In Situ Preparation of Copper-Loaded Carbon-Based Catalyst with Chelate Resin and Its Application on Persulfate Activation for X-3B Degradation
Abstract
1. Introduction
2. Results and Discussion
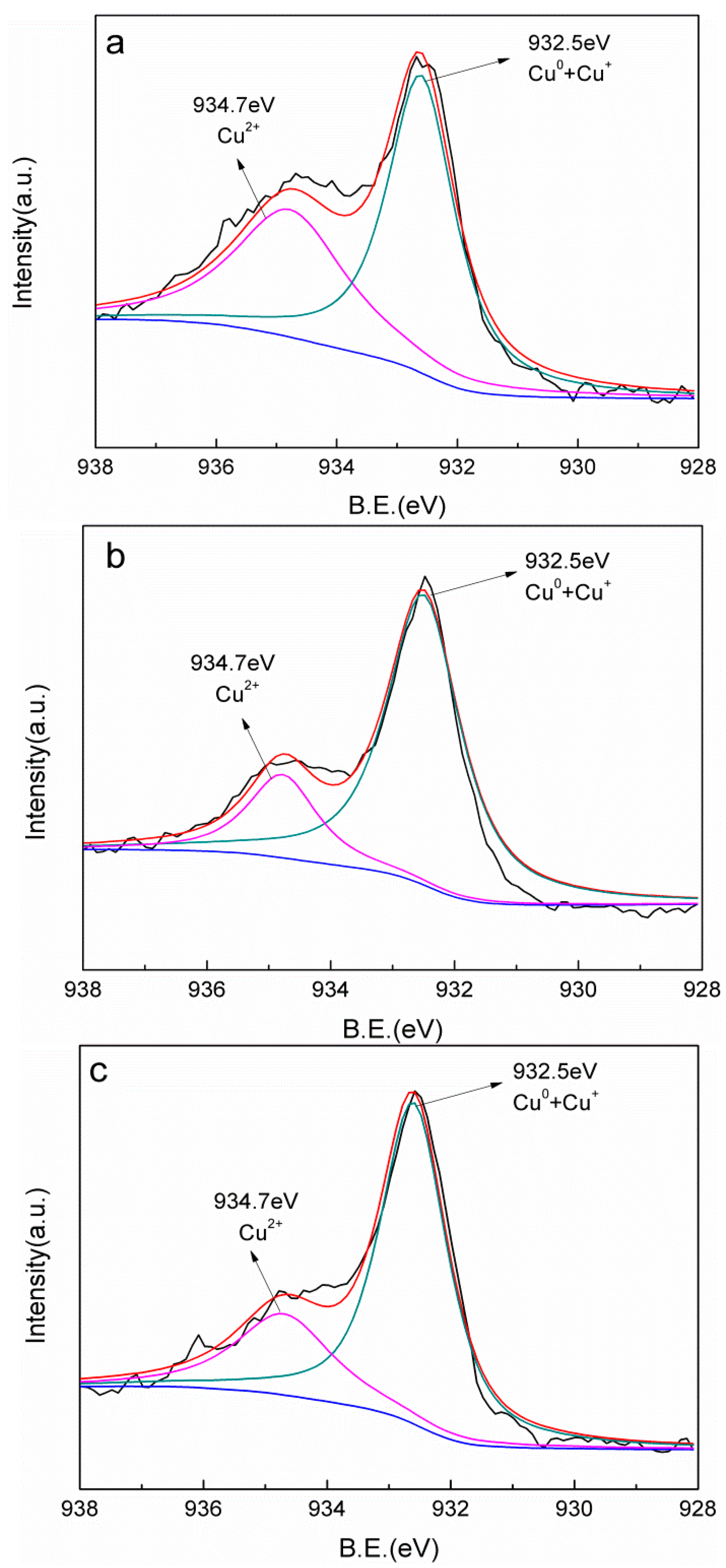
2.1. Catalyst Characteristics
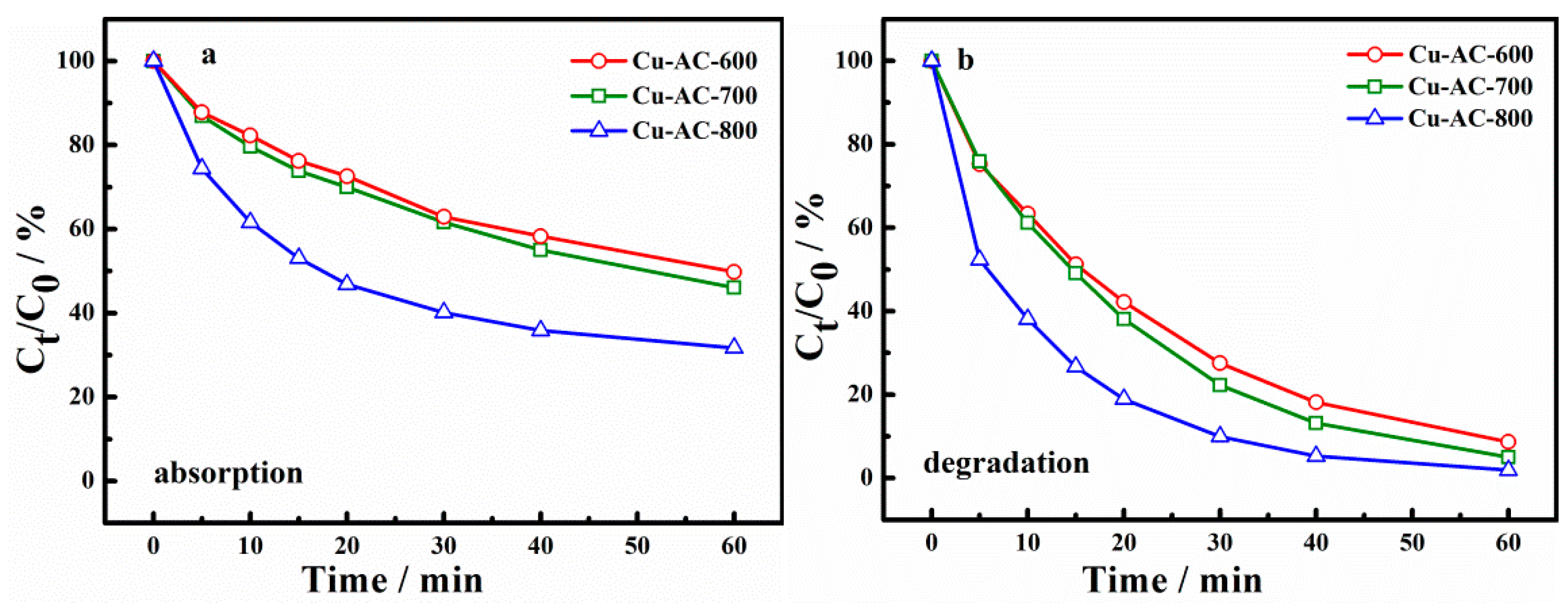
2.2. Catalytic Activity of Catalysts
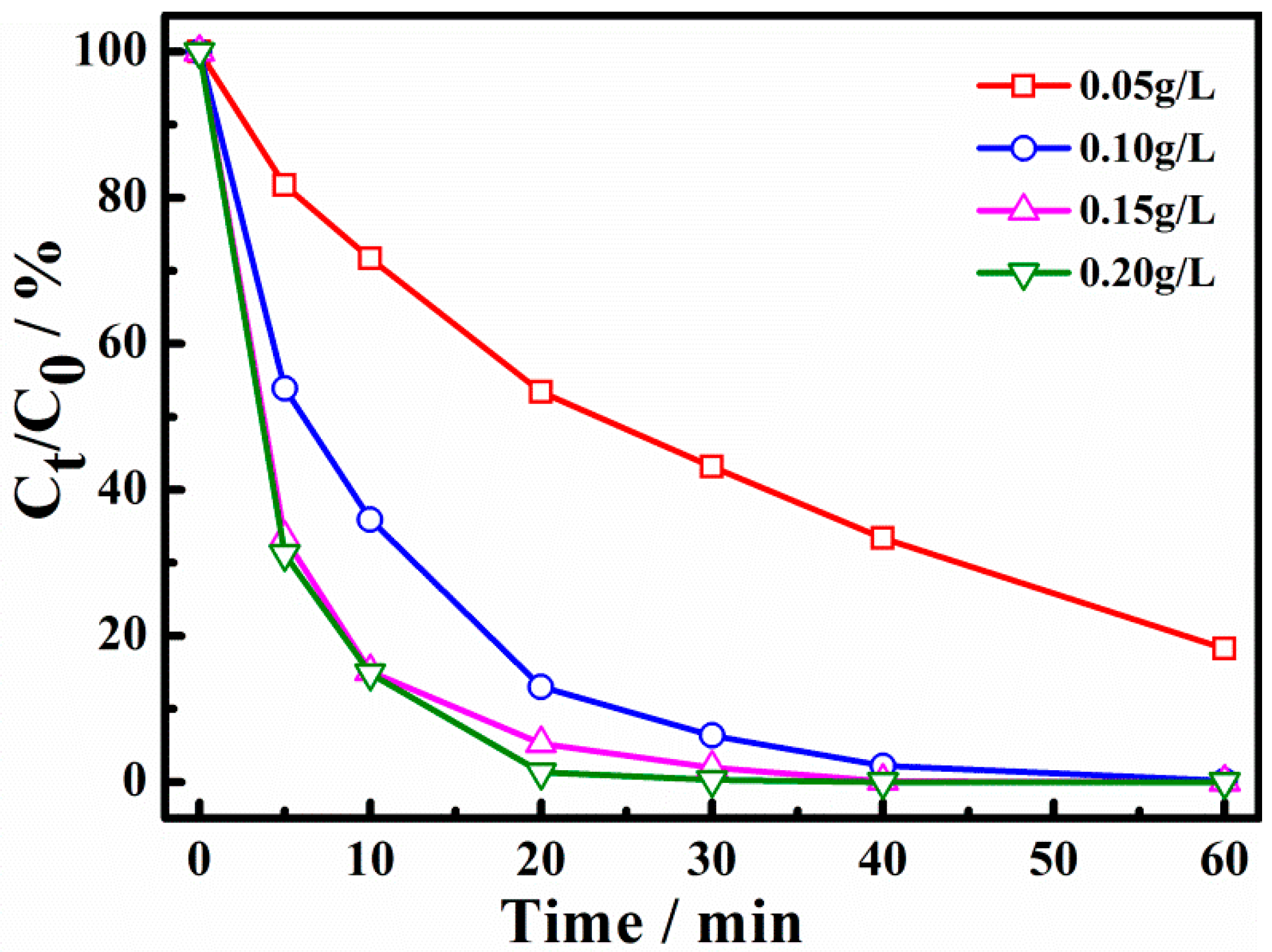
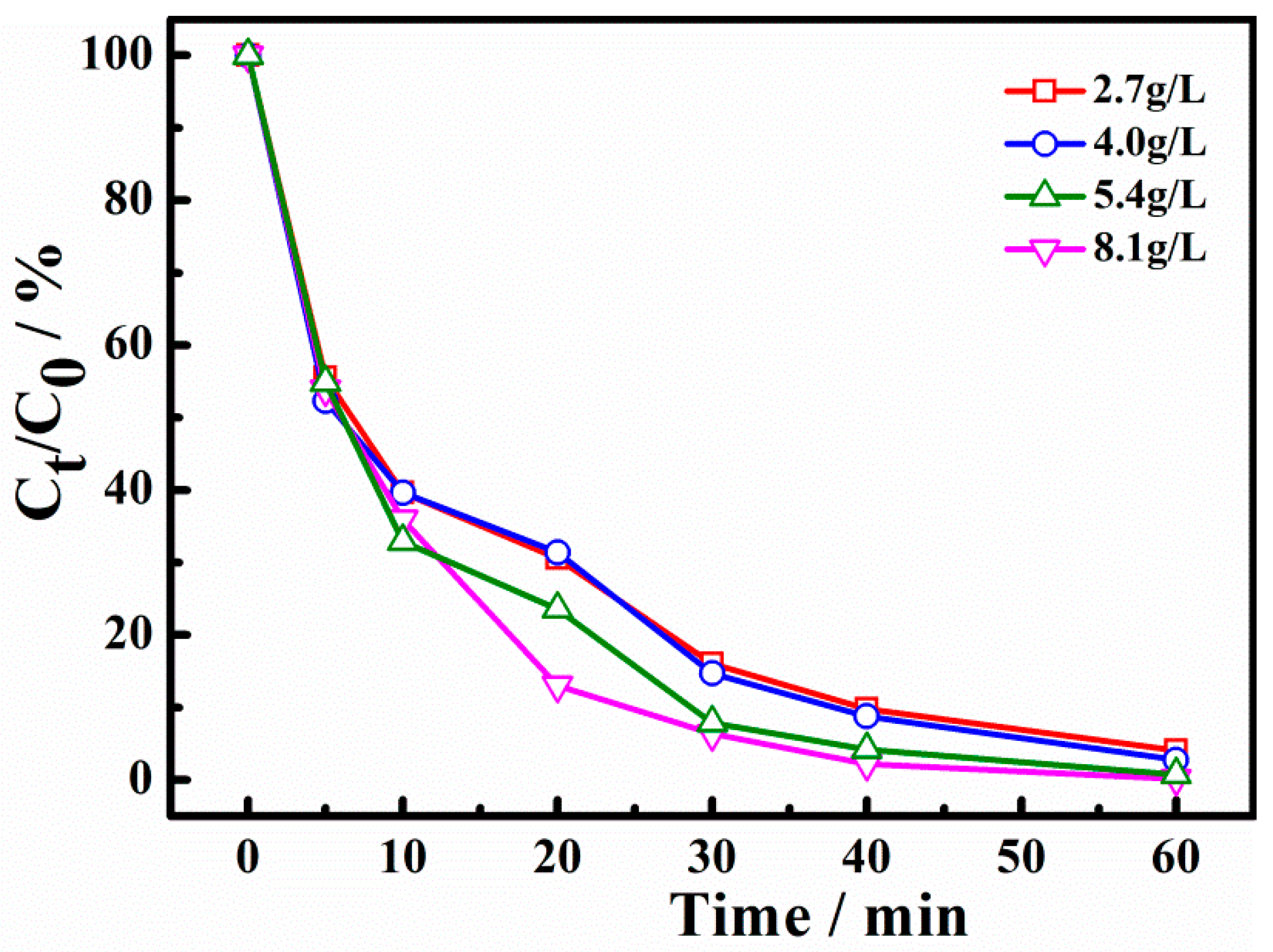
2.3. Influence of the Operation Conditions on X-3B Degradation
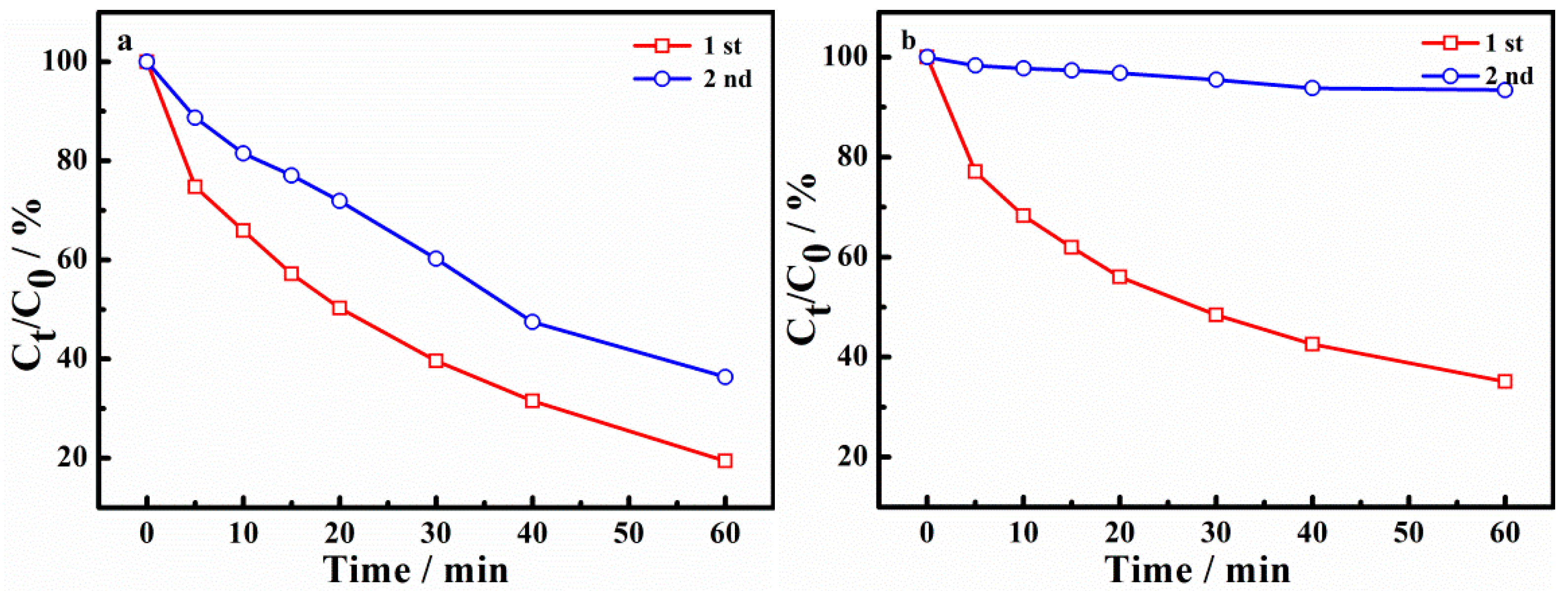
2.4. Reusability of the Catalyst
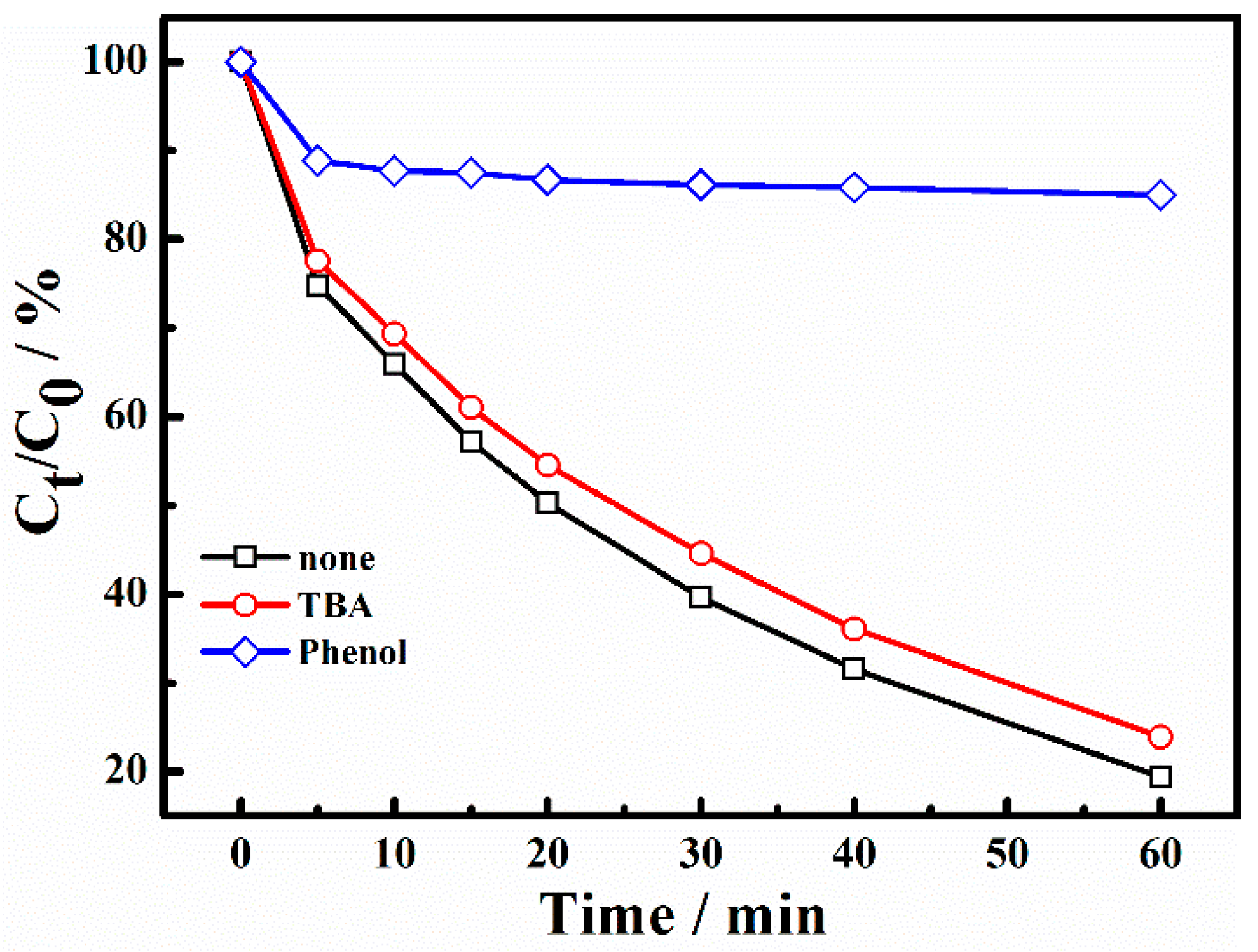
2.5. Radical Quenching Experiment
3. Experimental Materials and Methods
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Catalysis and Adsorption Experiments
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kang, X.; Cheng, Y.; Wen, Y.; Qi, J.; Li, X. Bio-inspired co-deposited preparation of GO composite loose nanofiltration membrane for dye contaminated wastewater sustainable treatment. J. Hazard. Mater. 2020, 400, 123121. [Google Scholar] [CrossRef] [PubMed]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Dominguez, J.R.; Beltran, J.; Rodriguez, O. Vis and UV photocatalytic detoxification methods (using TiO2, TiO2/H2O2, TiO2/O3, TiO2/S2O82−, O3, H2O2, S2O82−, Fe3+/H2O2 and Fe3+/H2O2/C2O42−) for dyes treatment. Catal. Today 2005, 101, 389–395. [Google Scholar] [CrossRef]
- Li, X.; Liao, F.; Ye, L.; Yeh, L. Controlled pyrolysis of MIL-88A to prepare iron/carbon composites for synergistic persulfate oxidation of phenol: Catalytic performance and mechanism. J. Hazard. Mater. 2020, 398, 122938. [Google Scholar] [CrossRef]
- Hazime, R.; Nguyen, Q.H.; Ferronato, C.; Salvador, A.; Jaber, F.; Chovelon, J.M. Comparative study of imazalil degradation in three systems: UV/TiO2, UV/K2S2O8 and UV/TiO2/K2S2O8. Appl. Catal. B Environ. 2014, 144, 286–291. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Z.; Huang, Z.; Cui, Y. Acceleration of Persulfate Activation by MIL-101(Fe) with Vacuum Thermal Activation: Effect of FeII/FeIII Mixed-Valence Center. Catalysts 2019, 9, 906. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Du, X.Z.; Huang, W.L. Temperature effect on the kinetics of persulfate oxidation of p-chloroaniline. Chin. Chem. Lett. 2011, 22, 358–361. [Google Scholar] [CrossRef]
- Liang, C.J.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate-thiosulfate redox couple. Chemosphere 2004, 55, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Yamamoto, A.; Hayakawa, E.; Taniyasu, S.; Yamashita, N.; Kutsuna, S. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.R.; Wang, S.B.; Ang, H.M.; Tade, M.O. Photocatalytic oxidation of phenolic compounds using zinc oxide and sulphate radicals under artificial solar light. Sep. Purif. Technol. 2010, 70, 338–344. [Google Scholar] [CrossRef]
- Xing, B.; Dong, J.W.; Yang, G.; Jiang, N.; Liu, X.Y.; Yuan, J.G. An insight into N, S-codoped activated carbon for the catalytic persulfate oxidation of organic pollutions in water: Effect of surface functionalization. Appl. Catal. A Gen. 2020, 602, 9. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Shao, X.; Niu, R.; Wang, L. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Arsalani, N.; Eftekhari-Sis, B.; Amini, M.; Gohari, G.; Jabbari, E. Cube-octameric silsesquioxane (POSS)-capped magnetic iron oxide nanoparticles for the efficient removal of methylene blue. Front. Chem. Sci. Eng. 2019, 13, 563–573. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Nengzi, L.-C.; Meng, L.; Song, Q.; Gou, J.; Cheng, X. In situ preparation of carbon-based Cu-Fe oxide nanoparticles from CuFe Prussian blue analogues for the photo-assisted heterogeneous peroxymonosulfate activation process to remove lomefloxacin. Chem. Eng. J. 2020, 398, 125556. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Zuo, C.Y.; Wu, H.Y. One step for synthesis of magnetic CuFe2O4 composites as photo-fenton catalyst for degradation organics. Mater. Chem. Phys. 2019, 237, 6. [Google Scholar] [CrossRef]
- Deng, J.; Xu, M.; Chen, Y.; Li, J.; Qiu, C.; Li, X.; Zhou, S. Highly-efficient removal of norfloxacin with nanoscale zero-valent copper activated persulfate at mild temperature. Chem. Eng. J. 2019, 366, 491–503. [Google Scholar] [CrossRef]
- Ghorbanian, Z.; Asgari, G.; Samadi, M.T.; Seid-mohammadi, A. Removal of 2,4 dichlorophenol using microwave assisted nanoscale zero-valent copper activated persulfate from aqueous solutions: Mineralization, kinetics, and degradation pathways. J. Mol. Liq. 2019, 296, 111873. [Google Scholar] [CrossRef]
- Ni, Y.-J.; Cheng, Y.-Q.; Xu, M.-Y.; Qiu, C.-G.; Ma, X.-Y.; Li, J.; Deng, J. Nanoscale Zero-valent Copper-Activated Molecular Oxygen for the Degradation of Enrofloxacin in Water. Huan Jing Ke Xue = Huanjing Kexue 2019, 40, 293–299. [Google Scholar]
- Qu, G.; Chu, R.; Wang, H.; Wang, T.; Zhang, Z.; Qiang, H.; Liang, D.; Hu, S. Simultaneous removal of chromium(VI) and tetracycline hydrochloride from simulated wastewater by nanoscale zero-valent iron/copper-activated persulfate. Environ. Sci. Pollut. Res. Int. 2020, 27, 40826–40836. [Google Scholar] [CrossRef]
- Ferreira de Sousa, P.V.; de Oliveira, A.F.; da Silva, A.A.; Vaz, B.G.; Lopes, R.P. Study of ciprofloxacin degradation by zero-valent copper nanoparticles. Chem. Pap. 2019, 73, 249–260. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, J.; Zhang, Y.; Zhang, G.; Li, W.; Wei, C.; Liang, J.; Liu, Y.; Shu, S. Degradation of 2,4-dichlorophenol by activating persulfate and peroxomonosulfate using micron or nanoscale zero-valent copper. J. Hazard. Mater. 2018, 344, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, G.Q.; Yan, L.F.; Kong, L.Q.; Zheng, H.Y.; Mi, J.; Li, Z. Carbon nanotube-supported Cu-based catalysts for oxidative carbonylation of methanol to methyl carbonate: Effect of nanotube pore size. Catal. Sci. Technol. 2020, 10, 2615–2626. [Google Scholar] [CrossRef]
- Ren, J.; Wang, W.; Wang, D.L.; Zuo, Z.J.; Lin, J.Y.; Li, Z. A theoretical investigation on the mechanism of dimethyl carbonate formation on Cu/AC catalyst. Appl. Catal. A Gen. 2014, 472, 47–52. [Google Scholar] [CrossRef]
- Liu, L.; Bao, R.; Yi, J.H. Mono-dispersed and homogeneous CNT/Cu composite powder preparation through forming Cu2O intermediates. Powder Technol. 2018, 328, 430–435. [Google Scholar] [CrossRef]
- Ulloa, L.; Martínez-Minchero, M.; Bringas, E.; Cobo, A.; San-Román, M.F. Split regeneration of chelating resins for the selective recovery of nickel and copper. Sep. Purif. Technol. 2020, 253, 117516. [Google Scholar] [CrossRef]
- Sirola, K.; Laatikainen, M.; Lahtinen, M.; Paatero, E. Removal of copper and nickel from concentrated ZnSO4 solutions with silica-supported chelating adsorbents. Sep. Purif. Technol. 2008, 64, 88–100. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Liu, Y.B.; Liu, Y. Fenton-like degradation of sulfamerazine at nearly neutral pH using Fe-Cu-CNTs and Al-0-CNTs for in-situ generation of H2O2/(OH)-O-center dot/O-2(center dot-). Chem. Eng. J. 2020, 396, 11. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gu, P.; Li, X.Y.; Zhang, G.H. Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O/Cu modified activated carbon. Chem. Eng. J. 2017, 322, 129–139. [Google Scholar] [CrossRef]
- Wu, X.; Meng, H.; Du, Y.L.; Liu, J.N.; Hou, B.H.; Xie, X.M. Insight into Cu2O/CuO collaboration in the selective catalytic reduction of NO with NH3: Enhanced activity and synergistic mechanism. J. Catal. 2020, 384, 72–87. [Google Scholar] [CrossRef]
- Wang, B.Q.; Fu, T.; An, B.H.; Liu, Y. UV light-assisted persulfate activation by Cu-0-Cu2O for the degradation of sulfamerazine. Sep. Purif. Technol. 2020, 251, 11. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, Z.; Liu, Y.; Huang, Z.; Cui, Y.; Yang, J. One-pot synthesis of sulfur doped activated carbon as a superior metal-free catalyst for the adsorption and catalytic oxidation of aqueous organics. J. Mater. Chem. A 2018, 6, 4055–4067. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, Z.; Zhu, Y.; Huang, Z.; Cui, Y.; Yang, J. Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene. Appl. Catal. B Environ. 2018, 220, 635–644. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Ocampo-Perez, R.; Leyva-Ramos, R.; Sanchez-Polo, M.; Rivera-Utrilla, J.; Mendez-Diaz, J.D. Removal of diethyl phthalate from water solution by adsorption, photo-oxidation, ozonation and advanced oxidation process (UV/H2O2, O3/H2O2 and O3/activated carbon). Sci. Total. Environ. 2013, 442, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lutze, H.V.; Kerlin, N.; Schmidt, T.C. Sulfate radical-based water treatment in presence of chloride: Formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate. Water Res. 2015, 72, 349–360. [Google Scholar] [CrossRef]
- Huang, Z.F.; Lin, Q.T.; Luo, H.Y.; Guo, P.R.; Weng, Q.S.; Lei, Y.Q.; Cheng, S.L.; Liu, S.S. Degradation of progesterone by coexisting free radical and nonradical pathways in the CuO/HNTs-PS system. Chem. Eng. J. 2020, 398, 10. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Deng, L. Performance of CuO/Oxone system: Heterogeneous catalytic oxidation of phenol at ambient conditions. Chem. Eng. J. 2011, 178, 239–243. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Wang, Y.X.; Kang, J.; Wang, S.B. N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. ACS Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
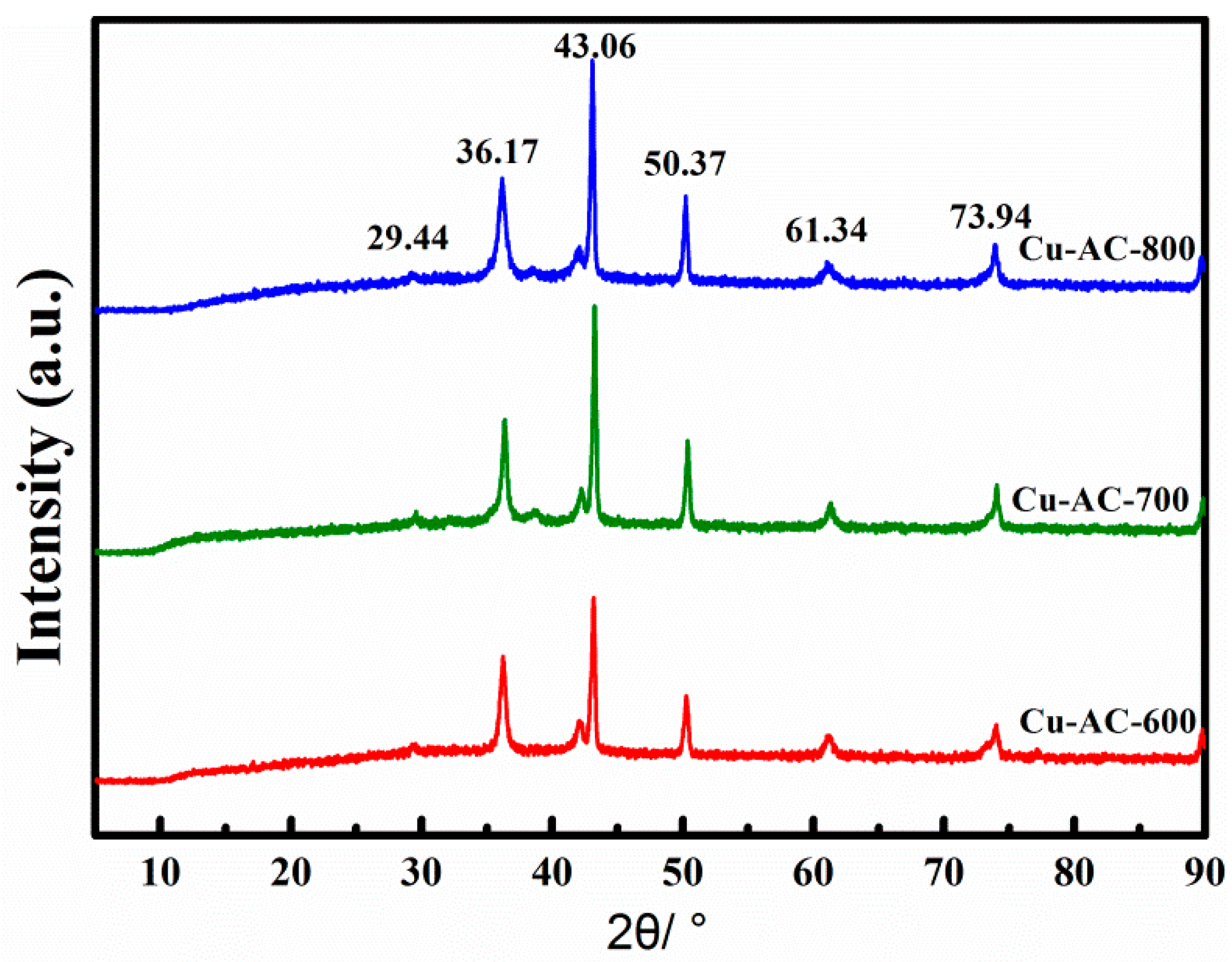



| Sample | SBET (m2·g−1) | Total Volume (cm3·g−1) | Pore Size (nm) | Micropore Volume (cm3·g−1) |
|---|---|---|---|---|
| Cu-AC-600 | 1170.6 | 0.68 | 0.57 | 0.52 |
| Cu-AC-700 | 1183.3 | 0.67 | 1.27 | 0.55 |
| Cu-AC-800 | 1448.8 | 0.93 | 0.85 | 0.72 |
| Sample | Cu0 + Cu+ % | Cu2+ % |
|---|---|---|
| Cu-AC-600 | 58.60 | 41.40 |
| Cu-AC-700 | 68.97 | 31.03 |
| Cu-AC-800 | 73.05 | 26.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Ran, X.; Zeng, Z.; Huang, Z.; Yang, J. In Situ Preparation of Copper-Loaded Carbon-Based Catalyst with Chelate Resin and Its Application on Persulfate Activation for X-3B Degradation. Catalysts 2020, 10, 1333. https://doi.org/10.3390/catal10111333
Cui Y, Ran X, Zeng Z, Huang Z, Yang J. In Situ Preparation of Copper-Loaded Carbon-Based Catalyst with Chelate Resin and Its Application on Persulfate Activation for X-3B Degradation. Catalysts. 2020; 10(11):1333. https://doi.org/10.3390/catal10111333
Chicago/Turabian StyleCui, Yan, Xuemei Ran, Zequan Zeng, Zhanggen Huang, and Jieyang Yang. 2020. "In Situ Preparation of Copper-Loaded Carbon-Based Catalyst with Chelate Resin and Its Application on Persulfate Activation for X-3B Degradation" Catalysts 10, no. 11: 1333. https://doi.org/10.3390/catal10111333
APA StyleCui, Y., Ran, X., Zeng, Z., Huang, Z., & Yang, J. (2020). In Situ Preparation of Copper-Loaded Carbon-Based Catalyst with Chelate Resin and Its Application on Persulfate Activation for X-3B Degradation. Catalysts, 10(11), 1333. https://doi.org/10.3390/catal10111333





