 catalyst. Energy. Convers. Manag. 2017, 150, 331–342. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, X.; Liang, S.; Zheng, X.; Shen, L.; Liu, F.; Cao, Y.; Wei, Z.; Jiang, L. Fe-doped γ-Al2O3 porous hollow microspheres for enhanced oxidative desulfurization: Facile fabrication and reaction mechanism. Green Chem. 2018, 20, 4645–4654. [Google Scholar] [CrossRef]
- Denardin, F.; Perez-Lopez, O.W. Tuning the acidity and reducibility of Fe/ZSM-5 catalysts for methane dehydroaromatization. Fuel 2019, 236, 1293–1300. [Google Scholar] [CrossRef]
- Kwon, E.E.; Kim, Y.T.; Kim, H.J.; Andrew Lin, K.Y.; Kim, K.H.; Lee, J.; Huber, G.W. Production of high-octane gasoline via hydrodeoxygenation of sorbitol over palladium-based bimetallic catalysts. J. Environ. Manage. 2018, 227, 329–334. [Google Scholar] [CrossRef]
- Scaldaferri, C.A.; Pasa, V.M.D. Production of jet fuel and green diesel range biohydrocarbons by hydro-processing of soybean oil over niobium phosphate catalyst. Fuel 2019, 245, 458–466. [Google Scholar] [CrossRef]
- Phimsen, S.; Kiatkittipong, W.; Yamada, H.; Tagawa, T.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Oil extracted from spent coffee grounds for bio-hydrotreated diesel production. Energy Convers. Manag. 2016, 126, 1028–1036. [Google Scholar] [CrossRef]
- Choi, I.H.; Hwang, K.R.; Han, J.S.; Lee, K.H.; Yun, J.S.; Lee, J.S. The direct production of jet-fuel from non-edible oil in a single-step process. Fuel 2015, 158, 98–104. [Google Scholar] [CrossRef]
- Makvisai, W.; Promdee, K.; Tanatavikorn, H.; Vitidsant, T. Catalytic cracking of used lubricating oil over Fe/Al2O3 and Fe/SiO2-Al2O3. Pet. Coal 2016, 58, 83–94. [Google Scholar]
| Compositions | %wt. |
|---|---|
| Capric acid (C10:0) | 0.11 ± 0.03 |
| Lauric acid (C12:0) | 1.58 ± 0.05 |
| Myristic acid (C14:0) | 4.74 ± 0.02 |
| Palmitic acid (C16:0) | 1.55 ± 0.01 |
| Palmitoleic (C16:1) | 12.50 ± 0.03 |
| Stearic acid (C18:0) | 1.82 ± 0.04 |
| Oleic acid (C18:1) | 76.27 ± 0.05 |
| Linoleic acid (C18:2) | 0.16 ± 0.06 |
| Linolenic acid (C18:3) | 0.08 ± 0.03 |
| Arachidic acid (C20:0) | 0.82 ± 0.06 |
| Erucic acid (C22:1) | 0.37 ± 0.04 |
| Catalyst | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Ave. Pore Diameter (nm) |
|---|---|---|---|
| Pd cat | 135.77 ± 0.05 | 0.37 ± 0.03 | 8.13 ± 0.06 |
| PF1 | 157.32 ± 0.05 | 0.47 ± 0.05 | 9.10 ± 0.03 |
| PF2 | 150.04 ± 0.03 | 0.48 ± 0.02 | 8.69 ± 0.04 |
| PF3 | 173.92 ± 0.04 | 0.53 ± 0.04 | 9.27 ± 0.05 |
| Catalyst. | Metal Loading (wt.%) | Crude Biofuel Yield (%) | Refined Biofuel Yield (%) |
|---|---|---|---|
| Pd cat | 0.5 Pd | 91 ± 0.05 | 86 ± 0.03 |
| PF1 | 0.38 Pd–0.12 Fe | 89 ± 0.07 | 74 ± 0.05 |
| PF2 | 0.25 Pd–0.25 Fe | 92 ± 0.04 | 66 ± 0.04 |
| PF3 | 0.12 Pd–0.38 Fe | 94 ± 0.08 | 82 ± 0.02 |
| Feedstocks | Catalysts | Conditions | Green Kerosene Yield (%) | Green Diesel Yield (%) | Refs. |
|---|---|---|---|---|---|
| Palm oil | Pd Cat | 0.9% catalyst, 400 °C, 60 bar H2, 2 h | 43.02 | 50.00 | This work |
| Palm oil | PF1 | 0.9% catalyst, 400 °C, 60 bar H2, 2 h | 40.54 | 51.35 | This work |
| Palm oil | PF2 | 0.9% catalyst, 400 °C, 60 bar H2, 2 h | 36.36 | 56.06 | This work |
| Palm oil | PF3 | 0.9% catalyst, 400 °C, 60 bar H2, 2 h | 31.71 | 62.20 | This work |
| Crude palm oil | 5%Pd/C | 5% catalyst, 400 °C, 40 bar H2, 3 h | Not reported | 51.00 | [11] |
| Soybean oil | NbOPO4 | 25% catalyst, 350 °C, 10 bar H2, 5 h | 62.00 | 40.00 | [29] |
| Coffee ground oil | 5%Pd/C | 5% catalyst, 400 °C, 40 bar H2, 2 h | Not reported | 22.30 | [30] |
| Soybean | Pd-based zeolite | 20% catalyst, 270 °C, 15 bar N2, 1 h | 31.00 | Not reported | [31] |
| PFAD | Pd-based zeolite | 20% catalyst, 270 °C, 15 bar N2, 1 h | 31.00 | Not reported | [31] |
| Used lubricating oil | 5%Fe/Al2O3 | 4% catalyst, 450 °C, 6.8 bar H2, 1.25 h | 24.16 | Not reported | [32] |
| Used lubricating oil | 0.5%Fe/SiO2-Al2O3 | 4% catalyst, 430 °C, 6.8 bar H2, 1 h | 11.41 | Not reported | [32] |
| Catalysts | Metals Loading onto Al2O3 Support (g) | |
|---|---|---|
| Palladium (Pd) | Iron (Fe) | |
| Pd cat | 0.50 | - |
| PF1 | 0.38 | 0.12 |
| PF2 | 0.25 | 0.25 |
| PF3 | 0.12 | 0.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srihanun, N.; Dujjanutat, P.; Muanruksa, P.; Kaewkannetra, P. Biofuels of Green Diesel–Kerosene–Gasoline Production from Palm Oil: Effect of Palladium Cooperated with Second Metal on Hydrocracking Reaction. Catalysts 2020, 10, 241. https://doi.org/10.3390/catal10020241
Srihanun N, Dujjanutat P, Muanruksa P, Kaewkannetra P. Biofuels of Green Diesel–Kerosene–Gasoline Production from Palm Oil: Effect of Palladium Cooperated with Second Metal on Hydrocracking Reaction. Catalysts. 2020; 10(2):241. https://doi.org/10.3390/catal10020241
Chicago/Turabian StyleSrihanun, Nithinun, Praepilas Dujjanutat, Papasanee Muanruksa, and Pakawadee Kaewkannetra. 2020. "Biofuels of Green Diesel–Kerosene–Gasoline Production from Palm Oil: Effect of Palladium Cooperated with Second Metal on Hydrocracking Reaction" Catalysts 10, no. 2: 241. https://doi.org/10.3390/catal10020241
APA StyleSrihanun, N., Dujjanutat, P., Muanruksa, P., & Kaewkannetra, P. (2020). Biofuels of Green Diesel–Kerosene–Gasoline Production from Palm Oil: Effect of Palladium Cooperated with Second Metal on Hydrocracking Reaction. Catalysts, 10(2), 241. https://doi.org/10.3390/catal10020241




