High Resistance of SO2 and H2O over Monolithic Mn-Fe-Ce-Al-O Catalyst for Low Temperature NH3-SCR
Abstract
1. Introduction
2. Results and Discussion
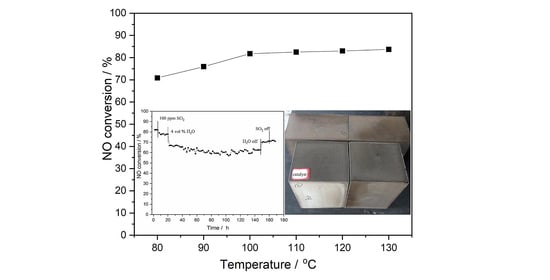
2.1. DeNOx Activity of Mn-Fe-Ce-Al-O Catalyst at Low Temperatures
2.2. Resistance of Mn-Fe-Ce-Al-O Catalyst to SO2 and H2O
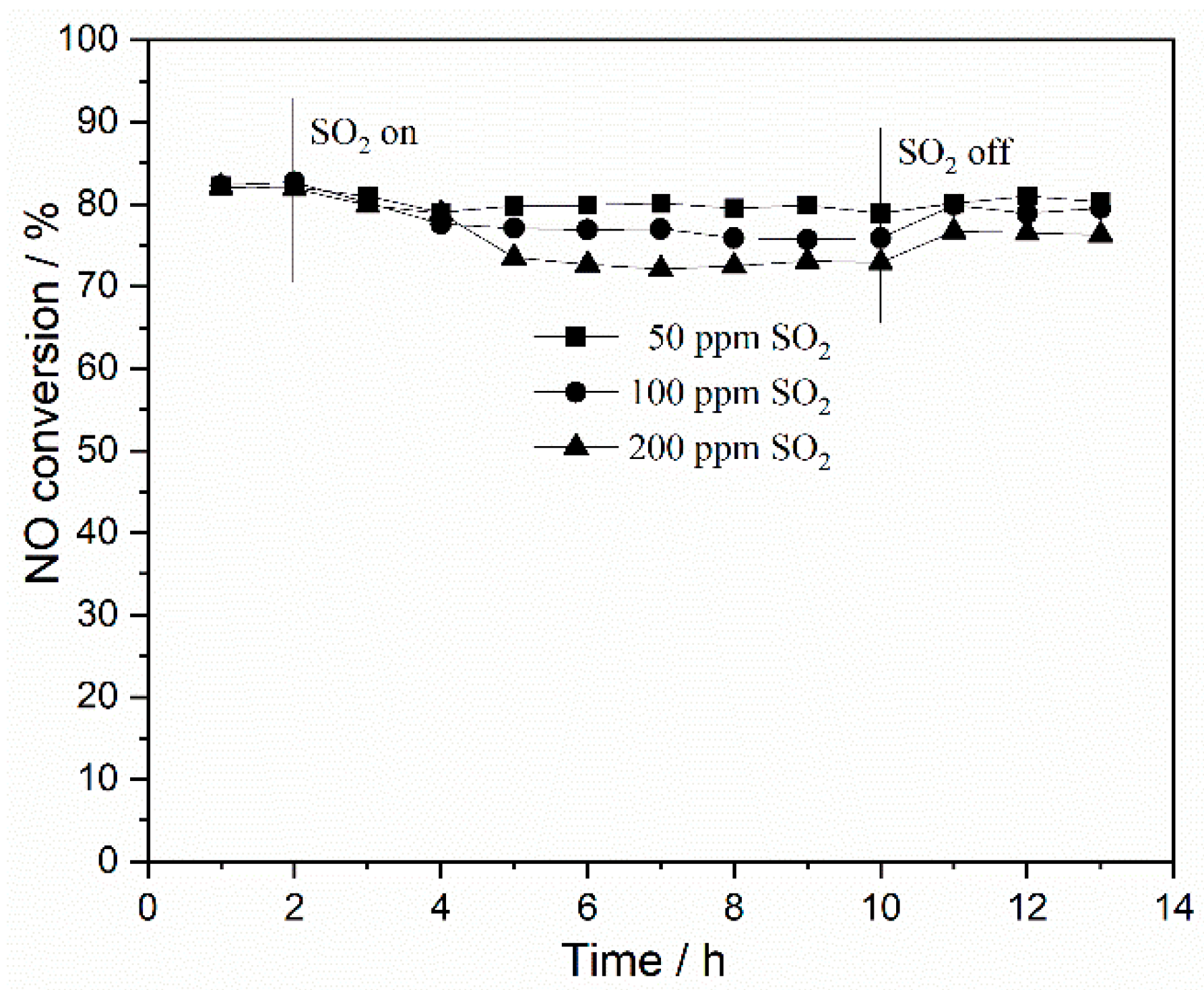
2.2.1. Resistance of Catalyst to SO2
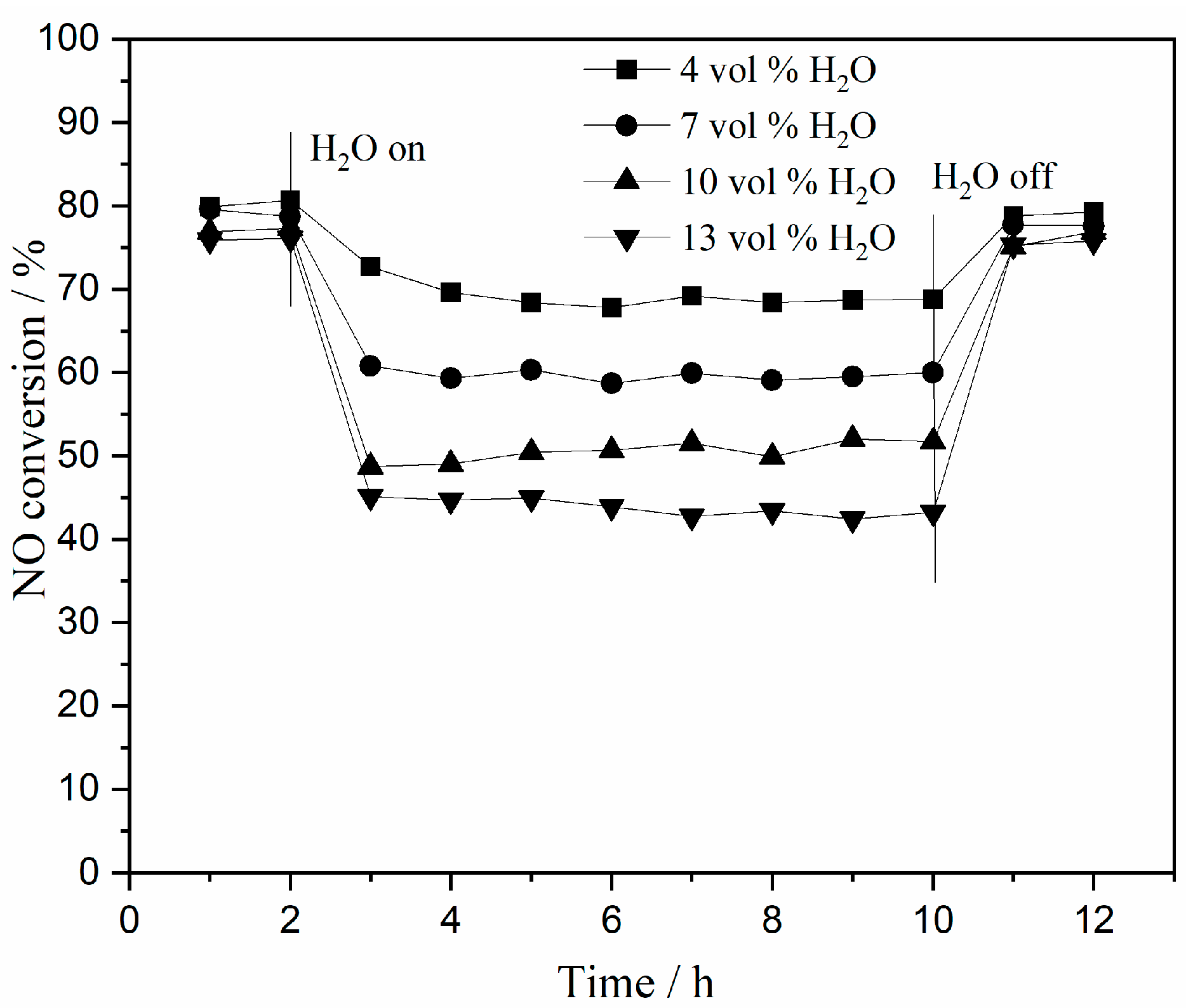
2.2.2. Resistance of Catalyst to H2O
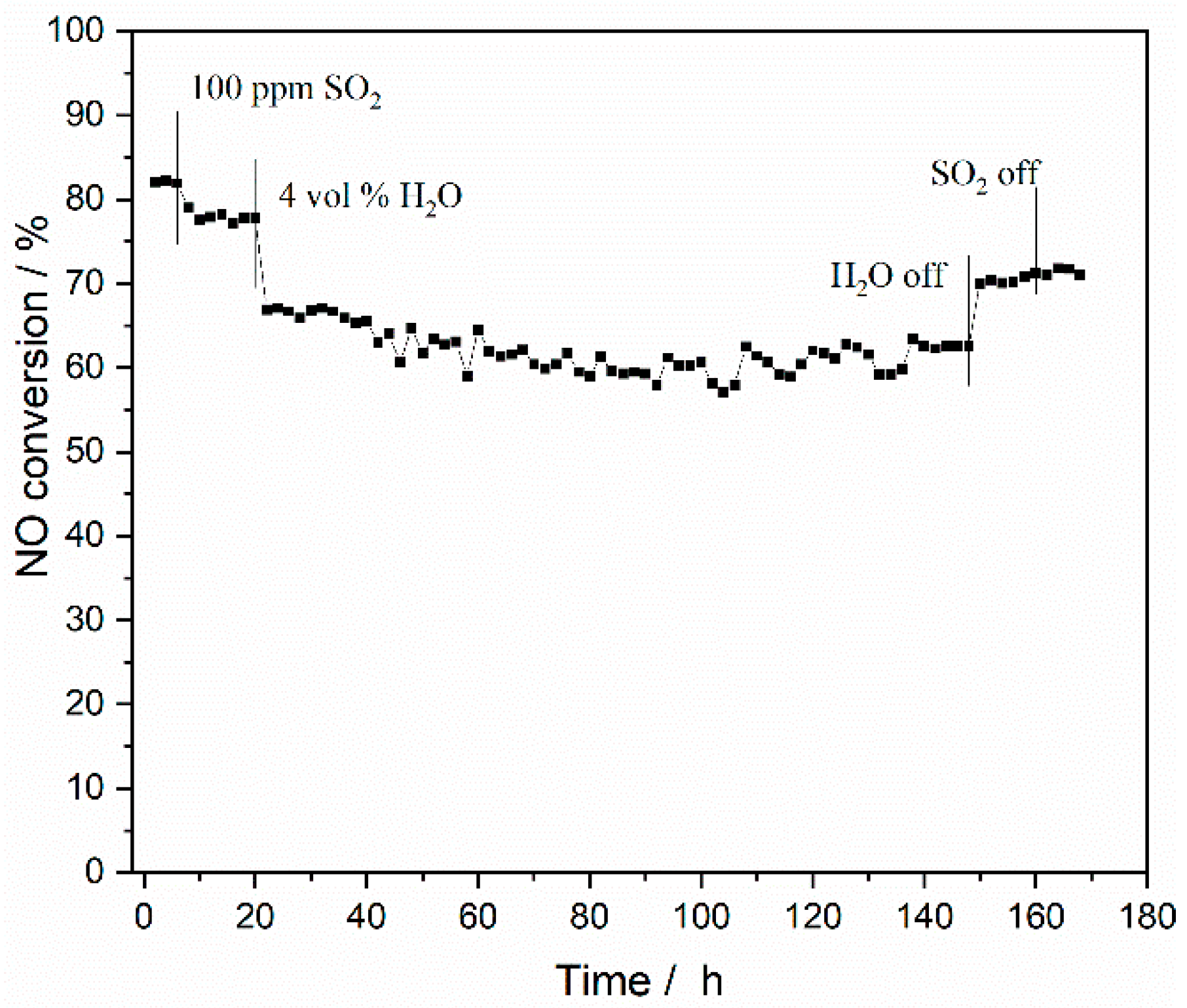
2.2.3. Resistance of the Catalyst against SO2 and H2O for 168 h
2.3. Physiochemical Characterization of Monolithic Mn-Fe-Ce-Al-O Catalyst before and after Reaction
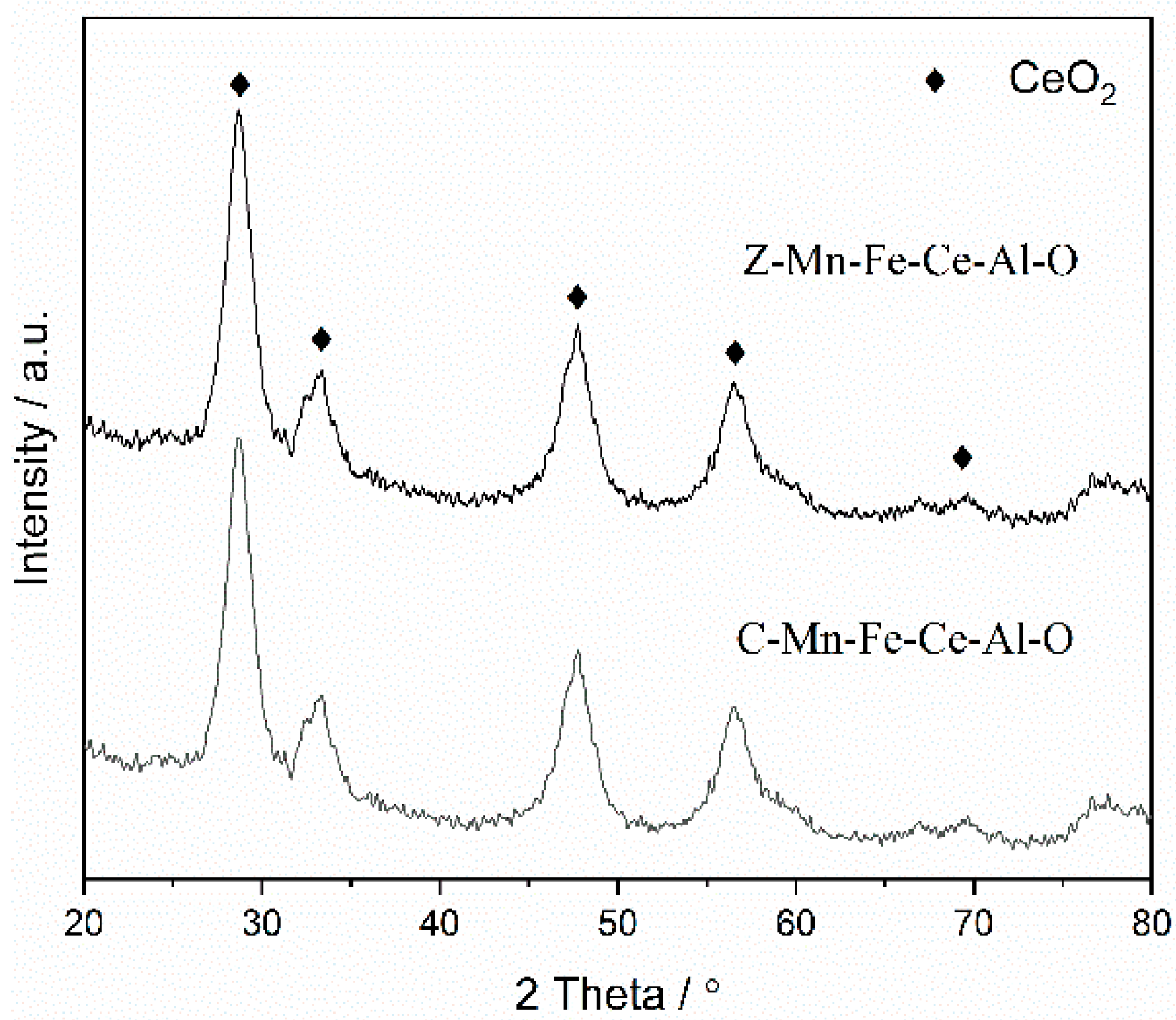
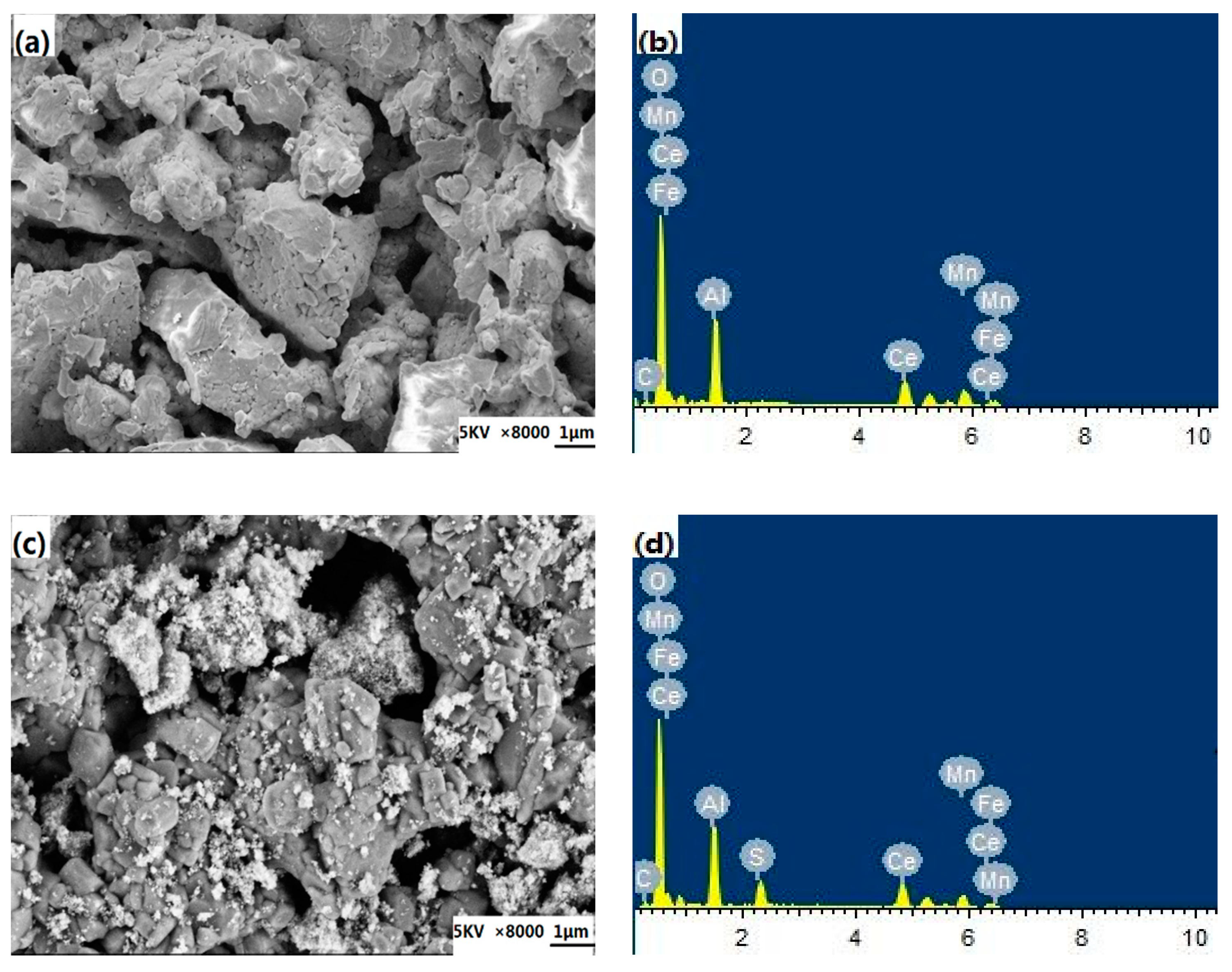
2.3.1. XRD, BET, SEM, and EDS
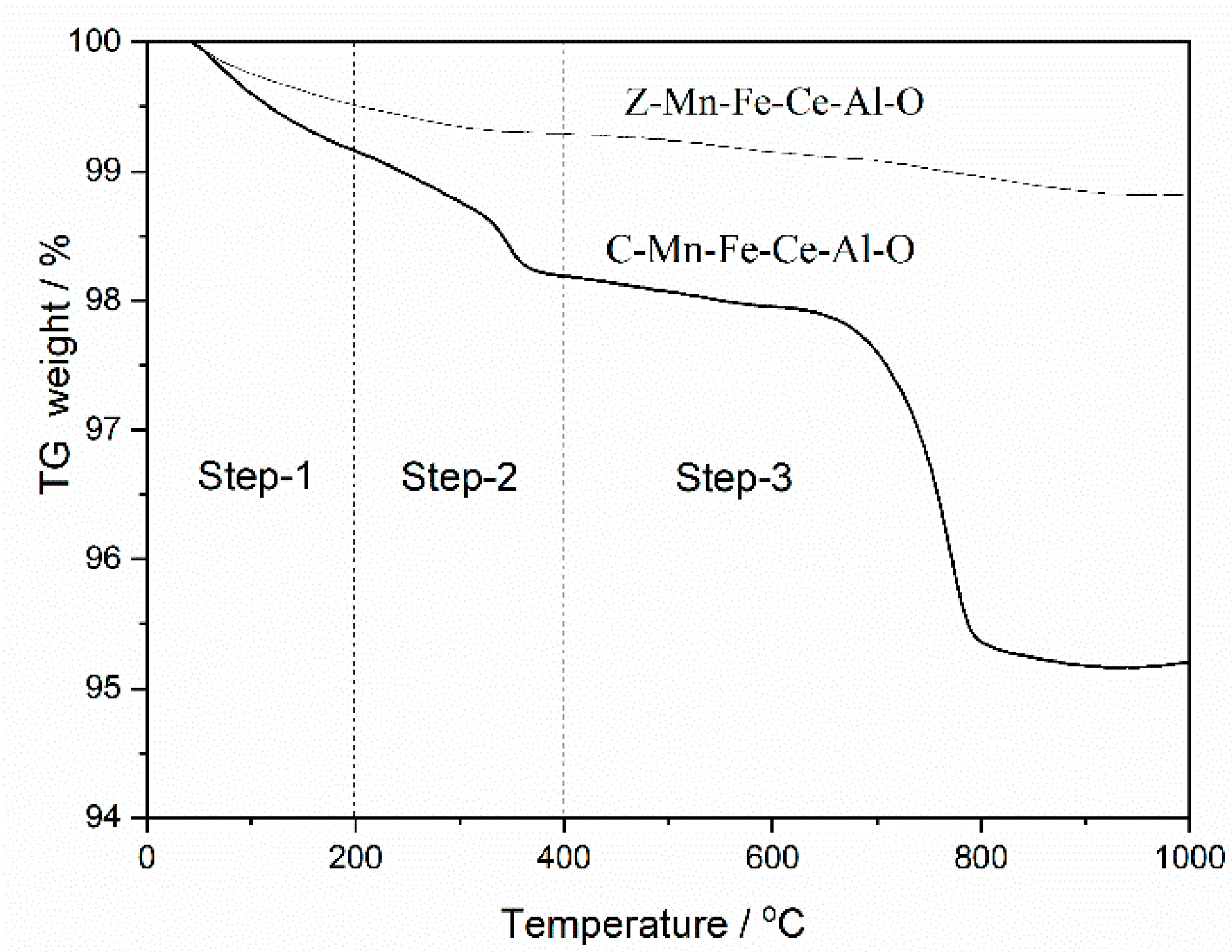
2.3.2. TG Analysis
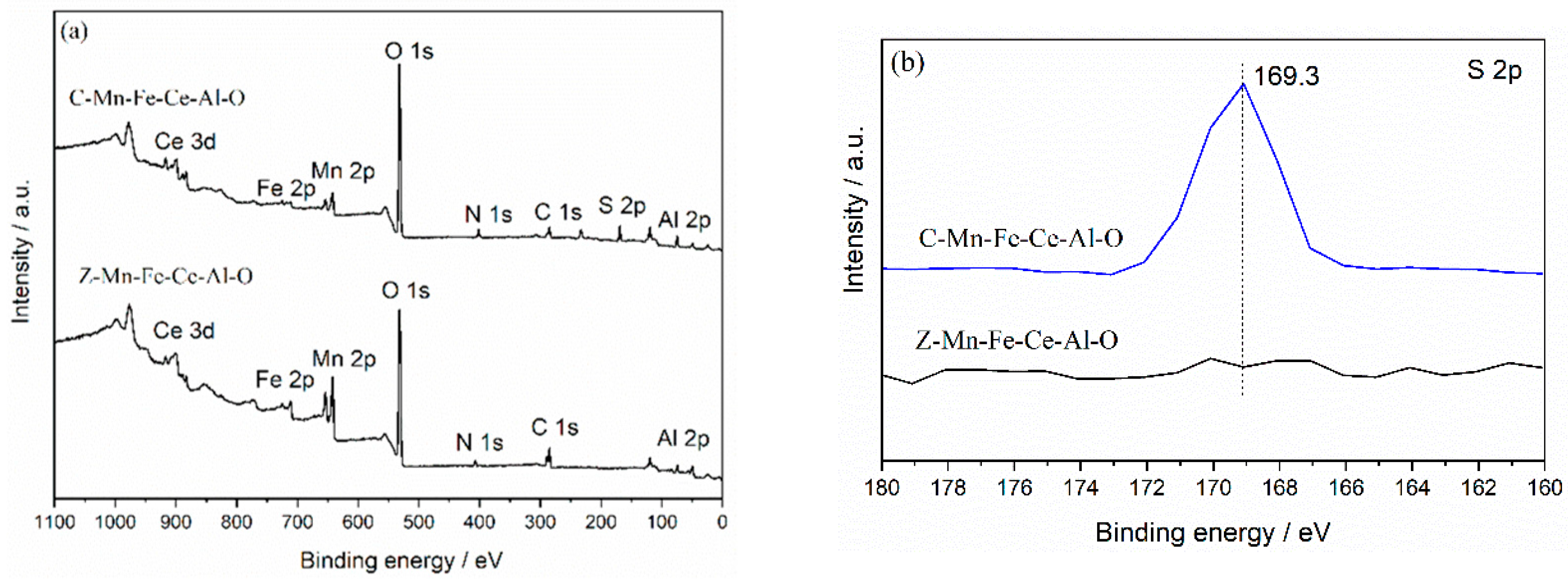
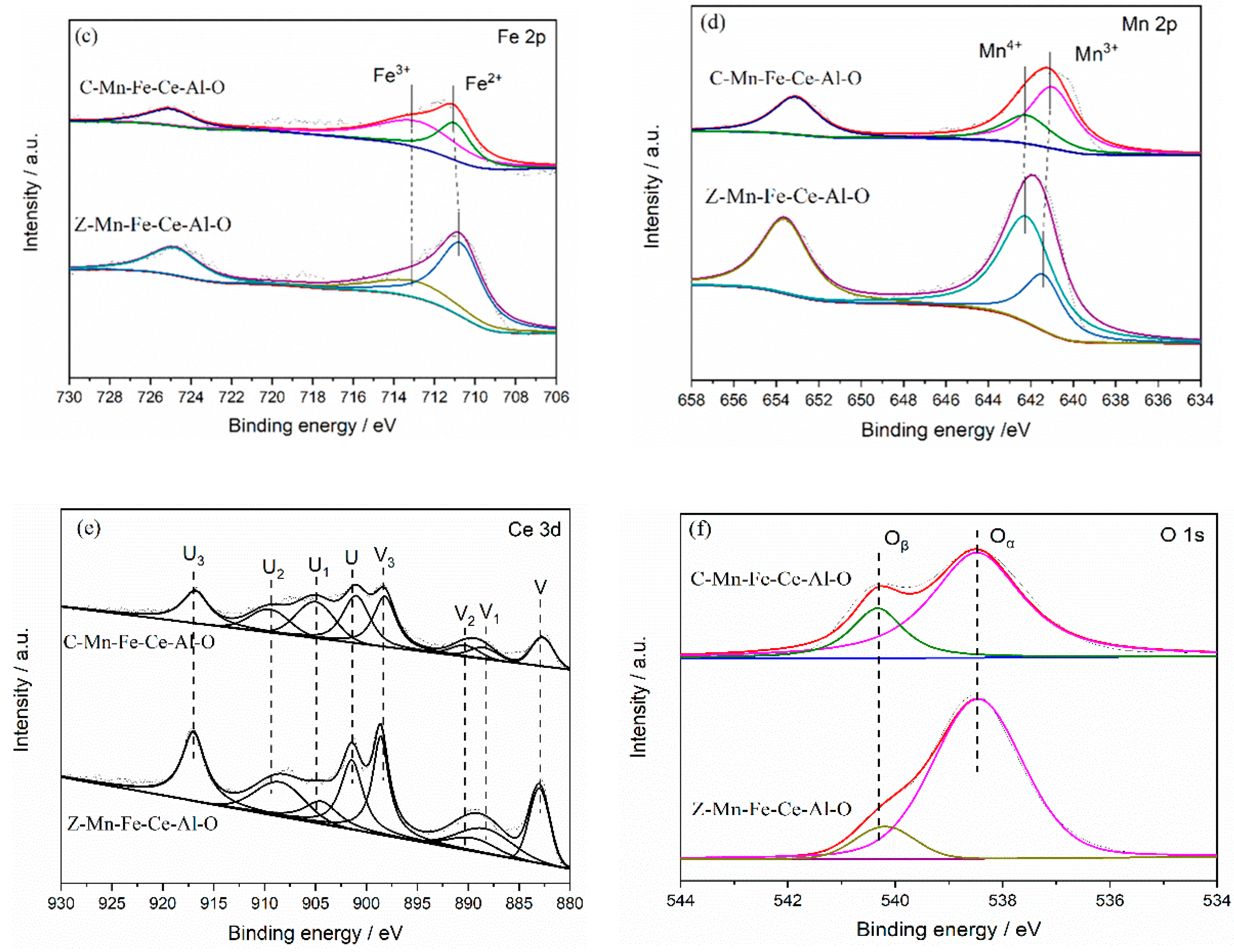
2.3.3. XPS Analysis
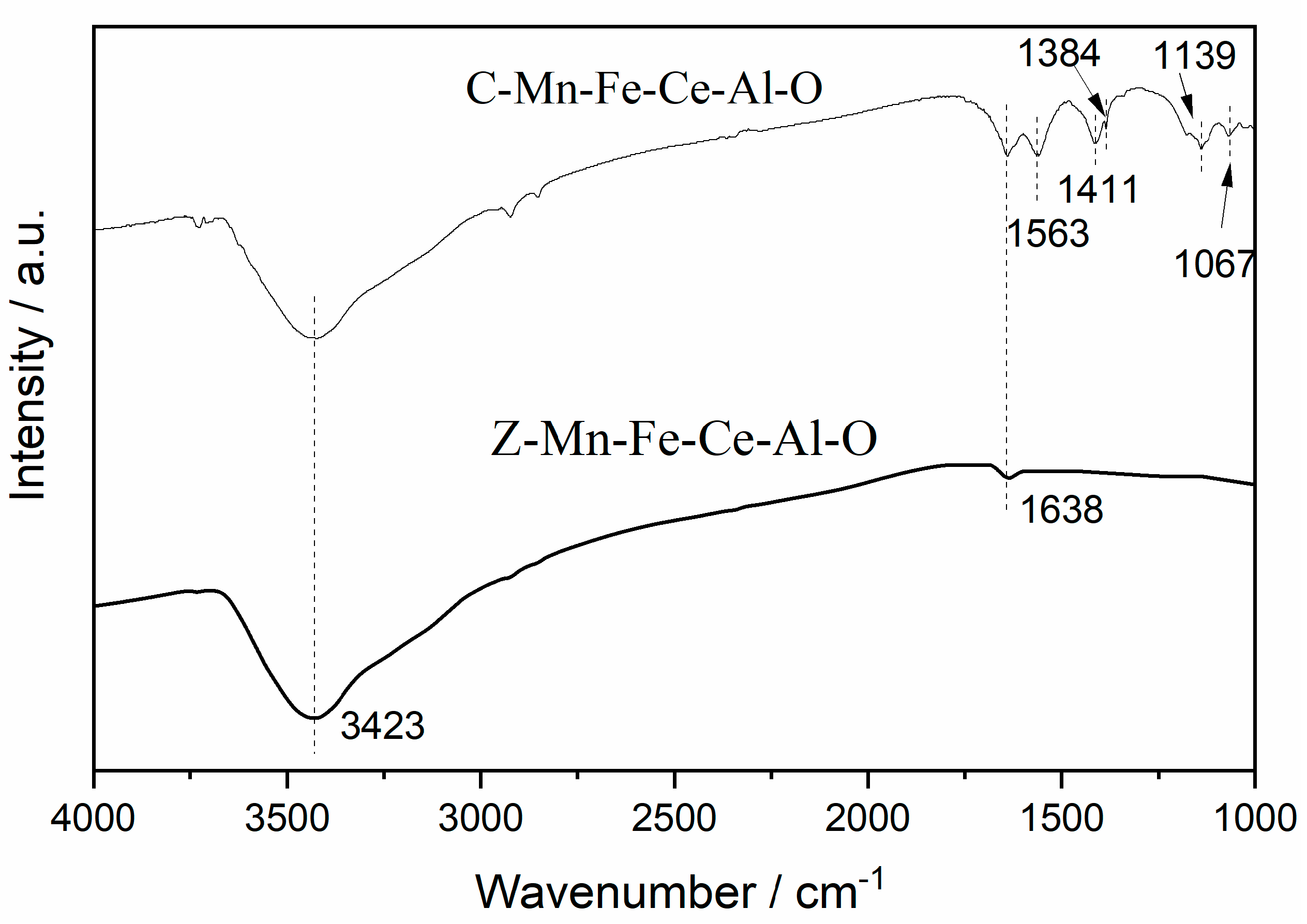
2.3.4. FT-IR Analysis
3. Materials and Methods
3.1. Catalyst Preparation
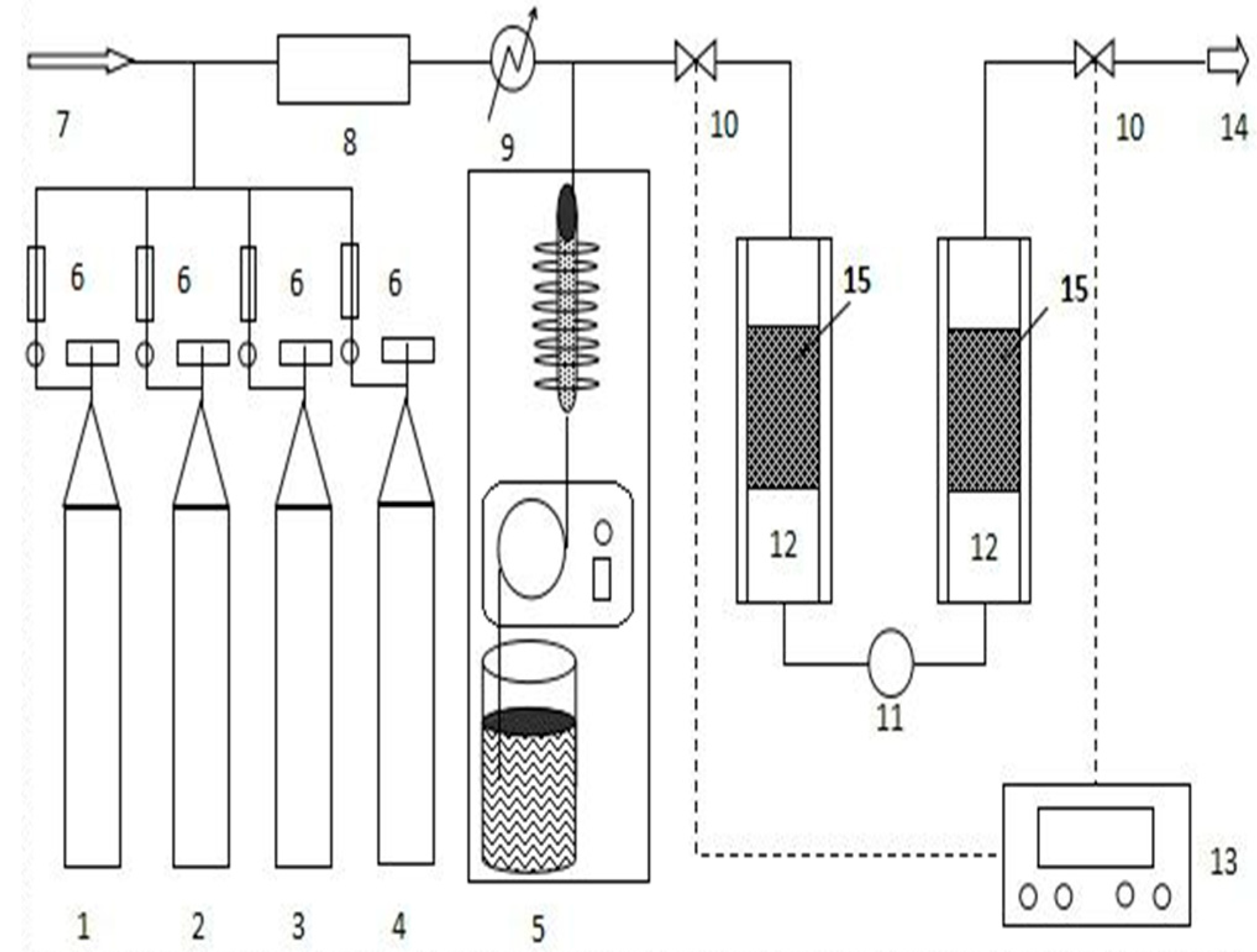
3.2. Catalytic Activity Test
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, H. Low-temperature selective catalytic reduction of NOx with NH3 over Fe–Cu mixed oxide/ZSM-5 catalysts containing Fe2CuO4 phase. Res. Chem. Intermed. 2014, 41, 4961–4975. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Yao, X.; Ma, K.; Zou, W.; He, S.; An, J.; Yang, F.; Dong, L. Influence of preparation methods on the physicochemical properties and catalytic performance of MnOx-CeO2 catalysts for NH3-SCR at low temperature. Chin. J. Catal. 2017, 38, 146–159. [Google Scholar] [CrossRef]
- Tang, X.; Hao, J.; Xu, W.; Li, J. Low temperature selective catalytic reduction of NOx with NH3 over amorphous MnOx catalysts prepared by three methods. Catal. Commun. 2007, 8, 329–334. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania. Appl. Catal. B Environ. 2003, 44, 217–225. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, H.; Dong, L. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 1248–1264. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, C.; Yao, X.; Zhang, L.; Li, L.; Wang, X.; Deng, Y.; Gao, F.; Dong, L. Effect of metal ions doping (M = Ti4+, Sn4+) on the catalytic performance of MnOx/CeO2 catalyst for low temperature selective catalytic reduction of NO with NH3. Appl. Catal. A Gen. 2015, 495, 206–216. [Google Scholar] [CrossRef]
- Yao, X.; Li, L.; Zou, W.; Yu, S.; An, J.; Li, H.; Yang, F.; Dong, L. Preparation, characterization, and catalytic performance of high efficient CeO2-MnOx-Al2O3 catalysts for NO elimination. Chin. J. Catal. 2016, 37, 1369–1380. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, W.; Ma, K.; Cao, Y.; Xiong, Y.; Wu, S.; Tang, C.; Gao, B.; Dong, L. Sulfated Temperature Effects on the Catalytic Activity of CeO2 in NH3-Selective Catalytic Reduction Conditions. J. Phys. Chem. C 2015, 119, 1155–1163. [Google Scholar] [CrossRef]
- Zhang, F.; Tian, G.; Wang, H.; Wang, H.; Zhang, C.; Cui, Y.; Huang, J.; Shu, Y. CeO2/TiO2 Monolith catalyst for the selective catalytic reduction of NOx with NH3: influence of H2O and SO2. Chem. Res. Chin. Univ. 2015, 32, 461–467. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Zhao, Y.; Yan, X.; Cao, P. Poisoning Effect of SO2 on Honeycomb Cordierite-Based Mn–Ce/Al2O3Catalysts for NO Reduction with NH3 at Low Temperature. Appl. Sci. 2018, 8, 95. [Google Scholar] [CrossRef]
- Xiao, X.; Xiong, S.; Shi, Y.; Shan, W.; Yang, S. Effect of H2O and SO2 on the Selective Catalytic Reduction of NO with NH3 Over Ce/TiO2 Catalyst: Mechanism and Kinetic Study. J. Phys. Chem. C 2016, 120, 1066–1076. [Google Scholar] [CrossRef]
- Ma, K.; Guo, K.; Li, L.; Zou, W.; Tang, C.; Dong, L. Cavity size dependent SO2 resistance for NH3-SCR of hollow structured CeO2-TiO2 catalysts. Catal. Commun. 2019, 128, 105719–105723. [Google Scholar] [CrossRef]
- Huang, J.; Tong, Z.; Huang, Y.; Zhang, J. Selective catalytic reduction of NO with NH3 at low temperatures over iron and manganese oxides supported on mesoporous silica. Appl. Catal. B Environ. 2008, 78, 309–314. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. DRIFT study on cerium−tungsten/titanium catalyst for selective catalytic reduction of NOx with NH3. Environ. Sci. Technol. 2010, 44, 9590–9596. [Google Scholar] [CrossRef]
- Guan, B.; Lin, H.; Zhu, L.; Huang, Z. Selective catalytic reduction of NOx with NH3 over Mn, Ce substitution Ti0.9V0.1O2-δ nanocomposites catalysts prepared by self-propagating high-temperature synthesis method. J. Phys. Chem. C 2011, 115, 12850–12863. [Google Scholar] [CrossRef]
- Chang, H.; Chen, X.; Li, J.; Ma, L.; Wang, C.; Liu, C.; Schwank, J.W.; Hao, J. Improvement of Activity and SO2 Tolerance of Sn-Modified MnOx–CeO2 Catalysts for NH3-SCR at Low Temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef]
- Zhang, B.; Liebau, M.; Suprun, W.; Liu, B.; Zhang, S.; Gläser, R. Suppression of N2O formation by H2O and SO2 in the selective catalytic reduction of NO with NH3 over a Mn/Ti–Si catalyst. Catal. Sci. Technol. 2019, 9, 4759–4770. [Google Scholar] [CrossRef]
- Krishna, K.; Seijger, G.B.F.; Bleek, C.M.V.D.; Makkee, M.; Mul, G.; Calis, H.P.A. Selective Catalytic Reduction of NO with NH3 over Fe-ZSM-5 Catalysts Prepared by Sublimation of FeCl3 at Different Temperatures. Catal. Lett. 2003, 86, 121–132. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, W.; An, D.; Wang, X.; Liu, A.; Zou, W.; Tang, C.; Ge, C.; Tong, Q.; Sun, J.; et al. Insight into the SO2 resistance mechanism on γ-Fe2O3 catalyst in NH3-SCR reaction: A collaborated experimental and DFT study. Appl. Catal. B Environ. 2021, 281, 119544. [Google Scholar] [CrossRef]
- Li, L.; Wu, Y.; Hou, X.; Chu, B.; Nan, B.; Qin, Q.; Fan, M.; Sun, C.-Z.; Li, B.; Dong, L.; et al. Investigation of Two-Phase Intergrowth and Coexistence in Mn–Ce–Ti–O Catalysts for the Selective Catalytic Reduction of NO with NH3: Structure–Activity Relationship and Reaction Mechanism. Ind. Eng. Chem. Res. 2019, 58, 849–862. [Google Scholar] [CrossRef]
- Geng, Y.; Shan, W.; Yang, S.; Liu, F. W-Modified Mn–Ti Mixed Oxide Catalyst for the Selective Catalytic Reduction of NO with NH3. Ind. Eng. Chem. Res. 2018, 57, 9112–9119. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, W.; Jiang, Z.; Chen, Z.; Wang, J. The surface properties and the activities in catalytic wet air oxidation over CeO2–TiO2 catalysts. Appl. Surf. Sci. 2006, 252, 8499–8505. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Ge, C.; Li, T.; Li, C.; Li, S.; Xiong, F.; Dong, L. Promoting N2 Selectivity of CeMnOx Catalyst by Supporting TiO2 in NH3-SCR Reaction. Ind. Eng. Chem. Res. 2019, 58, 6325–6332. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A Highly Effective Catalyst of Sm-MnOx for the NH3-SCR of NOx at Low Temperature: Promotional Role of Sm and Its Catalytic Performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Cao, Y.; Yao, X.; Ge, C.; Gao, F.; Deng, Y.; Tang, C.; Dong, L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2015, 165, 589–598. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoing resistant mesoporous Mn-base catalyst for low temperature SCR of NO with NH3. Appl. Catal. B Environ. 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The determination of the activities of different iron species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B Environ. 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Liu, L.; Chen, Y.; Zhang, Z.; Wang, X. In situ DRIFTS investigation on the SCR of NO with NH3 over V2O5 catalyst supported by activated semi-coke. Appl. Surf. Sci. 2014, 313, 660–669. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Cao, J.; Chen, Y.; Tian, M.; Yang, F.; Sun, J.; Tang, C.; Dong, L. Enhancing the deNO performance of MnO/CeO2-ZrO2 nanorod catalyst for low-temperature NH3-SCR by TiO2 modification. Chem. Eng. J. 2019, 369, 46–56. [Google Scholar] [CrossRef]

| Catalyst | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| Z-Mn-Fe-Ce-Al-O | 77.90 | 0.19 | 9.59 |
| C-Mn-Fe-Ce-Al-O | 52.34 | 0.15 | 11.21 |
| Catalyst | O | C | Al | Mn | Ce | Fe | S |
|---|---|---|---|---|---|---|---|
| Z-Mn-Fe-Ce-Al-O | 64.55 | 9.11 | 12.44 | 5.98 | 6.18 | 1.73 | - |
| C-Mn-Fe-Ce-Al-O | 64.38 | 9.11 | 11.13 | 4.97 | 5.26 | 1.87 | 3.27 |
| Catalyst | Ce3+/Ce4+ | Fe2+/Fe3+ | Oβ/(Oα + Oβ) | Mn4+/(Mn4+ + Mn3+) |
|---|---|---|---|---|
| Z-Mn-Fe-Ce-Al-O | 0.22 | 2.26 | 0.19 | 0.72 |
| C-Mn-Fe-Ce-Al-O | 0.27 | 0.56 | 0.39 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, S.; Cai, Y.; Sun, C.; Sun, J.; Tang, C.; Dong, L. High Resistance of SO2 and H2O over Monolithic Mn-Fe-Ce-Al-O Catalyst for Low Temperature NH3-SCR. Catalysts 2020, 10, 1329. https://doi.org/10.3390/catal10111329
Hao S, Cai Y, Sun C, Sun J, Tang C, Dong L. High Resistance of SO2 and H2O over Monolithic Mn-Fe-Ce-Al-O Catalyst for Low Temperature NH3-SCR. Catalysts. 2020; 10(11):1329. https://doi.org/10.3390/catal10111329
Chicago/Turabian StyleHao, Shijie, Yandi Cai, Chuanzhi Sun, Jingfang Sun, Changjin Tang, and Lin Dong. 2020. "High Resistance of SO2 and H2O over Monolithic Mn-Fe-Ce-Al-O Catalyst for Low Temperature NH3-SCR" Catalysts 10, no. 11: 1329. https://doi.org/10.3390/catal10111329
APA StyleHao, S., Cai, Y., Sun, C., Sun, J., Tang, C., & Dong, L. (2020). High Resistance of SO2 and H2O over Monolithic Mn-Fe-Ce-Al-O Catalyst for Low Temperature NH3-SCR. Catalysts, 10(11), 1329. https://doi.org/10.3390/catal10111329