Influences of Magnesium Content in Rehydrated Mixed Oxides on Furfural Conversion
Abstract
1. Introduction
2. Results and Discussion
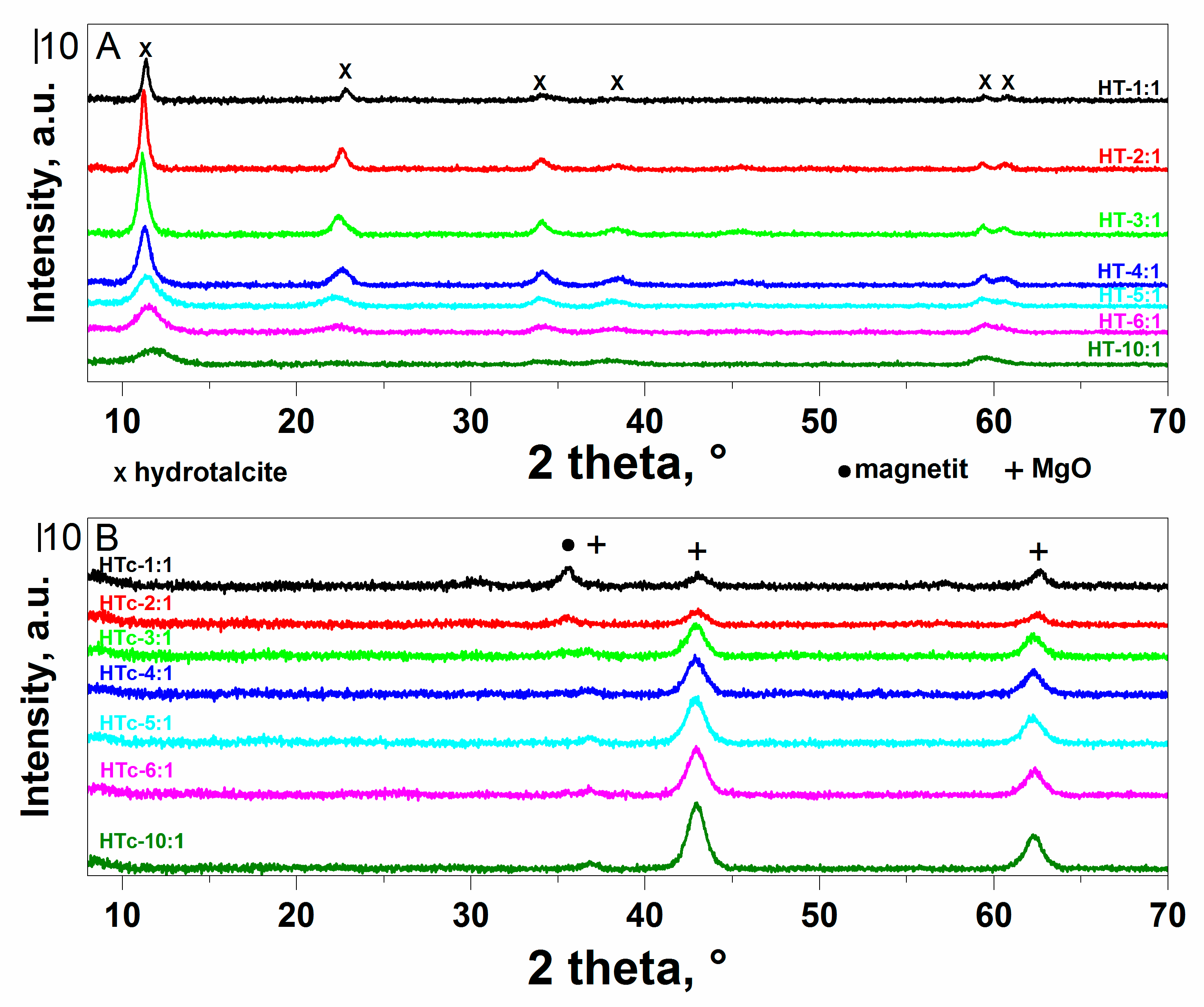
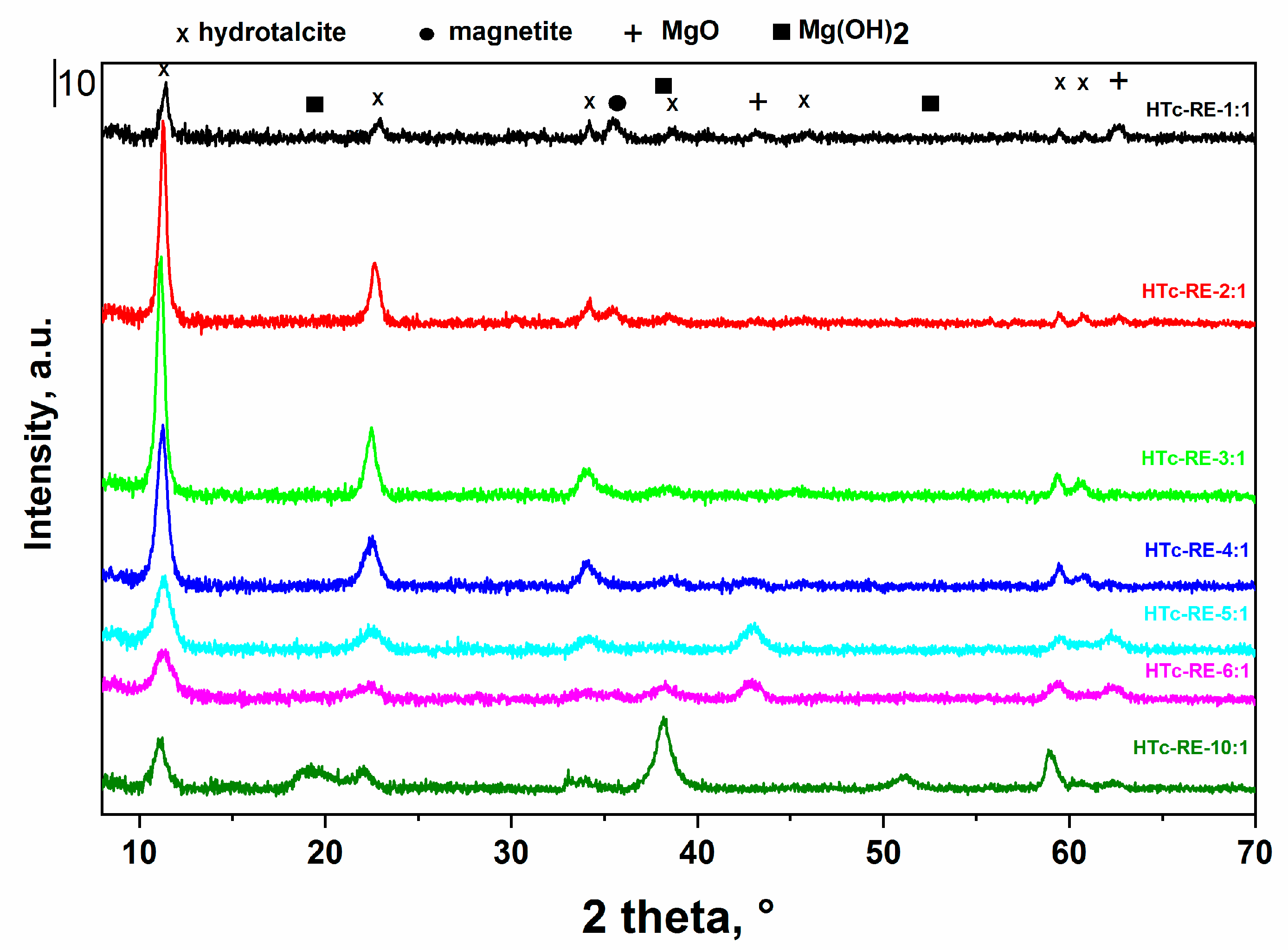
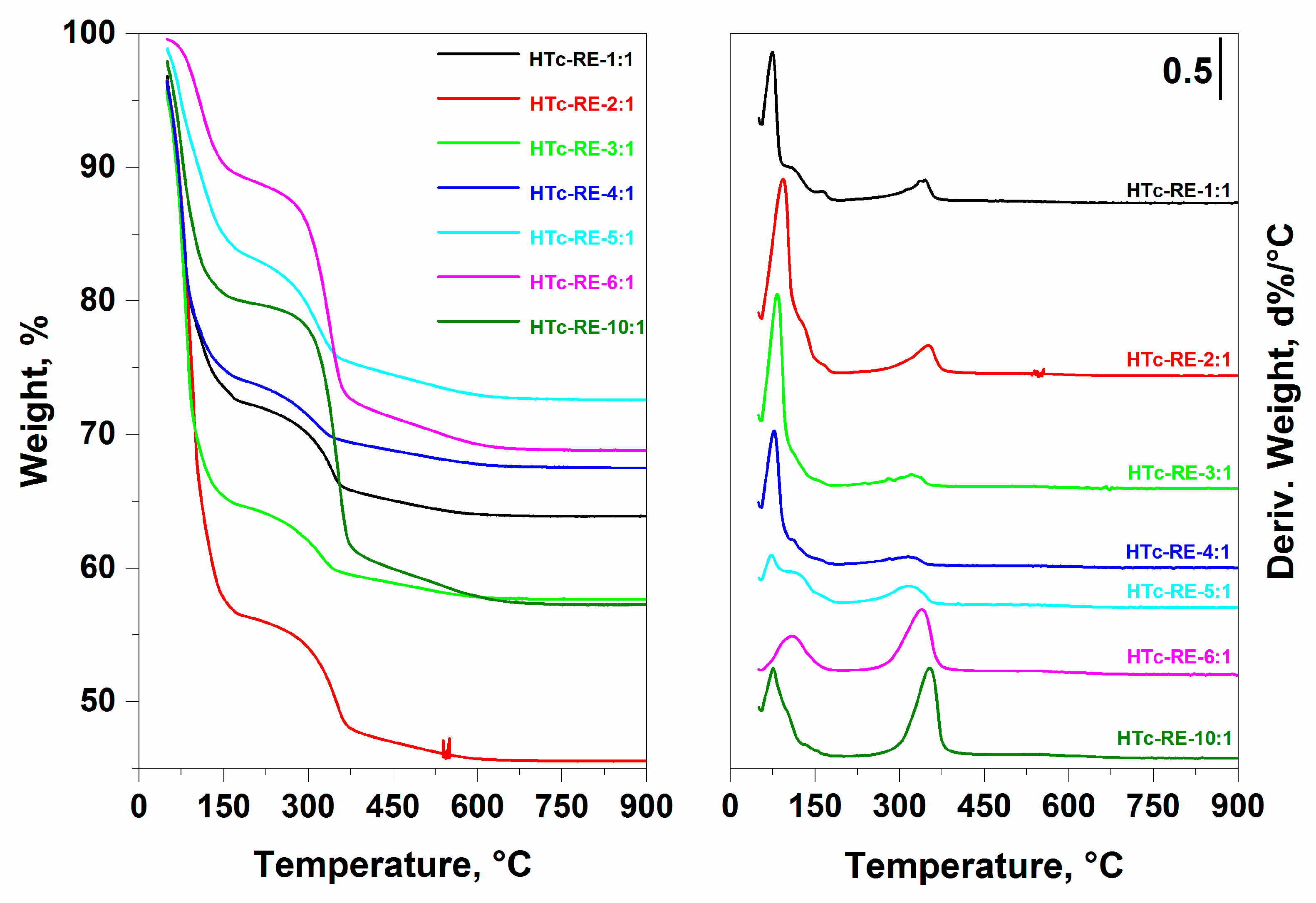
2.1. Structural and Textural Properties of Mg-Fe LDH and Mixed Oxide
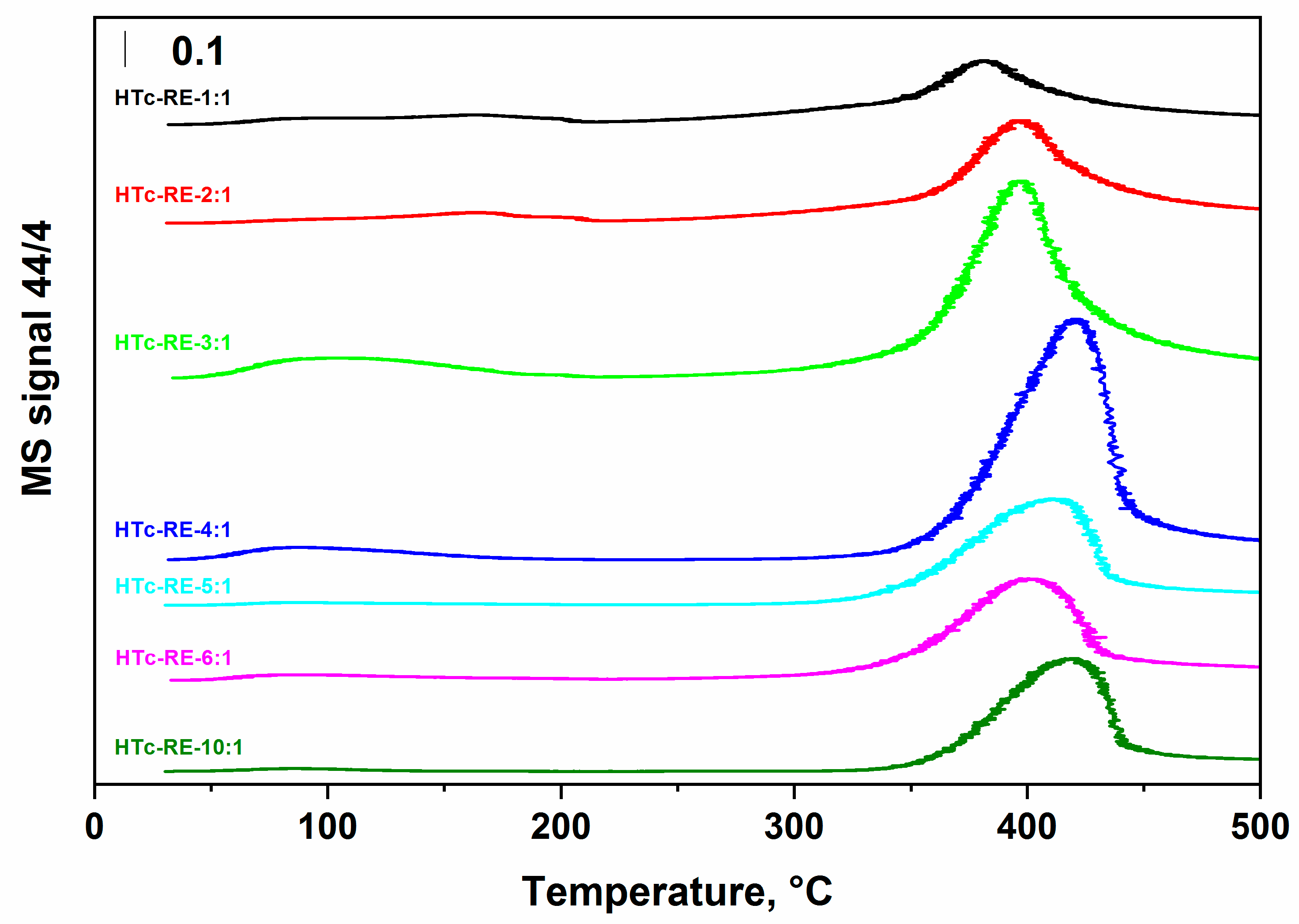
2.2. Basic Properties
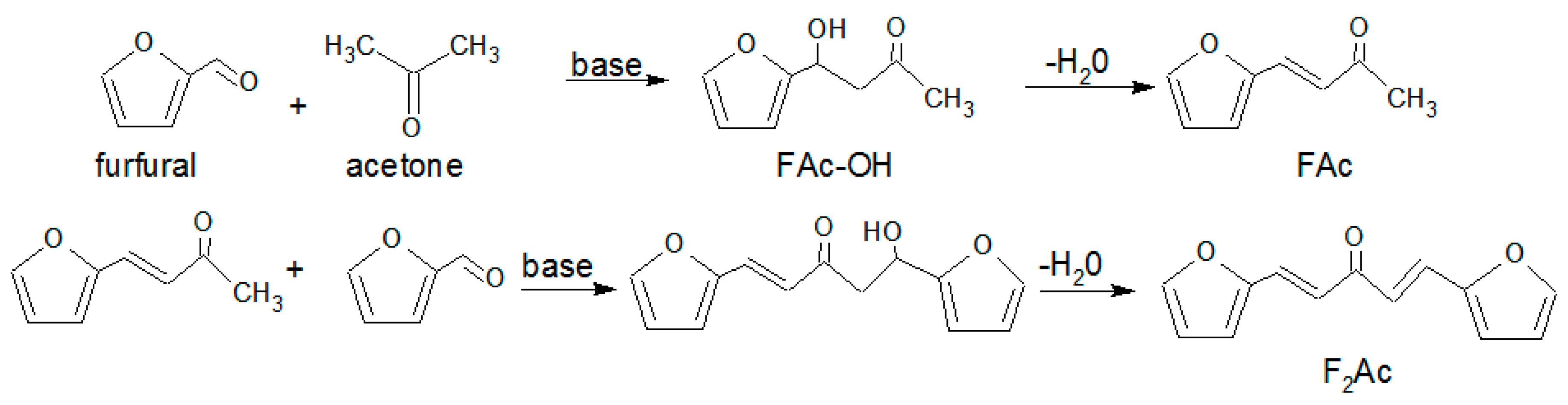
2.3. Aldol Condensation
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Characterisation of Catalyst
3.3. Catalytic Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghorbel, S.B.; Medina, F.; Ghorbel, A.; Segarra, A.M. Phosphoric acid intercalated Mg–Al hydrotalcite-like compounds for catalytic carboxylation reaction of methanol in a continuous system. Appl. Catal. A Gen. 2015, 493, 142–148. [Google Scholar] [CrossRef]
- Nawfal, M.; Gennequin, C.; Labaki, M.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Hydrogen production by methane steam reforming over Ru supported on Ni–Mg–Al mixed oxides prepared via hydrotalcite route. Int. J. Hydrog. Energy 2015, 40, 1269–1277. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Haldar, K.; Pal, S.K.; Chakraborty, S. Synthesis of Cu–Al layered double hydroxide nanofluid and characterization of its thermal properties. Appl. Clay Sci. 2015, 107, 98–108. [Google Scholar] [CrossRef]
- Xie, W.; Peng, H.; Chen, L. Calcined Mg–Al hydrotalcites as solid base catalysts for methanolysis of soybean oil. J. Mol. Catal. A Chem. 2006, 246, 24–32. [Google Scholar] [CrossRef]
- Hajek, M.; Kutalek, P.; Smolakova, L.; Troppova, I.; Capek, L.; Kubicka, D.; Kocik, J.; Thanh, D.N. Transesterification of rapeseed oil by Mg-Al mixed oxides with various Mg/Al molar ratio. Chem. Eng. J. 2015, 263, 160–167. [Google Scholar] [CrossRef]
- Kocík, J.; Frolich, K.; Perková, I.; Horáček, J. Pyroaurite-based Mg Fe mixed oxides and their activity in aldol condensation of furfural with acetone: Effect of oxide composition. J. Chem. Technol. Biotechnol. 2019, 94, 435–445. [Google Scholar] [CrossRef]
- Prescott, H.A.; Li, Z.J.; Kemnitz, E.; Trunschke, A.; Deutsch, J.; Lieske, H.; Auroux, A. Application of calcined Mg-Al hydrotalcites for Michael additions: An investigation of catalytic activity-and acid-base properties. J. Catal. 2005, 234, 119–130. [Google Scholar] [CrossRef]
- Dumbre, D.K.; Mozammel, T.; Selvakannan, P.R.; Hamid, S.B.A.; Choudhary, V.R.; Bhargava, S.K. Thermally decomposed mesoporous Nickel Iron hydrotalcite: An active solid-base catalyst for solvent-free Knoevenagel condensation. J. Colloid Interface Sci. 2015, 441, 52–58. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Primo, J. Base Catalysis for Fine Chemicals Production-Claisen-Schmidt Condensation on Zeolites and Hydrotalcites for the Production of Chalcones and Flavanones of Pharmaceutical Interest. J. Catal. 1995, 151, 60–66. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Tisler, Z.; Velvarska, R.; Kubicka, D. Reconstructed Mg-Al hydrotalcites prepared by using different rehydration and drying time: Physico-chemical properties and catalytic performance in aldol condensation. Appl. Catal. A-Gen. 2017, 536, 85–96. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Thanh, D.N.; Kikhtyanin, O.; Ramos, R.; Kothari, M.; Ulbrich, P.; Munshi, T.; Kubicka, D. Nanosized TiO2—A promising catalyst for the aldol condensation of furfural with acetone in biomass upgrading. Catal. Today 2016, 277, 97–107. [Google Scholar] [CrossRef]
- Hua, D.R.; Wu, Y.L.; Liu, Y.F.; Chen, Y.; Yang, M.D.; Lu, X.N.; Li, J. Preparation of furfural and reaction kinetics of xylose dehydration to furfural in high-temperature water. Pet. Sci. 2016, 13, 167–172. [Google Scholar] [CrossRef]
- Marchal, R.; Ropars, M.; Pourquié, J.; Fayolle, F.; Vandecasteele, J.P. Large-scale enzymatic hydrolysis of agricultural lignocellulosic biomass. Part 2: Conversion into acetone-butanol. Bioresour. Technol. 1992, 42, 205–217. [Google Scholar] [CrossRef]
- Shekoohi, K.; Hosseini, F.S.; Haghighi, A.H.; Sahrayian, A. Synthesis of some Mg/Co-Al type nano hydrotalcites and characterization. MethodsX 2017, 4, 86–94. [Google Scholar] [CrossRef]
- Lavalley, J.C. Infrared spectrometric studies of the surface basicity of metal oxides and zeolites using adsorbed probe molecules. Catal. Today 1996, 27, 377–401. [Google Scholar] [CrossRef]
- Constantino, V.R.L.; Pinnavaia, T.J. Basic Properties of Mg1-X(2+)Alx(3+) Layered Double Hydroxides Intercalated by Carbonate, Hydroxide Chloride and Sulfate Anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Yun, S.K.; Pinnavaia, T.J. Water-Content and Particle Texture of Synthetic Hydrotalcite-Like Layered Double Hydroxides. Chem. Mater. 1995, 7, 348–354. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Čapek, L.; Smoláková, L.; Tišler, Z.; Kadlec, D.; Lhotka, M.; Diblíková, P.; Kubička, D. Influence of Mg–Al Mixed Oxide Compositions on Their Properties and Performance in Aldol Condensation. Ind. Eng. Chem. Res. 2017, 56, 13411–13422. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, T.; Fang, H.; Li, S.; Yao, Y.; Fan, B.; Wang, J. A novel preparation of nanosized hexagonal Mg(OH)2 as a flame retardant. Particuology 2016, 24, 177–182. [Google Scholar] [CrossRef]
- Gong, W.; Wu, D.; Cheng, Z.; Pang, H.; Lin, Y.; Ning, G. Direct synthesis of porous Mg(OH)2 nanoplates from natural brucite. Mater. Res. Bull. 2013, 48, 1333–1337. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Capek, L.; Tisler, Z.; Velvarska, R.; Panasewicz, A.; Diblikova, P.; Kubicka, D. Physico-Chemical Properties of MgGa Mixed Oxides and Reconstructed Layered Double Hydroxides and Their Performance in Aldol Condensation of Furfural and Acetone. Front. Chem. 2018, 6, 176. [Google Scholar] [CrossRef] [PubMed]
- Hora, L.; Kelbichová, V.; Kikhtyanin, O.; Bortnovskiy, O.; Kubička, D. Aldol condensation of furfural and acetone over MgAl layered double hydroxides and mixed oxides. Catal. Today 2014, 223, 138–147. [Google Scholar] [CrossRef]

| Catalyst | a, nm | c, nm | D, nm | SBET, m2/g | Mg, wt% | Fe, wt% |
|---|---|---|---|---|---|---|
| HTc-Re-1:1 | 0.304 | 2.333 | 17 | 55 | 15.0 | 35.0 |
| HTc-Re-2:1 | 0.305 | 2.352 | 22 | 98 | 20.7 | 24.2 |
| HTc-Re-3:1 | 0.305 | 2.381 | 19 | 121 | 22.9 | 17.6 |
| HTc-Re-4:1 | 0.304 | 2.363 | 14 | 97 | 24.9 | 15.0 |
| HTc-Re-5:1 | 0.310 | 2.351 | 9 | 41 | 27.1 | 12.9 |
| HTc-Re-6:1 | 0.311 | 2.369 | 5 | 115 | 28.9 | 12.1 |
| HTc-Re-10:1 | 0.313 | 2.402 | 10 | 49 | 31.9 | 10.1 |
| Catalyst | Basicity, µmol/g | Density of Basic Sites, µmol/m2 |
|---|---|---|
| HTc-Re-1:1 | 580 | 10.55 |
| HTc-Re-2:1 | 838 | 8.55 |
| HTc-Re-3:1 | 1434 | 11.86 |
| HTc-Re-4:1 | 1506 | 15.53 |
| HTc-Re-5:1 | 956 | 23.34 |
| HTc-Re-6:1 | 872 | 7.61 |
| HTc-Re-10:1 | 779 | 15.91 |
| Catalyst | Mg, ppm | Fe, ppm |
|---|---|---|
| HTc-Re-1:1 | 0.157 | 0.456 |
| HTc-Re-2:1 | 0.1 | 0.28 |
| HTc-Re-3:1 | 0.32 | 0.355 |
| HTc-Re-4:1 | 5.28 | 3.46 |
| HTc-Re-5:1 | 4.34 | 2.35 |
| HTc-Re-6:1 | 3.84 | 1.92 |
| HTc-Re-10:1 | 3.17 | 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaroslav, K.; Jiří, K.; Uliana, A.; Zdeněk, T. Influences of Magnesium Content in Rehydrated Mixed Oxides on Furfural Conversion. Catalysts 2020, 10, 1484. https://doi.org/10.3390/catal10121484
Jaroslav K, Jiří K, Uliana A, Zdeněk T. Influences of Magnesium Content in Rehydrated Mixed Oxides on Furfural Conversion. Catalysts. 2020; 10(12):1484. https://doi.org/10.3390/catal10121484
Chicago/Turabian StyleJaroslav, Kocík, Kolena Jiří, Akhmetzyanova Uliana, and Tišler Zdeněk. 2020. "Influences of Magnesium Content in Rehydrated Mixed Oxides on Furfural Conversion" Catalysts 10, no. 12: 1484. https://doi.org/10.3390/catal10121484
APA StyleJaroslav, K., Jiří, K., Uliana, A., & Zdeněk, T. (2020). Influences of Magnesium Content in Rehydrated Mixed Oxides on Furfural Conversion. Catalysts, 10(12), 1484. https://doi.org/10.3390/catal10121484





