Novel Insights into the Existence of the Putative UDP-Glucuronate 5-Epimerase Specificity
Abstract
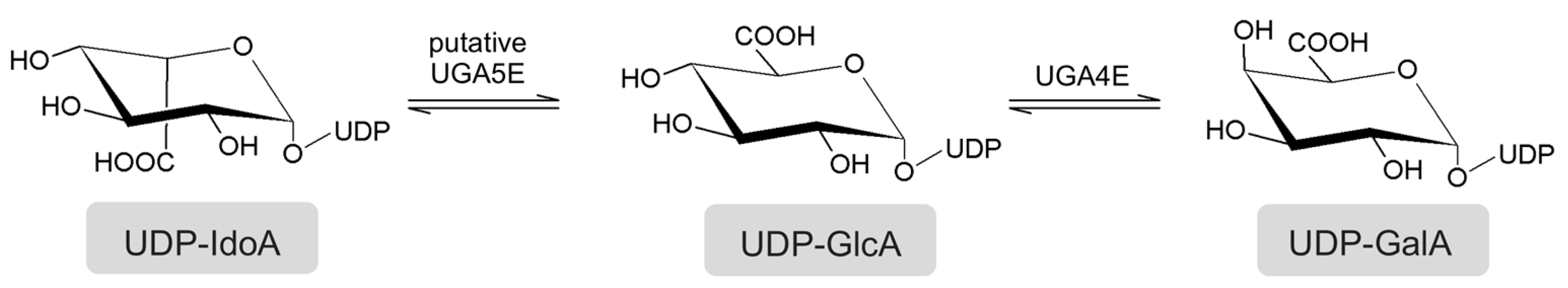
:1. Introduction
2. Results
2.1. Selection of a UGA5E Sequence
2.2. Evaluation of TgUGAE
2.3. In Silico Analysis of the UGA5E Specificity
2.4. Evaluation of ArUGAE
2.5. Analysis of the UGA5E Specificity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Gene Cloning and Transformation
4.3. Enzyme Production
4.4. Activity Testing
4.5. Sequence Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Samuel, J.; Tanner, M. Mechanistic aspects of enzymatic carbohydrate epimerization. Nat. Prod. Rep. 2002, 19, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Beerens, K.; Overtveldt, S.; Van Desmet, T. The “epimerring” highlights the potential of carbohydrate epimerases for rare sugar production. Biocatal. Biotransform. 2017, 35, 230–237. [Google Scholar] [CrossRef]
- Van Overtveldt, S.; Verhaeghe, T.; Joosten, H.J.; van den Bergh, T.; Beerens, K.; Desmet, T. A structural classification of carbohydrate epimerases: From mechanistic insights to practical applications. Biotechnol. Adv. 2015, 33, 1814–1828. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Schmaltz, R.M.; Bozeman, T.C.; Paul, R.; Rishel, M.J.; Tsosie, K.S.; Hecht, S.M. Selective tumor cell targeting by the disaccharide moiety of bleomycin. J. Am. Chem. Soc. 2013, 135, 2883–2886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathé, C.; Gosselin, G. l-Nucleoside enantiomers as antivirals drugs: A mini-review. Antivir. Res. 2006, 71, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Beerens, K.; Desmet, T.; Soetaert, W. Enzymes for the biocatalytic production of rare sugars. J. Ind. Microbiol. Biotechnol. 2012, 39, 823–834. [Google Scholar] [CrossRef]
- Gevaert, O.; Van Overtveldt, S.; Beerens, K.; Desmet, T. Characterization of the first bacterial and thermostable GDP-mannose 3,5-epimerase. Int. J. Mol. Sci. 2019, 20, 3530. [Google Scholar] [CrossRef] [Green Version]
- Major, L.L.; Wolucka, B.A.; Naismith, J.H. Structure and function of GDP-mannose-3′,5′-epimerase: An enzyme which performs three chemical reactions at the same active site. J. Am. Chem. Soc. 2005, 127, 18309–18320. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, B.; Davidson, E.A. UDP-d-glucuronic acid-5-epimerase and UDP-N-acetylglucosamine-4-epimerase of rabbit skin. BBA—Enzymol. Subj. 1962, 73, 145–151. [Google Scholar] [CrossRef]
- Damus, P.S.; Hicks, M.; Rosenberg, R.D. Anticoagulant action of heparin. Nature 1973, 246, 355–357. [Google Scholar] [CrossRef]
- Rabenstein, D. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Linhardt, R.J. Lessons learned from the contamination of heparin. Nat. Prod. Rep. 2009, 26, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulaney, S.B.; Huang, X. Strategies in synthesis of heparin/heparan sulfate oligosaccharides: 2000-present. Adv. Carbohydr. Chem. Biochem. 2012, 67, 95–136. [Google Scholar] [PubMed] [Green Version]
- Sugahara, K.; Mikami, T.; Uyama, T.; Mizuguchi, S.; Nomura, K.; Kitagawa, H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003, 13, 612–620. [Google Scholar] [CrossRef]
- Trowbridge, J.M.; Gallo, R.L. Dermatan sulfate: New functions from an old glycosaminoglycan. Glycobiology 2002, 12, 117R–125R. [Google Scholar] [CrossRef]
- Neufeld, E.F. UDP-d-galacturonic acid 4-epimerase from radish roots. Methods Enzymol. 1966, 8, 276–277. [Google Scholar]
- Dalessandro, G.; Northcote, D.H. Changes in enzymic activities of nucleoside diphosphate sugar interconversions during differentiation of cambium to xylem in pine and fir. Biochem. J. 1977, 162, 267–279. [Google Scholar] [CrossRef] [Green Version]
- Regué, M.; Hita, B.; Pique, N.; Izquierdo, L.; Merino, S.; Fresno, S.; Benedi, V.J.; Tomás, J.M. A gene, uge, is essential for Klebsiella pneumoniae virulence. Infect. Immun. 2004, 72, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, R.; Lopez, R.; De Frutos, M.; García, E. First molecular characterization of a uridine diphosphate galacturonate 4-epimerase: An enzyme required for capsular biosynthesis in Streptococcus pneumoniae type 1. Mol. Microbiol. 1999, 31, 703–713. [Google Scholar] [CrossRef]
- Sun, H.; Ko, T.P.; Liu, W.; Liu, W.; Zheng, Y.; Chen, C.C.; Guo, R.T. Structure of an antibiotic-synthesizing UDP-glucuronate 4-epimerase MoeE5 in complex with substrate. Biochem. Biophys. Res. Commun. 2019, 521, 31–36. [Google Scholar] [CrossRef]
- Kereszt, A.; Kiss, E.; Reuhs, B.L.; Carlson, R.W.; Kondorosi, Á.; Putnoky, P. Novel rkp gene clusters of Sinorhizobium meliloti involved in capsular polysaccharide production and invasion of the symbiotic nodule: The rkpK gene encodes a UDP-glucose dehydrogenase. J. Bacteriol. 1998, 180, 5426–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, J.D.; Labthavikul, S.T.; Otey, C.R.; Arnold, F.H. Protein stability promotes evolvability. Proc. Natl. Acad. Sci. USA 2006, 103, 5869–5874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eijsink, V.G.H.; Gaseidnes, S.; Borchert, T.V.; van den Burg, B. Directed evolution of enzyme stability. Biomol. Eng. 2005, 22, 21–30. [Google Scholar] [CrossRef]
- Elkins, J.G.; Hamilton-Brehm, S.D.; Lucas, S.; Han, J.; Lapidus, A.; Cheng, J.F.; Goodwin, L.A.; Pitluck, S.; Peters, L.; Mikhailova, N.; et al. Complete genome sequence of the hyperthermophilic sulfate-reducing bacterium Thermodesulfobacterium geofontis OPF15T. Genome Announc. 2013, 1, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, S.; Kamagata, Y.; Hanada, S.; Shoun, H. Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 2000, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Göker, M.; Daligault, H.; Mwirichia, R.; Lapidus, A.; Lucas, S.; Deshpande, S.; Pagani, I.; Tapia, R.; Cheng, J.F.; Goodwin, L.; et al. Complete genome sequence of the thermophilic sulfur-reducer Desulfurobacterium thermolithotrophum type strain (BSAT) from a deep-sea hydrothermal vent. Stand. Genomic Sci. 2011, 5, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radke-Mitchell, L.C.; Sandine, W.E. Influence of temperature on associative growth of Streptococcus thermophilus and Lactobacillus bulgaricus. J. Dairy Sci. 1986, 69, 2558–2568. [Google Scholar] [CrossRef]
- Kavanagh, K.L.; Jörnvall, H.; Persson, B.; Oppermann, U. The SDR superfamily: Functional and structural diversity within a family of metabolic and regulatory enzymes. Cell. Mol. Life Sci. 2008, 65, 3895–3906. [Google Scholar] [CrossRef] [Green Version]
- Raedts, J.; Kengen, S.W.M.; Van Der Oost, J. Occurrence of l-iduronic acid and putative d-glucuronyl C5-epimerases in prokaryotes. Glycoconj. J. 2011, 28, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Raedts, J.; Lundgren, M.; Kengen, S.W.M.; Li, J.P.; Van Der Oost, J. A novel bacterial enzyme with d-glucuronyl C5-epimerase activity. J. Biol. Chem. 2013, 288, 24332–24339. [Google Scholar] [CrossRef] [Green Version]
- Debarnot, C.; Monneau, Y.R.; Roig-Zamboni, V.; Delauzun, V.; Le Narvor, C.; Richard, E.; Hénault, J.; Goulet, A.; Fadel, F.; Vivès, R.R.; et al. Substrate binding mode and catalytic mechanism of human heparan sulfate d-glucuronyl C5 epimerase. Proc. Natl. Acad. Sci. USA 2019, 116, 6760–6765. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, B.; Maccarana, M.; Goodlett, D.R.; Malmström, A.; Malmström, L. Identification of the active site of DS-epimerase 1 and requirement of N-glycosylation for enzyme function. J. Biol. Chem. 2009, 284, 1741–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccarana, M.; Olander, B.; Malmström, J.; Tiedemann, K.; Aebersold, R.; Lindahl, U.; Li, J.P.; Malmström, A. Biosynthesis of dermatan sulfate: Chondroitin-glucuronate C5-epimerase is identical to SART2. J. Biol. Chem. 2006, 281, 11560–11568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; Thompson, J.D.; Higgins, D.G.; Mcwilliam, H.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| EC Number | Specificity |
|---|---|
| 5.1.3.2 | UDP-glucose 4-epimerase * |
| 5.1.3.10 | CDP-paratose 2-epimerase * |
| 5.1.3.6 | UDP-glucuronate 4-epimerase * |
| 5.1.3.7 | UDP-N-acetylglucosamine 4-epimerase * |
| 5.1.3.16 | UDP-glucosamine 4-epimerase |
| 5.1.3.25 | dTDP-l-rhamnose 4-epimerase |
| 5.1.3.26 | N-acetyl-α-d-glucosaminyl-diphospho-ditrans, octacis-undecaprenol 4-epimerase |
| 5.1.3.5 | UDP-l-arabinose 4-epimerase |
| 5.1.3.12 | UDP-glucuronate 5-epimerase |
| 5.1.3.20 | ADP-l-glyceromanno-heptose 6-epimerase * |
| / | UDP-2-acetamido-2,6-dideoxy-beta-l-arabino-4-hexulose 3-epimerase |
| 5.1.3.18 | GDP-mannose 3,5-epimerase * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gevaert, O.; Van Overtveldt, S.; Da Costa, M.; Beerens, K.; Desmet, T. Novel Insights into the Existence of the Putative UDP-Glucuronate 5-Epimerase Specificity. Catalysts 2020, 10, 222. https://doi.org/10.3390/catal10020222
Gevaert O, Van Overtveldt S, Da Costa M, Beerens K, Desmet T. Novel Insights into the Existence of the Putative UDP-Glucuronate 5-Epimerase Specificity. Catalysts. 2020; 10(2):222. https://doi.org/10.3390/catal10020222
Chicago/Turabian StyleGevaert, Ophelia, Stevie Van Overtveldt, Matthieu Da Costa, Koen Beerens, and Tom Desmet. 2020. "Novel Insights into the Existence of the Putative UDP-Glucuronate 5-Epimerase Specificity" Catalysts 10, no. 2: 222. https://doi.org/10.3390/catal10020222
APA StyleGevaert, O., Van Overtveldt, S., Da Costa, M., Beerens, K., & Desmet, T. (2020). Novel Insights into the Existence of the Putative UDP-Glucuronate 5-Epimerase Specificity. Catalysts, 10(2), 222. https://doi.org/10.3390/catal10020222





