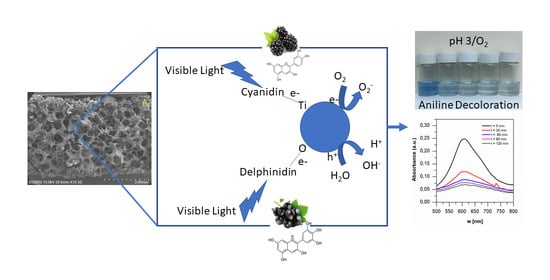
Visible-Light Photocatalytic Degradation of Aniline Blue by Stainless-Steel Foam Coated with TiO2 Grafted with Anthocyanins from a Maqui-Blackberry System
Abstract
1. Introduction
2. Results
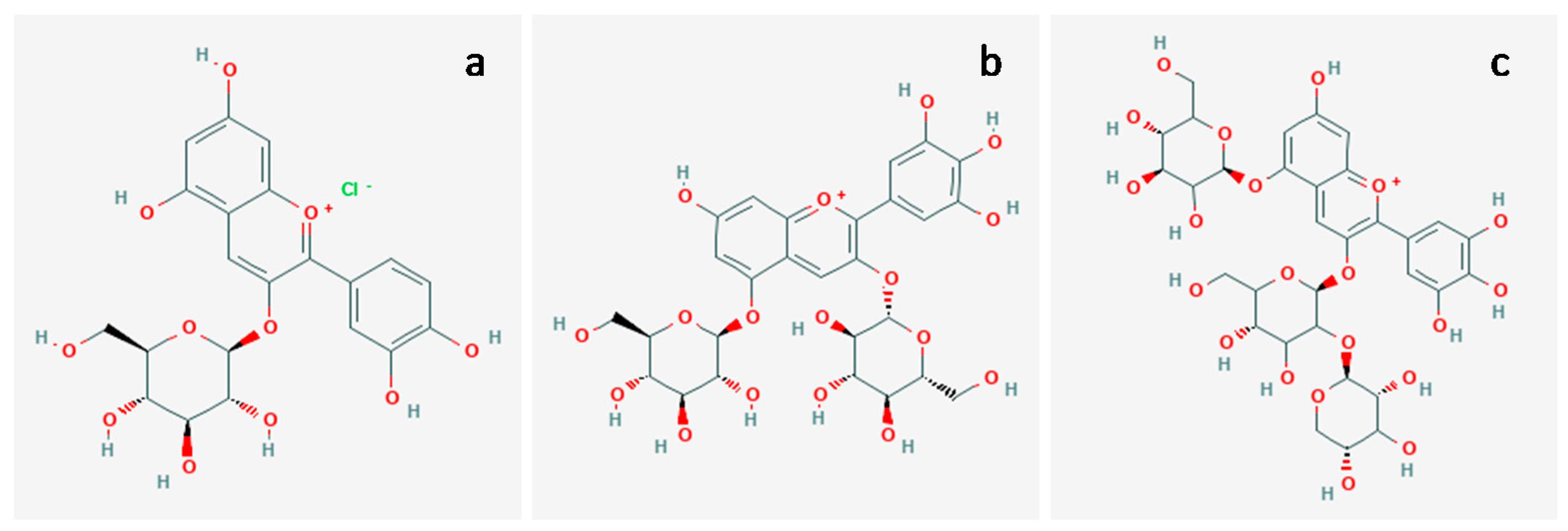
2.1. Anthocyanin Extraction
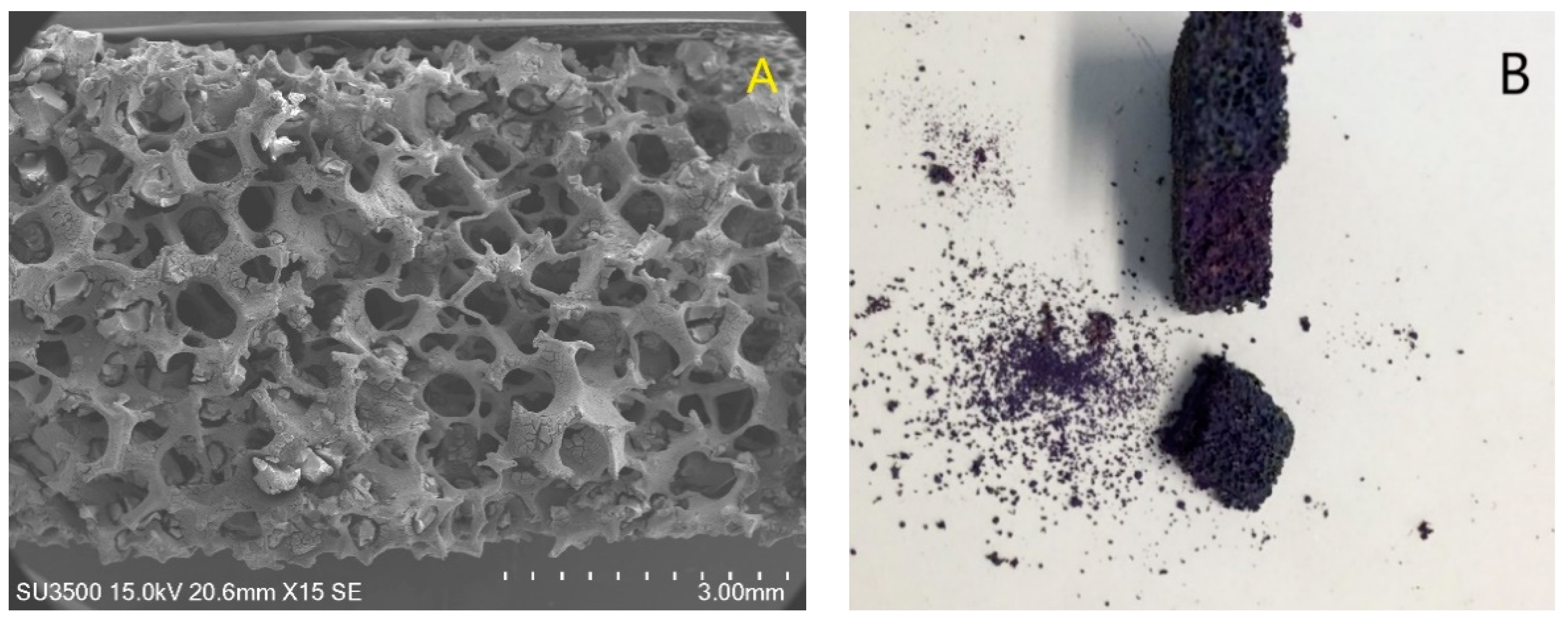
2.2. Stainless-Steel Foam Coated with TiO2
2.3. Stainless-Steel Foam Coated with TiO2-Anthocyanin
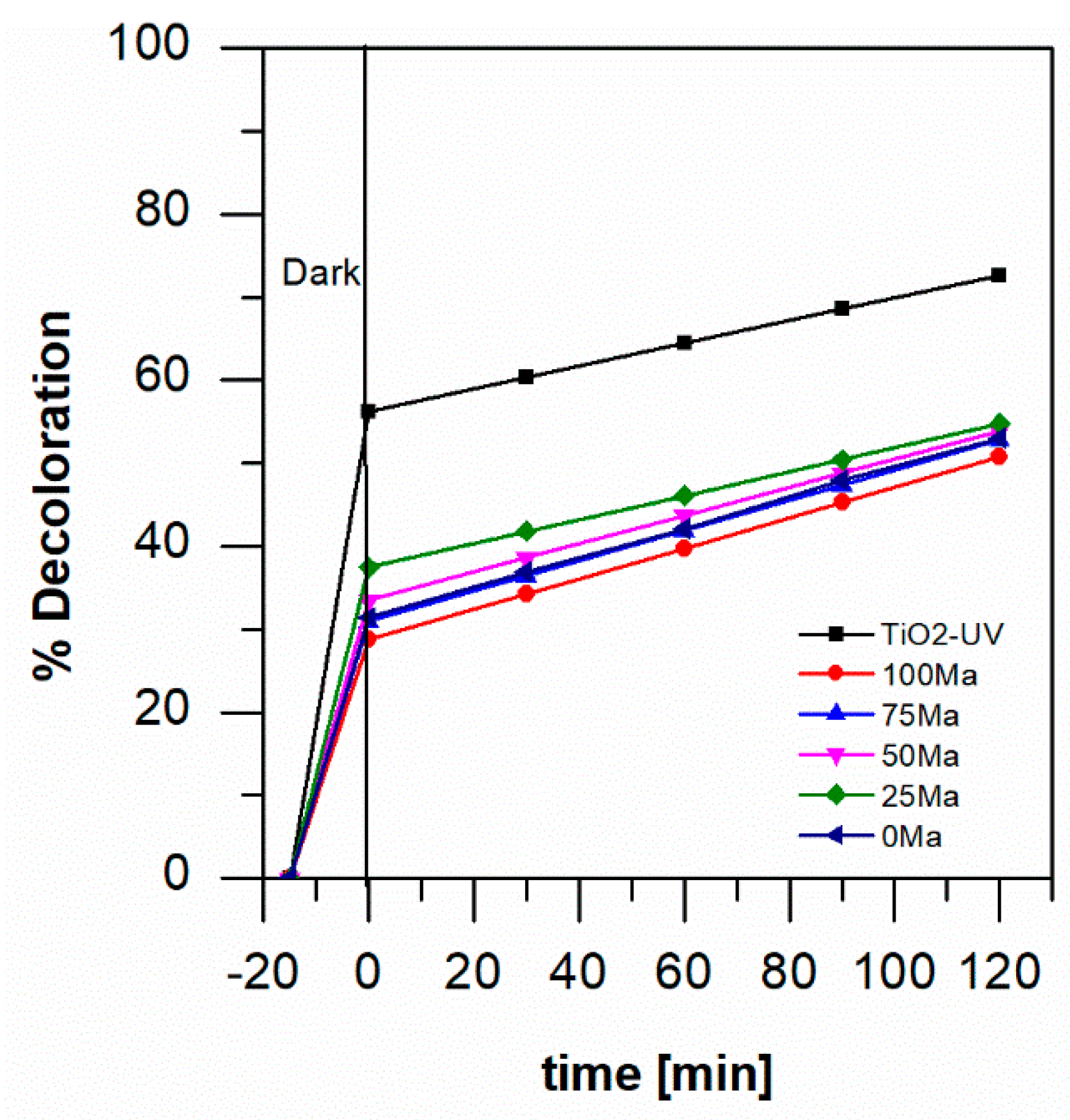
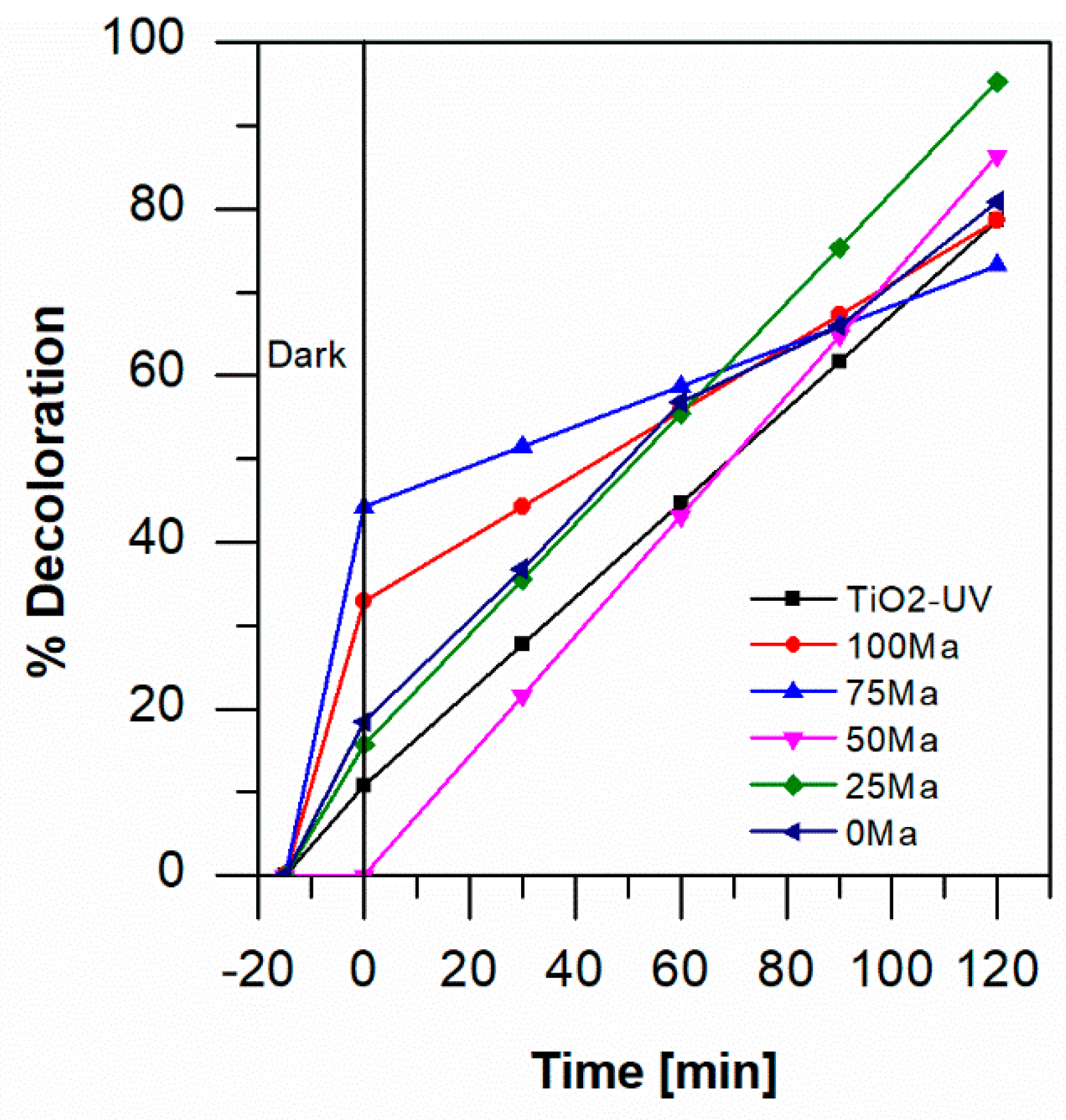
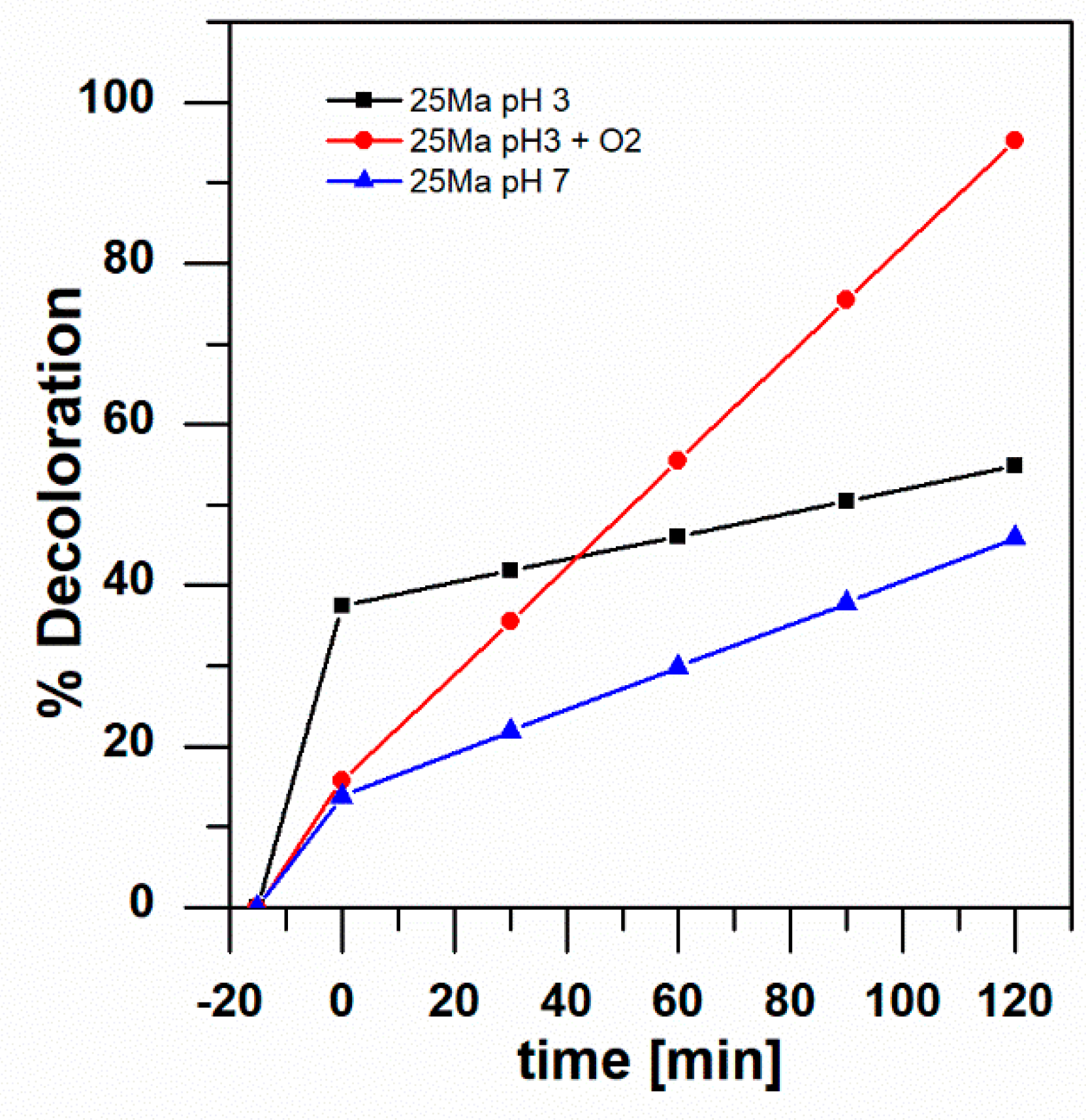
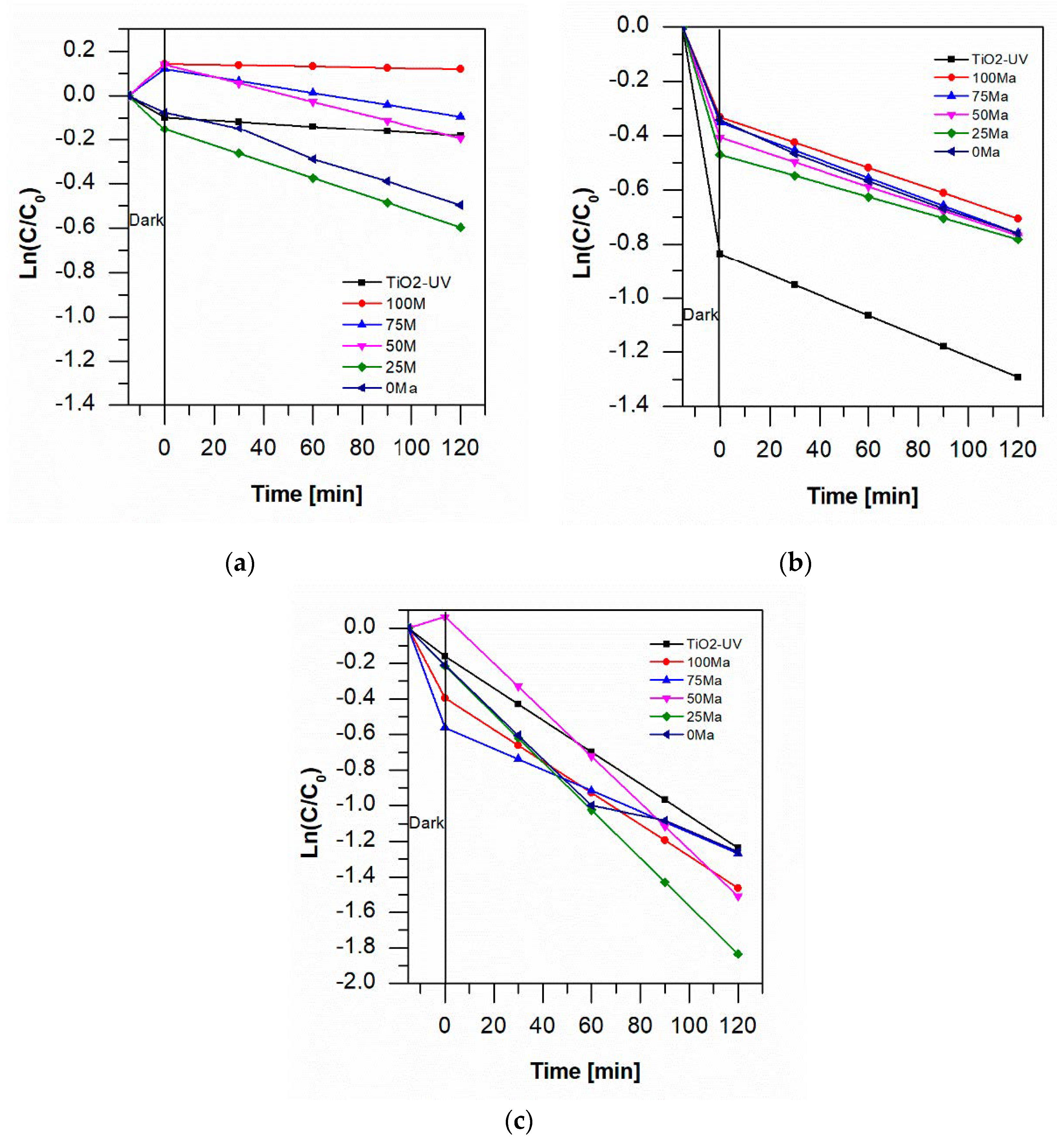
2.4. Photocatalytic Degradation of Aniline Blue
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Methods
4.2.1. Anthocyanin Ultrasound-Assisted Extraction
4.2.2. Stainless-Steel Foam Dip-Coating
4.2.3. Degradation Method
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pirsaheb, M.; Shahmoradi, B.; Beikmohammadi, M.; Azizi, E.; Hossini, H.; Ashraf, G.M. Photocatalytic degradation of aniline from aqueous solutions under sunlight illumination using immobilized Cr: ZnO nanoparticles. Sci. Rep. 2017, 7, 1473. [Google Scholar] [CrossRef] [PubMed]
- Egzar, H.K.; Mashkour, M.S.; Juda, A.M. Study the Photodegradation of Aniline Blue dye in aqueous Phase by using Different Photocatalysts. Asian Trans. Basic Appl. Sci. 2013, 3, 23–28. [Google Scholar]
- Wenhua, L.; Hong, L.; Sao’an, C.; Jianqing, Z.; Chunan, C. Kinetics of photocatalytic degradation of aniline in water over TiO2 supported on porous nickel. J. Photochem. Photobiol. A Chem. 2000, 131, 125–132. [Google Scholar] [CrossRef]
- Shahrezaei, F.; Mansouri, Y.; Zinatizadeh, A.A.L.; Akhbari, A. Photocatalytic degradation of aniline using TiO2 nanoparticles in a vertical circulating photocatalytic reactor. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; Martín, I.S.; Merino, S. Photocatalytic degradation of aniline using an autonomous rotating drum reactor with both solar and UV-C artificial radiation. J. Environ. Manag. 2018, 210, 122–130. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Jorgetto, A.; Milbrat, A.; Schneider, J.F.; Li, Z.; Giammaria, G.; Saeki, M.J.; Gianeti, T.M.R.; Lima, G.P.P.; de Albuquerque Pedrosa, V.; Mul, G.; et al. Magnetically-extractable hybrid of magnetite, mesoporous silica and titania for the photo-degradation of organic compounds in water. Appl. Surf. Sci. 2018, 457, 121–133. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [PubMed]
- Toe, E.D.; Kurniawan, W.; Mariquit, E.G.; Hinode, H. Synthesis of N-doped mesoporous TiO2 by a facile one-step solvothermal process for visible light photocatalytic degradation of organic pollutant. J. Environ. Chem. Eng. 2018, 6, 5125–5134. [Google Scholar] [CrossRef]
- Shukla, A.; Singha, R.K.; Sasaki, T.; Adak, S.; Bhandari, S.; Prasad, V.V.; Bordoloi, A.; Bal, R. Room temperature selective reduction of nitroarenes to azoxy compounds over Ni-TiO2 catalyst. Mol. Catal. 2020, 490, 110943. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Han, X.; An, L.; Hu, Y.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. Ti3C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation. Appl. Catal. B Environ. 2020, 265, 118539. [Google Scholar] [CrossRef]
- Pedroza-Herrera, G.; Medina-Ramírez, I.E.; Lozano-Álvarez, J.A.; Rodil, S.E. Evaluation of the Photocatalytic Activity of Copper Doped TiO2 nanoparticles for the Purification and Disinfection of Industrial Effluents. Catal. Today 2020, 341, 37–48. [Google Scholar] [CrossRef]
- Diaz-Angulo, J.; Lara-Ramos, J.; Mueses, M.; Hernández-Ramírez, A.; Li Puma, G.; Machuca-Martínez, F. Enhancement of the oxidative removal of diclofenac and of the TiO2 rate of photon absorption in dye-sensitized solar pilot scale CPC photocatalytic reactors. Chem. Eng. J. 2020, 381, 122520. [Google Scholar] [CrossRef]
- Bohnenkamp, B.; Linnemann, J.H.; Junger, I.J.; Schwenzfeier-Hellkamp, E.; Ehrmann, A. Influence of different solvents on the electrical properties of dye-sensitized solar cells. J. Renew. Sustain. Energy 2018, 10, 282–286. [Google Scholar] [CrossRef]
- Fredes, C.; Yousef, G.G.; Robert, P.; Grace, M.H.; Lila, M.A.; Gómez, M.; Gebauer, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef]
- Ryu, D.; Koh, E. Optimization of Ultrasound-Assisted Extraction of Anthocyanins and Phenolic Compounds from Black Soybeans (Glycine max L.). Food Anal. Methods 2019, 12, 1382–1389. [Google Scholar] [CrossRef]
- Leyrer, J.; Rubilar, M.; Morales, E.; Pavez, B.; Leal, E.; Hunter, R. Factor Optimization in the Manufacturing Process of Dye-Sensitized Solar Cells Based on Naturally Extracted Dye from a Maqui and Blackberry Mixture (Aristotelia Chilensis and Rubus Glaucus). J. Electron. Mater. 2018, 47, 6136–6143. [Google Scholar] [CrossRef]
- Jaime Guerrero, C.; Luigi Ciampi, P.; Andrea Castilla, C.; Fernando Medel, S.; Heidi Schalchli, S.; Emilio Hormazabal, U.; Emma Bensch, T.; Miren Alberdi, L. Capacidad antioxidante, antocianinas y fenoles totales de berries silvestres y cultivados en Chile. Chil. J. Agric. Res. 2010, 70, 537–544. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanins in berries of Maqui (Aristotelia chilensis (Mol.) Stuntz). Phytochem. Anal. 2006, 17, 8–14. [Google Scholar] [CrossRef]
- Fan-Chiang, H.J.; Wrolstad, R.E. Anthocyanin pigment composition of blackberries. J. Food Sci. 2005, 70, C198–C202. [Google Scholar] [CrossRef]
- Marcano, E. DFT study of anthocyanidin and anthocyanin pigments for Dye-Sensitized Solar Cells: Electron injecting from the excited states and adsorption onto TiO2 (anatase) surface. Phys. Sci. Rev. 2019, 2, 29–38. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Y.; Huang, W.; Huang, S. Degradation kinetics and mechanism of aniline by heat-assisted persulfate oxidation. J. Environ. Sci. 2012, 24, 821–826. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sakamoto, H.; Fujiwara, K.; Ichikawa, S.; Hirai, T. Selective Photocatalytic Oxidation of Aniline to Nitrosobenzene by Pt Nanoparticles Supported on TiO2 under Visible Light Irradiation. ACS Catal. 2014, 4, 2418–2425. [Google Scholar] [CrossRef]



| Sample | 100 Ma | 75 Ma | 50 Ma | 25 Ma | 100 Ba |
|---|---|---|---|---|---|
| TA (mg 100 g−1) | 12.023 | 14.175 | 17.575 | 18.798 | 32.600 |
| k [min−1] | TiO2 | 100 Ma | 75 Ma | 50 Ma | 25 Ma | 0 Ma |
|---|---|---|---|---|---|---|
| pH 7 | 0.0007 | 0.0002 | 0.0018 | 0.0028 | 0.0037 | 0.0036 |
| pH 3 | 0.0038 | 0.0031 | 0.0034 | 0.003 | 0.0026 | 0.0035 |
| pH 3 + O2 | 0.009 | 0.0089 | 0.0059 | 0.0131 | 0.0135 | 0.0086 |
| Catalyst | Photocatalytic Parameters | Aniline | Reference |
|---|---|---|---|
| TiO2 powder | Solar/UV-artificial | 89% degradation | Durán, A., et al. [5] |
| lamp/H2O2/TiO2; pH 4; 2 h | 100% discoloration (10 min) | ||
| Solar/H2O2/TiO2; pH 4; 2 h | 85% mineralization in 2 h | ||
| ZnO powder | 12 min; pH 4; 0.1 g catalyst; UV light; 323 K | 75% discoloration | Egzar, H.K., et al. [2] |
| ZnS powder | 12 min; pH 5; 0.5 g catalyst; UV light; 323 K | 24.5% discoloration | |
| SnO2 powder | 12 min; pH 4; 1 g catalyst; UV light; 323 K | 100% discoloration | |
| Cr:ZnO nanoparticles | Solar light; pH 9; 6 h | 93% degradation | Pirsaheb, et al. [1] |
| TiO2/Ni foam | pH 7; 2 h | 3% degradation | Wenhua, L., et al. [3] |
| pH 6.8; 2 h | 20% degradation | ||
| pH 6; 0.4 H2O2 mlh−1; 8 h | 100% degradation | ||
| None | UV light; 2 h | 3% degradation | Wenhua, L., et al. [3] |
| TiO2-25 Ma/SS-foam | TiO2/SS-foam with UV light; 2 h; pH 7 | 12% discoloration | Present study |
| TiO2/SS-foam with UV light; 2 h; pH 3 | 73% discoloration | ||
| TiO2/SS-foam with UV light; 2 h; pH 3; aerated | 73% discoloration | ||
| TiO2-25Ma/SS-foam with visible light; 2 h; pH 7 | 46% discoloration | ||
| TiO2-25Ma/SS-foam with visible light; 2 h; pH 3 | 55% discoloration | ||
| TiO2-25Ma/SS-foam with visible light; 2 h; pH 3; aerated | 95% discoloration |
| Name of the Mix | vol. % Maqui Juice | vol.% Blackberry Juice |
|---|---|---|
| 0 Ma | 0 | 100 |
| 25 Ma | 25 | 75 |
| 50 Ma | 50 | 50 |
| 75 Ma | 75 | 25 |
| 100 Ma | 100 | 0 |
| Aniline Blue Concentration [mM] | pH | Type of Light | |
|---|---|---|---|
| UV | Visible | ||
| 1 | 3 | TiO2-foam | 0 Ma |
| 25 Ma | |||
| 50 Ma | |||
| 75 Ma | |||
| 100 Ma | |||
| 7 | TiO2-foam | 0 Ma | |
| 25 Ma | |||
| 50 Ma | |||
| 75 Ma | |||
| 100 Ma | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez, D.; Palominos, F.; Martínez, S. Visible-Light Photocatalytic Degradation of Aniline Blue by Stainless-Steel Foam Coated with TiO2 Grafted with Anthocyanins from a Maqui-Blackberry System. Catalysts 2020, 10, 1245. https://doi.org/10.3390/catal10111245
Vásquez D, Palominos F, Martínez S. Visible-Light Photocatalytic Degradation of Aniline Blue by Stainless-Steel Foam Coated with TiO2 Grafted with Anthocyanins from a Maqui-Blackberry System. Catalysts. 2020; 10(11):1245. https://doi.org/10.3390/catal10111245
Chicago/Turabian StyleVásquez, Dreidy, Francisca Palominos, and Sebastián Martínez. 2020. "Visible-Light Photocatalytic Degradation of Aniline Blue by Stainless-Steel Foam Coated with TiO2 Grafted with Anthocyanins from a Maqui-Blackberry System" Catalysts 10, no. 11: 1245. https://doi.org/10.3390/catal10111245
APA StyleVásquez, D., Palominos, F., & Martínez, S. (2020). Visible-Light Photocatalytic Degradation of Aniline Blue by Stainless-Steel Foam Coated with TiO2 Grafted with Anthocyanins from a Maqui-Blackberry System. Catalysts, 10(11), 1245. https://doi.org/10.3390/catal10111245