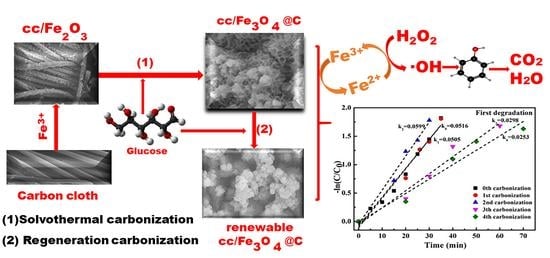
A High-Efficient Carbon-Coated Iron-Based Fenton-Like Catalyst with Enhanced Cycle Stability and Regenerative Performance
Abstract
1. Introduction
2. Results and Discussion
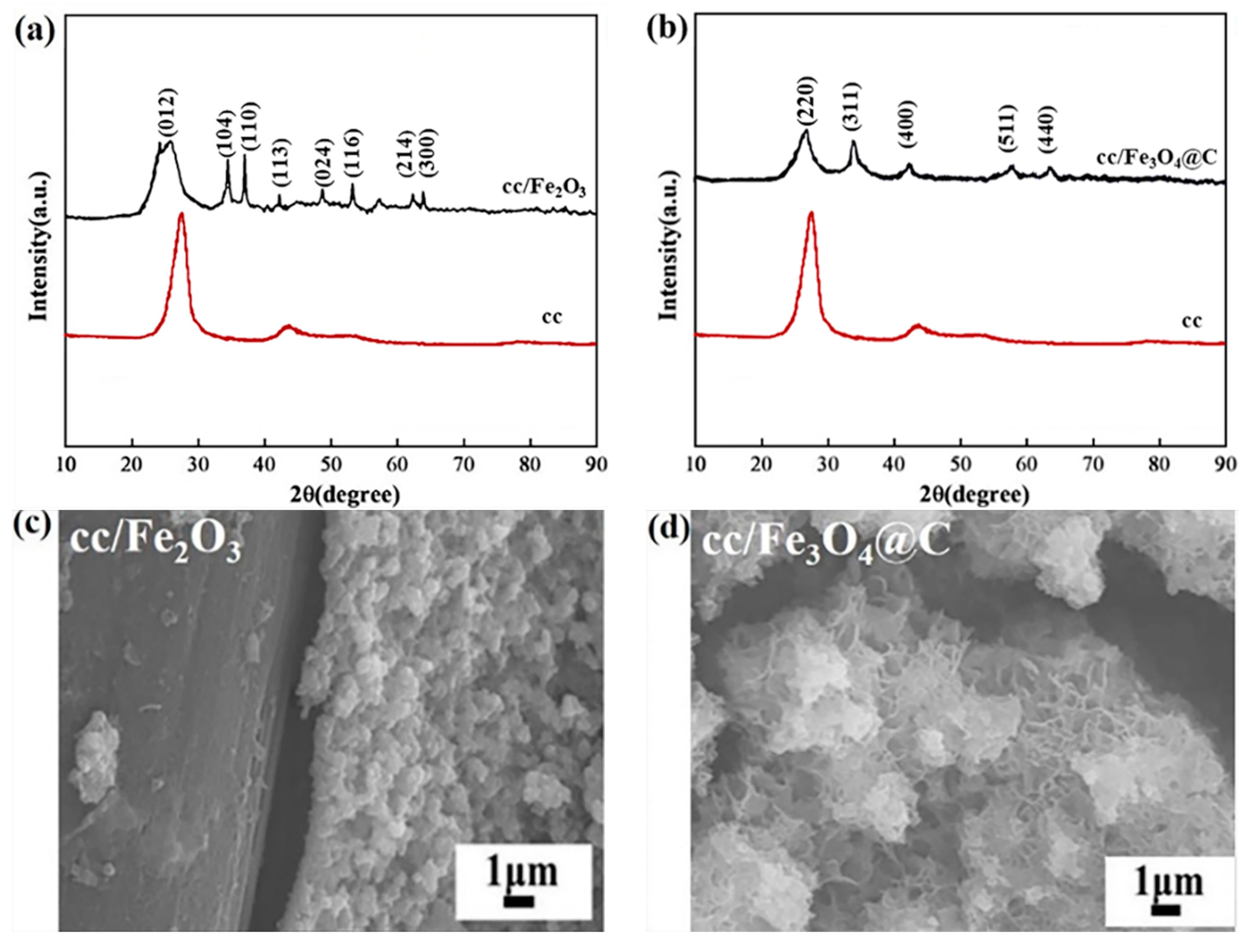
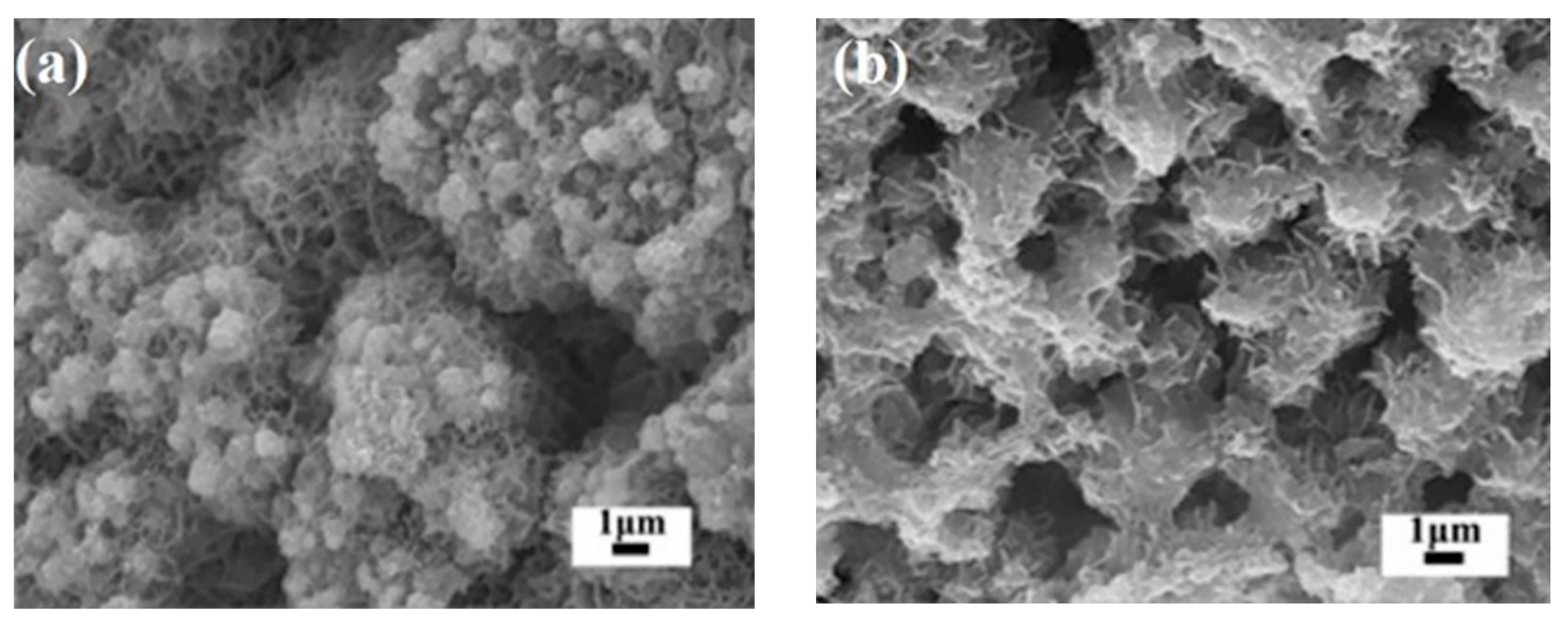
2.1. Characterization of Iron Oxide Fenton-Like Catalysts
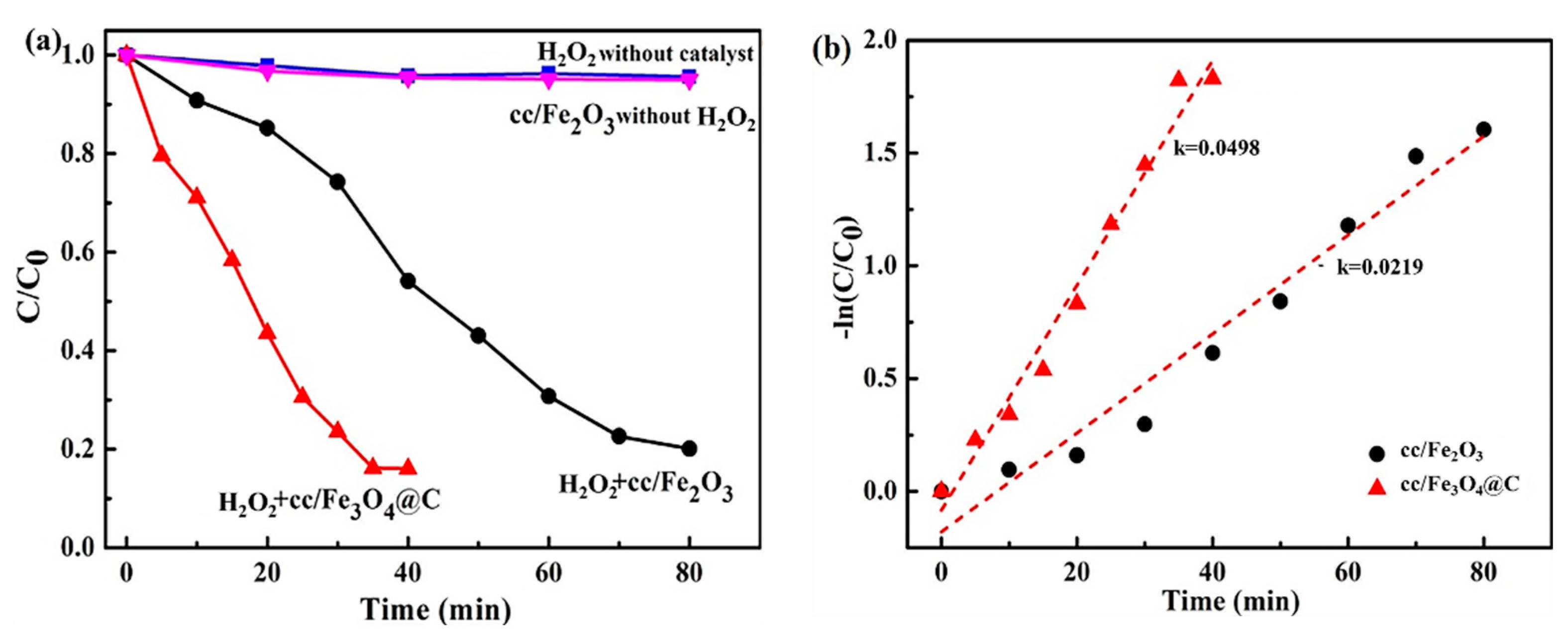
2.2. Degradation Performance of Iron Oxide Fenton-Like Catalysts
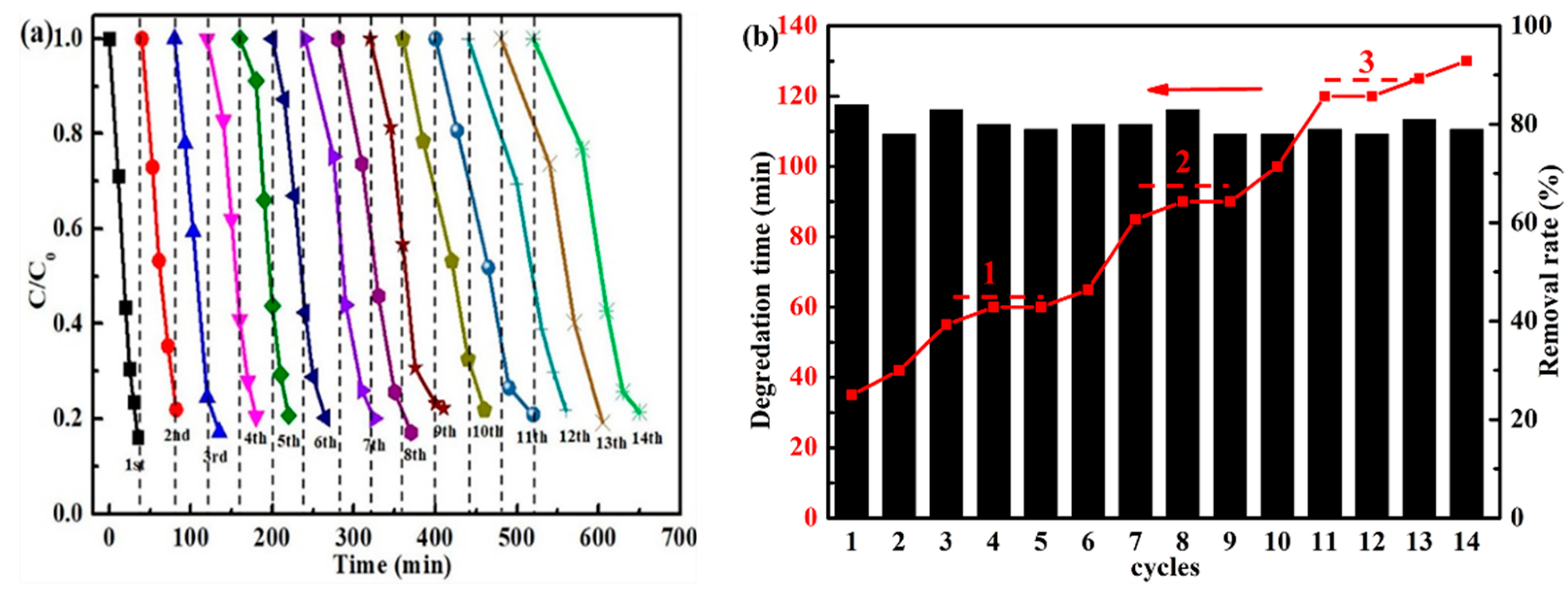
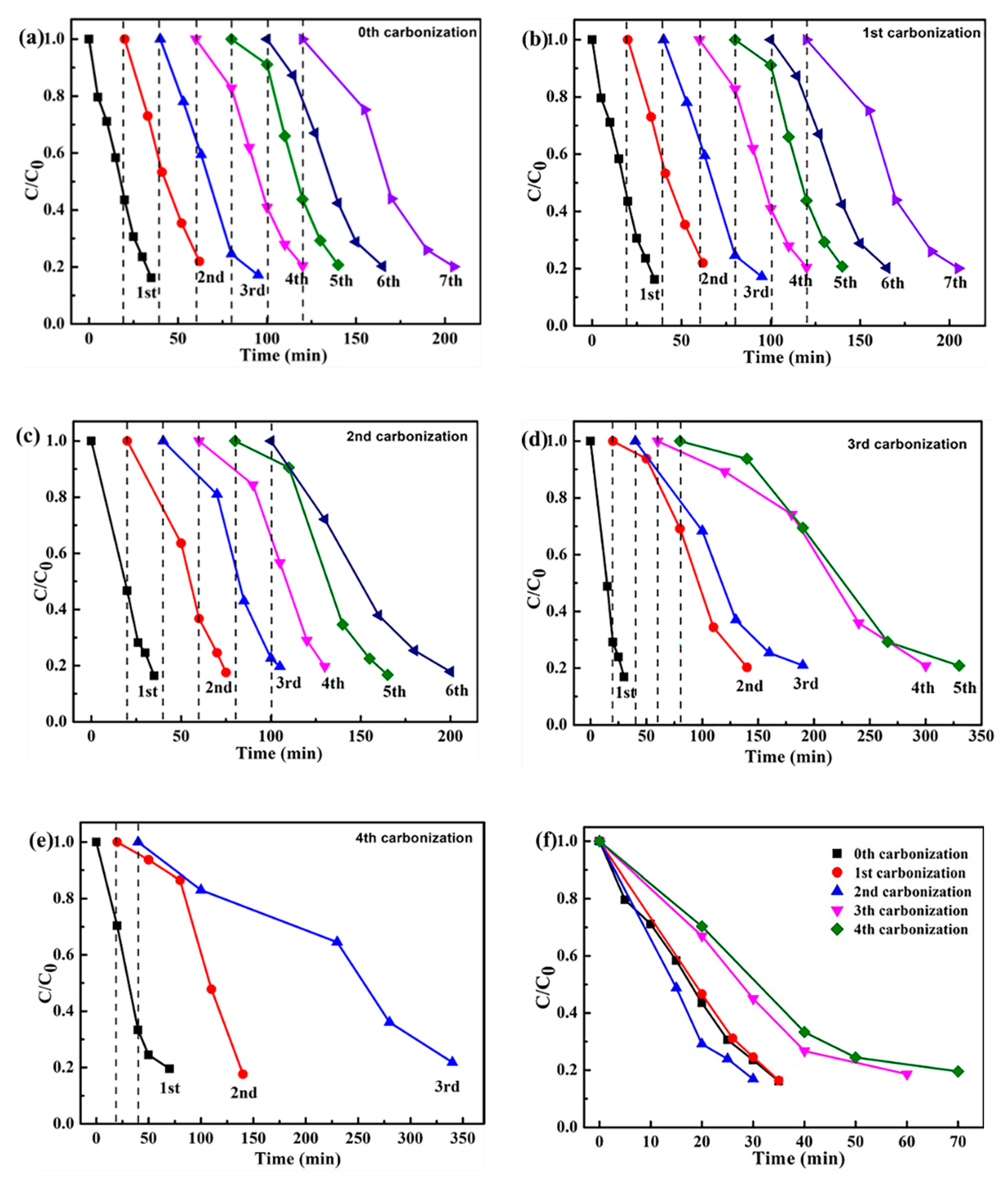
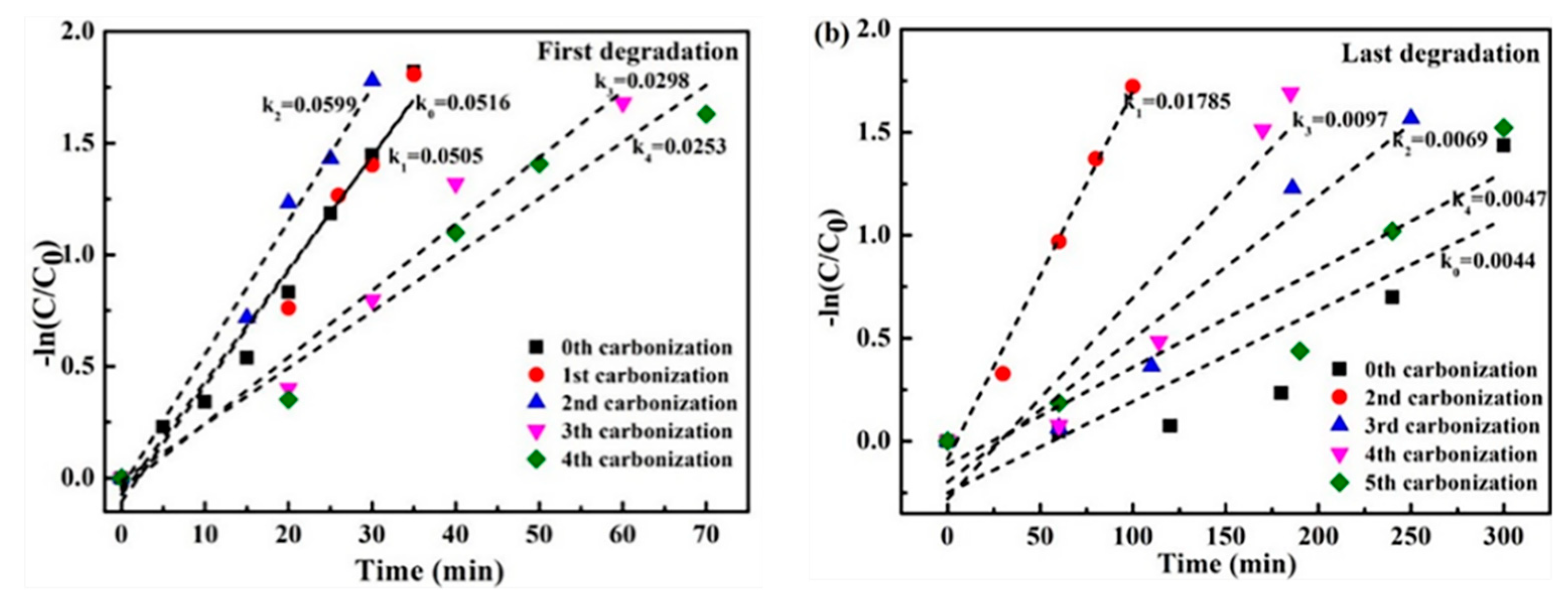
2.3. Cyclic Stability of Iron Oxide Fenton-Like Catalyst
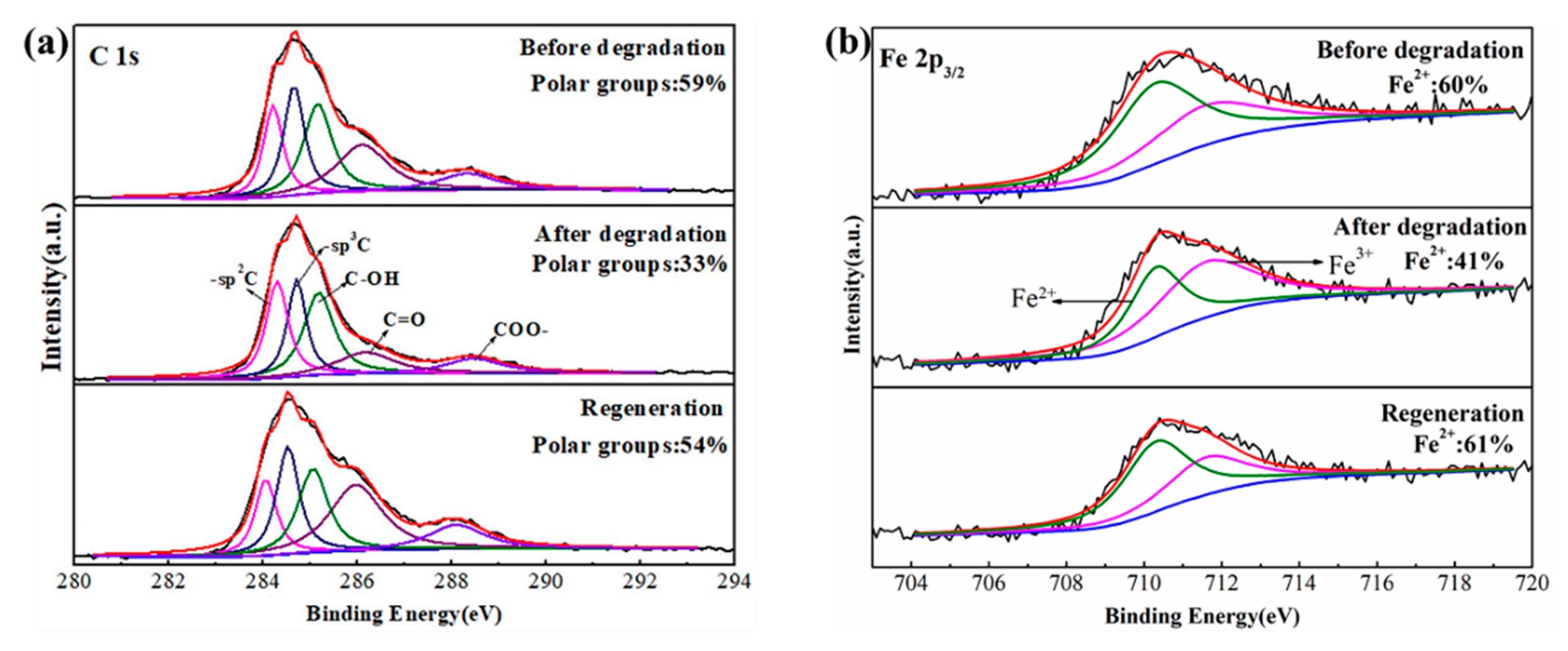
2.4. Renewability of Iron Oxide Fenton-Like Catalyst
3. Experimental
3.1. Preparation of Carbon-Coated Iron Oxide Fenton-Like Catalyst
3.2. Characterization Methods
3.3. Catalytic Performance Test
4. Conclusions
- (1)
- Glucose works as a carbon source during the ethanol solvothermal treatment, which both transforms Fe2O3 into Fe3O4 through reduction effect, and coats on Fe3O4 in a flaky cross-linked network structure with polar C-groups.
- (2)
- The cc/Fe3O4@C shows excellent catalytic activities, the removal rate of 35 ppm phenol reaches 84% within 35 min when H2O2 is 6 mmol and pH is 4. The degradation process corresponds to the first-order kinetic curve (k = 0.0498 min−1). The catalysts present the enhanced cycle stability. Although the degradation time is prolonged to 120 min, the removal rate is still around 80 % in the 14th cycle.
- (3)
- Ethanol solvothermal treatment with glucose as carbon source for the used catalysts can realize the regeneration. The kinetic constants of the first two regenerated catalysts are consistent with that of the fresh one. However, the last two regenerations’ constant is lowered to half of the original, which may originate from the inevitable iron oxide loss.
- (4)
- The formation of the amorphous carbon layer on catalysts’ surface determines the enhanced cycle stability and regenerative performance. The carbon layer makes the consumed Fe2+ replenished in time by reducing the Fe3+ while cutting back the iron leaching. Besides, polar C-groups of the carbon layer are conducive to the adsorption of phenol and H2O2, further promoting the catalyst’s degradation performance.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gou, N.; Yuan, S.; Lan, J.; Gao, C.; Alshawabkeh, A.N.; Gu, A.Z. A Quantitative Toxic Genomics Assay Reveals the Evolution and Nature of Toxicity during the Transformation of Environmental Pollutants. Environ. Sci. Technol. 2014, 48, 8855–8863. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vítor, J.P.V. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Envrion. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Zepp, R.G.; Faust, B.C.; Holgne, J. Hydroxyl radical formation in aqueous reactions (pH 3–8) of iron(ii) with hydrogen peroxide: The Photo-Fenton reaction. Environ. Sci. Technol. 1992, 26, 313–319. [Google Scholar] [CrossRef]
- Chen, Y.P.; Li, M.Y.; Chen, P.J.; Zheng, Y.M. Electrospun spongy zero-valent iron as excellent electro-Fenton catalyst for enhanced sulfathiazole removal by a combination of adsorption and electro-catalytic oxidation. J. Hazard. Mater. 2019, 371, 576–585. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 1, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Rusevova, K.; Kopinke, F.D.; Georgi, A. Nano-sized magnetic iron oxides as catalysts for heterogeneous Fenton-like reactions-Influence of Fe (II)/Fe (III) ratio on catalytic performance. J. Hazard. Mater. 2012, 241–242, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Aghabarari, B.; Alizadeh, M.; Khanlarkhani, A.; Abu-Zahra, N. High efficiency decolorization of wastewater by Fenton catalyst: Magnetic iron-copper hybrid oxides. J. Water Process. Eng. 2020, 37, 101540. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Kurosu, S.; Suzuki, M.; Kawase, Y. Hydroxyl radical generation by zero-valent iron/Cu (ZVI/Cu) bimetallic catalyst in wastewater treatment: Heterogeneous Fenton/Fenton-like reactions by Fenton reagents formed in-situ under oxic conditions. Chem. Eng. J. 2018, 334, 1537–1549. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Hartmann, M.; Kullmann, S.; Keller, H. Wastewater Treatment with Heterogeneous Fenton-type Catalysts Based on Porous Materials. J. Mater. Chem. 2010, 20, 9002–9017. [Google Scholar] [CrossRef]
- Liang, L.; Cheng, L.; Zhang, Y.; Wang, Q.; Meng, X. Efficiency and mechanisms of rhodamine B degradation in Fenton-like systems based on zero-valent iron. RSC Adv. 2020, 10, 28509–28515. [Google Scholar] [CrossRef]
- Xue, X.; Hanna, K.; Deng, N. Fenton-like oxidation of Rhodamine B in the presence of two types of iron (II, III) oxide. J. Hazard. Mater. 2009, 166, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Liang, X.; He, Z.; Liao, Y.; Xie, Z.; Liu, P.; Ji, S.; He, H.; Li, D.; Zhang, J. Heterogeneous activation of Oxone by substituted magnetites Fe3–xMxO4 (Cr, Mn, Co, Ni) for degradation of Acid Orange II at neutral pH. J. Mol. Catal. A Chem. 2015, 398, 86–94. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Hussain, I.; Li, C.; Li, J.; Sun, X.; Shen, Z.; Han, W.; Wang, L. Iron-copper bimetallic nanoparticles supported on hollow mesoporous silica sphere: The effect of Fe/Cu ratio on heterogeneous Fenton degradation of dye. RSC Adv. 2016, 59, 54623–54635. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J. Degradation of sulfamethazine antibiotics using Fe3O4-Mn3O4 nanocomposite as a Fenton-like catalyst. J. Chem. Technol. Biotechnol. 2017, 92, 874–883. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Ai, Z.; Zhang, L. Hydrothermal synthesis of FeS2 as a high-efficiency Fenton reagent to degrade alachlor via superoxide-mediated Fe(II)/Fe(III) cycle. ACS Appl. Mater. Interfaces 2015, 7, 28534–28544. [Google Scholar] [CrossRef]
- Mesquita, I.; Matos, L.C.; Duarte, F.; Maldonado-Hodar, F.J.; Mendes, A.; Madeira, L.M. Treatment of azo dye-containing wastewater by a Fenton-like process in a continuous packed-bed reactor filled with activated carbon. J. Hazard. Mater. 2012, 237, 30–37. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhao, Y.; Dionysiou, D.D. Aligned α-FeOOH nanorods anchored on a graphene oxide-carbon nanotubes aerogel can serve as an effective Fenton-like oxidation catalyst. Appl. Catal. B Environ. 2017, 23, 74–86. [Google Scholar] [CrossRef]
- Hassani, A.; Çelikdağ, G.; Eghbali, P.; Sevim, M.; Karaca, S.; Metin, Ö. Heterogeneous sono-Fenton-like process using magnetic cobalt ferrite-reduced graphene oxide (CoFe2O4-rGO) nanocomposite for the removal of organic dyes from aqueous solution. Ultrason. Sonochem. 2018, 40, 841–852. [Google Scholar] [CrossRef]
- Barrios-Bermúdez, N.; González-Avendaño, M.; Lado-Touriño, I.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. Fe-Cu Doped Multiwalled Carbon Nanotubes for Fenton-like Degradation of Paracetamol Under Mild Conditions. Nanomaterials 2020, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yao, Z.; Zhang, D.; Li, D.; Zhang, Z.; Jiang, Z. Rational synthesis of micronano dendritic ZVI@Fe3O4 modified with carbon quantum dots and oxygen vacancies for accelerating Fenton-like oxidation. J. Sci. Total Environ. 2019, 671, 1056–1065. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Alvaro, M.; Garcia, H. Metal nanoparticles as heterogeneous Fenton catalysts. ChemSusChem 2020, 5, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.H.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Moreno-Castilla, C.; Costa, C.A.; Madeira, L.M. Azo-dye Orange II degradation by heterogeneous Fenton-like reaction using carbon-Fe catalysts. Appl. Catal. B Envrion. 2007, 75, 312–323. [Google Scholar] [CrossRef]
- Li, X.; Gai, F.; Guan, B.; Zhang, Y.; Liu, Y.; Huo, Q. Fe@ C core–shell and Fe@ C yolk–shell particles for effective removal of 4-chlorophenol. J. Mater. Chem. A 2015, 3, 3988–3994. [Google Scholar]
- Zhuang, Y.; Liu, J.; Yuan, S.; Ge, B.; Zhang, Y. Degradation of octane using an efficient and stable core-shell Fe3O4@C during Fenton processes: Enhanced mass transfer, adsorption and catalysis. Appl. Surf. Sci. 2020, 515, 146083. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Wu, R.; Yu, B.; Li, H.; Zhang, X.; Xie, J.; Yang, S. Fe3O4/SiO2/C nanocomposite as a high-performance Fenton-like catalyst in a neutral environment. RSC Adv. 2016, 10, 1039. [Google Scholar]
- Zhan, J.; Li, M.; Zhang, X.; An, Y.; Zhou, H. Aerosol-assisted submicron γ-Fe2O3/C spheres as a promising heterogeneous Fenton-like catalyst for soil and groundwater remediation: Transport, adsorption and catalytic ability. Chin. Chem. Lett. 2020, 31, 147–152. [Google Scholar] [CrossRef]
- Zhang, X.; He, M.; Liu, J.H.; Liao, R.; Zhao, L.; Xie, J.; Wang, R.; Yang, S.; Wang, H.; Liu, Y. Fe3O4@C nanoparticles as high-performance Fenton-like catalyst for dye decoloration. Chin. Sci. Bull. 2014, 59, 3406–3412. [Google Scholar] [CrossRef]
- Zubir, N.A.; Yacou, C.; Motuzas, J.; Zhang, X.; Zhao, X.; da Costa, J.C.D. The sacrificial role of graphene oxide in stabilising a Fenton-like catalyst GO-Fe3O4. Chem. Commun. 2015, 51, 9291–9293. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Yu, B.; Wu, R.; Mai, J.; Wang, R.; Chen, L.; Yang, S. Preparation of Fe3O4/TiO2/C nanocomposites and their application in Fenton-like catalysis for dye decoloration. Catalysts 2016, 6, 146. [Google Scholar] [CrossRef]
- Bohn, C.D.; Cleeton, J.P.; Müller, C.R.; Davidson, J.F.; Hayhurst, A.N.; Scott, S.A.; Dennis, J.S. The kinetics of the reduction of iron oxide by carbon monoxide mixed with carbon dioxide. AlChE J. 2010, 56, 1016–1029. [Google Scholar] [CrossRef]
- Li, J.; Li, B.W.; Zhang, B.W. Microwave carbothermic reduction from Fe2O3 to Fe3O4 powders. J. Univ. Sci. Technol. B. 2011, 33, 1127–1132. [Google Scholar]
- Jiang, Y.; Yu, Z.; Xia, Q.; Zhang, Y.; Wu, Z.; Jiang, Z.; Yao, Z.; Wu, Z. Structure and corrosion resistance of PEO ceramic coatings on AZ91D Mg alloy under three kinds of power modes. Int. J. Appl. Ceram. Technol. 2013, 10, E310–E317. [Google Scholar] [CrossRef]
- Guo, L.; Chen, F.; Fan, X.; Cai, W.; Zhang, J. S-doped α-Fe2O3 as a highly active heterogeneous Fenton-like catalyst towards the degradation of acid orange 7 and phenol. Appl. Catal. B Envrion. 2010, 96, 162–168. [Google Scholar] [CrossRef]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Gilarranz, M.A.; Rodríguez, J.J. Chemical pathway and kinetics of phenol oxidation by Fenton’s reagent. Environ. Sci. Technol. 2005, 39, 9295–9302. [Google Scholar] [CrossRef]
- Wei, X.; Wu, H.; He, G.; Guan, Y. Efficient degradation of phenol using iron-montmorillonite as a fenton catalyst: Importance of visible light irradiation and intermediates. J. Hazard. Mater. 2017, 321, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Bremner, D.H.; Burgess, A.E.; Houllemare, D.; Namkung, K.C. Phenol degradation using hydroxyl radicals generated from zero-valent iron and hydrogen peroxide. Appl. Catal. B Envrion. 2006, 63, 15–19. [Google Scholar] [CrossRef]
- Fan, Z.; Kai, W.; Yan, J.; Wei, T.; Zhi, L.; Feng, J.; Ren, Y.; Song, L.; Fei, W. Facile synthesis of graphene nanosheets via Fe reduction of exfoliated graphite oxide. ACS Nano 2010, 5, 191–198. [Google Scholar] [CrossRef]

| Catalyst | Preparation | Degradable SubStances | pH | Time Degradation | Cycles; Last Degradation | Ref. |
|---|---|---|---|---|---|---|
| Fe-AC (activated carbon) | impregnation of ferrous sulfate | Chicago Sky Blue (CSB) | 3 | 40–50 min 88% (pH = 3) | 3 88% | [18] |
| α-FeOOH@GCA | in-situ hydrolysis | Orange II (OII) | 3–10 | 10 min 23.7% | 6 6% | [19] |
| CoFe2O4-rGO | high temperature thermal decomposition | Acid Orange 7 (AO7) | 3–9 | 120 min 76.9% | 5 67.85% | [20] |
| Fe25Cu75/CNT | wetness impregnation | paracetamol | 3–6.6 | 120 min 87.8% (pH = 3) | 3 72.8% | [21] |
| Fe@Fe3O4/OVs@CQDs | solvothermal electrodeposition | phenol | 4–6.5 | 10 min 99% (pH = 4) | 4 99% | [22] |
| Fe@C yolk-shell | hydrothermal and thermal calcination | 4-chlorophenol | 4 | 12 min 100% (pH = 4) | 3 100% | [25] |
| core-shell Fe3O4@C | hydrothermal dehydrogenation | octane | 5 | 60 min 83.55% (pH = 7.5) | 5 83.55% | [26] |
| Fe3O4/SiO2 /C | hydrothermal dehydrogenation | methylene blue (MB) | 3.5–9.5 | 28 min 96% (pH = 7.5) | 8 82.0% | [27] |
| γ-Fe2O3/C | one-step aerosol-based procession | methylene blue (MB) | 3–9 | 240 min 100% (pH = 7) | 5 84.0% | [28] |
| Fe3O4@C NPs | co-precipitation hydrothermal dehydrogenation | methylene blue (MB) | 3–8 | 120 min 99.4% (pH = 3) | 4 74.3% | [29] |
| GO-Fe3O4 | codeposition and hummer method | Acid Orange 7 (AO7) | / | / | 7 98%–99% | [30] |
| Fe3O4/TiO2/C | hydrothermal dehydrogenation | methylene blue (MB) | 4–9 | 140 min 82% (pH = 6) | 8 >60% | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, J.; Zhang, X.; Hou, X.; Xu, H.; Yao, Z.; Jiang, Z. A High-Efficient Carbon-Coated Iron-Based Fenton-Like Catalyst with Enhanced Cycle Stability and Regenerative Performance. Catalysts 2020, 10, 1486. https://doi.org/10.3390/catal10121486
Li X, Wang J, Zhang X, Hou X, Xu H, Yao Z, Jiang Z. A High-Efficient Carbon-Coated Iron-Based Fenton-Like Catalyst with Enhanced Cycle Stability and Regenerative Performance. Catalysts. 2020; 10(12):1486. https://doi.org/10.3390/catal10121486
Chicago/Turabian StyleLi, Xin, Jiankang Wang, Xiao Zhang, Xianjin Hou, Hongbo Xu, Zhongping Yao, and Zhaohua Jiang. 2020. "A High-Efficient Carbon-Coated Iron-Based Fenton-Like Catalyst with Enhanced Cycle Stability and Regenerative Performance" Catalysts 10, no. 12: 1486. https://doi.org/10.3390/catal10121486
APA StyleLi, X., Wang, J., Zhang, X., Hou, X., Xu, H., Yao, Z., & Jiang, Z. (2020). A High-Efficient Carbon-Coated Iron-Based Fenton-Like Catalyst with Enhanced Cycle Stability and Regenerative Performance. Catalysts, 10(12), 1486. https://doi.org/10.3390/catal10121486