Optimization of Carbon Nanotube-Coated Monolith by Direct Liquid Injection Chemical Vapor Deposition Based on Taguchi Method
Abstract
1. Introduction
2. Results and Discussion
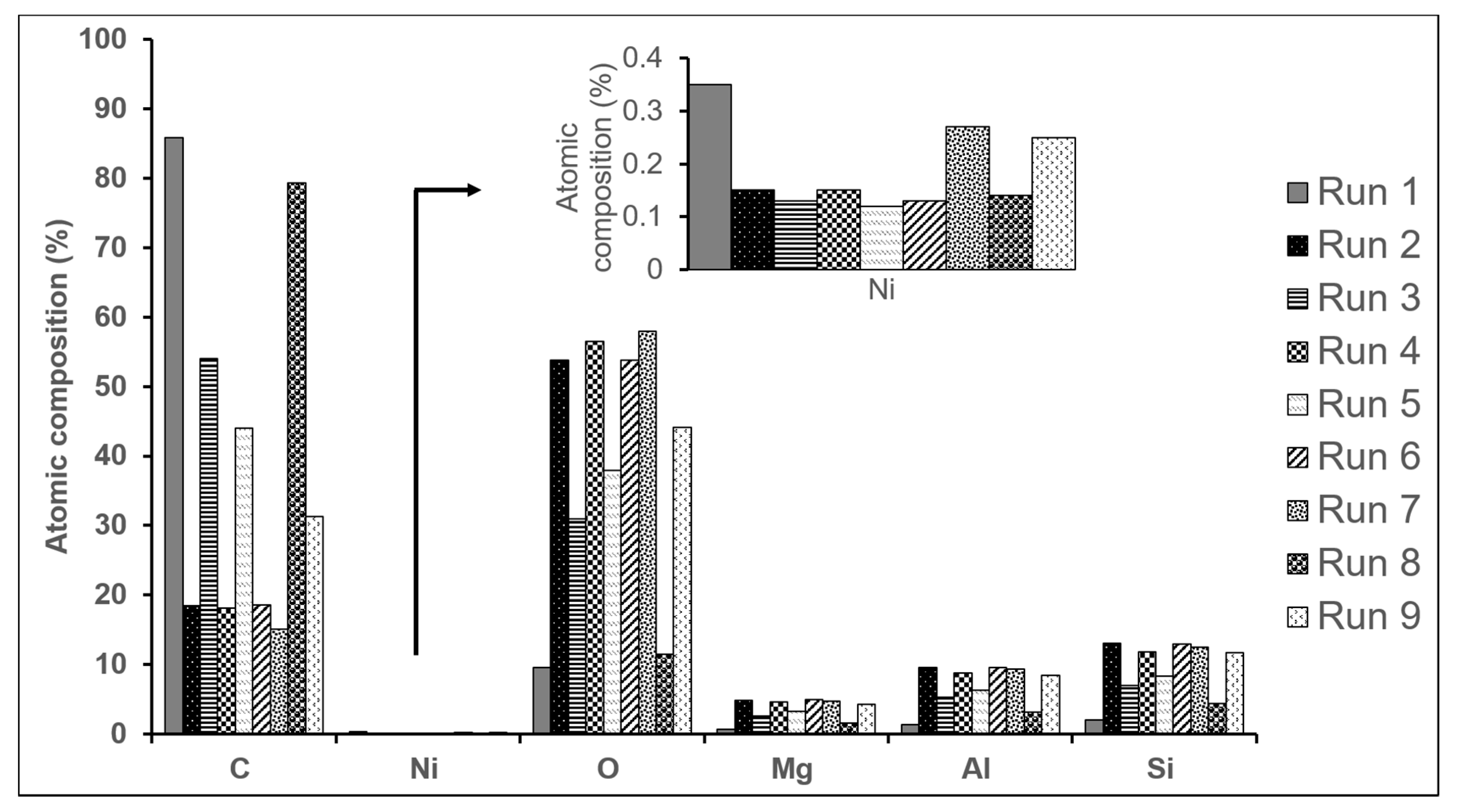
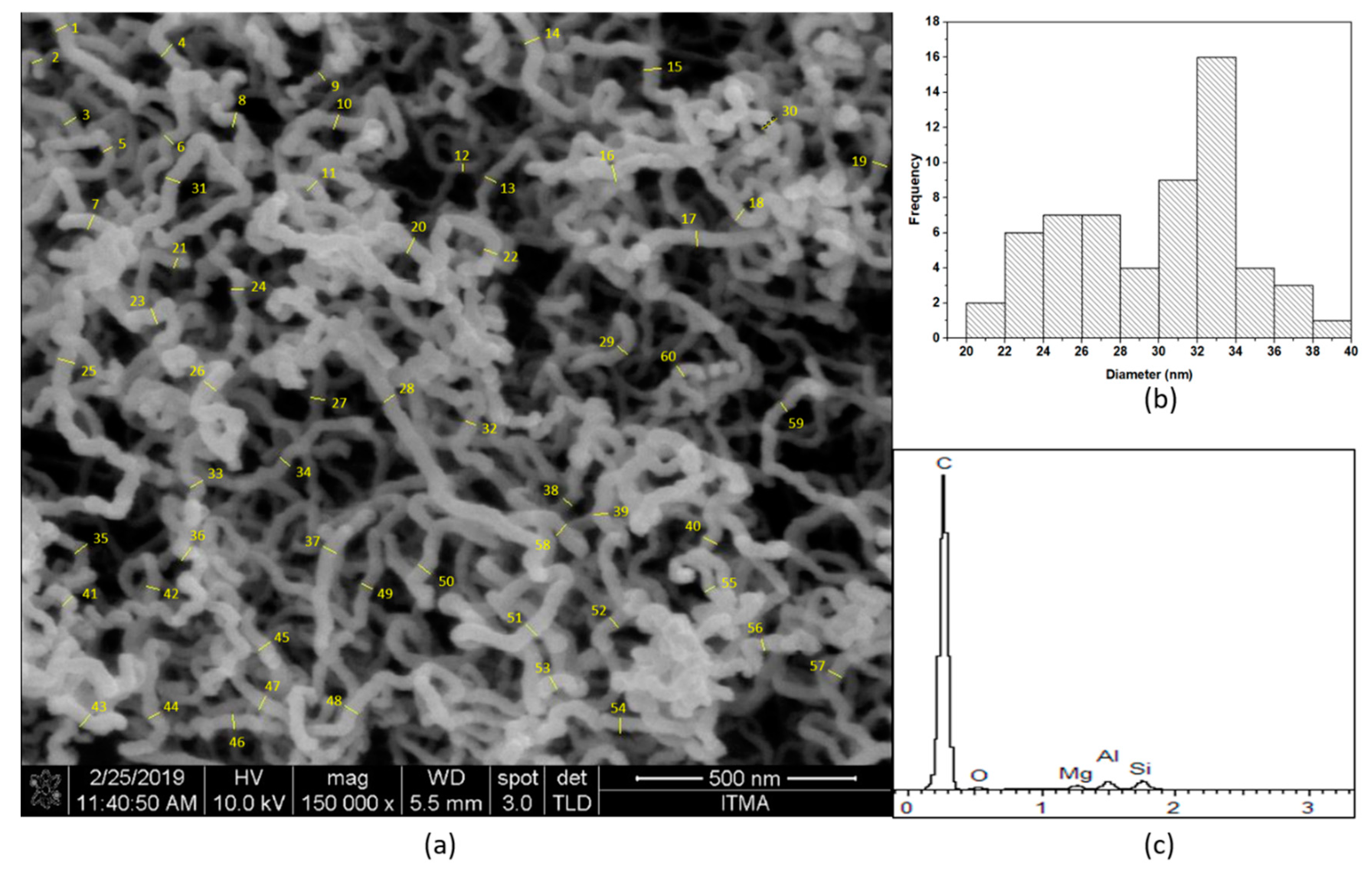
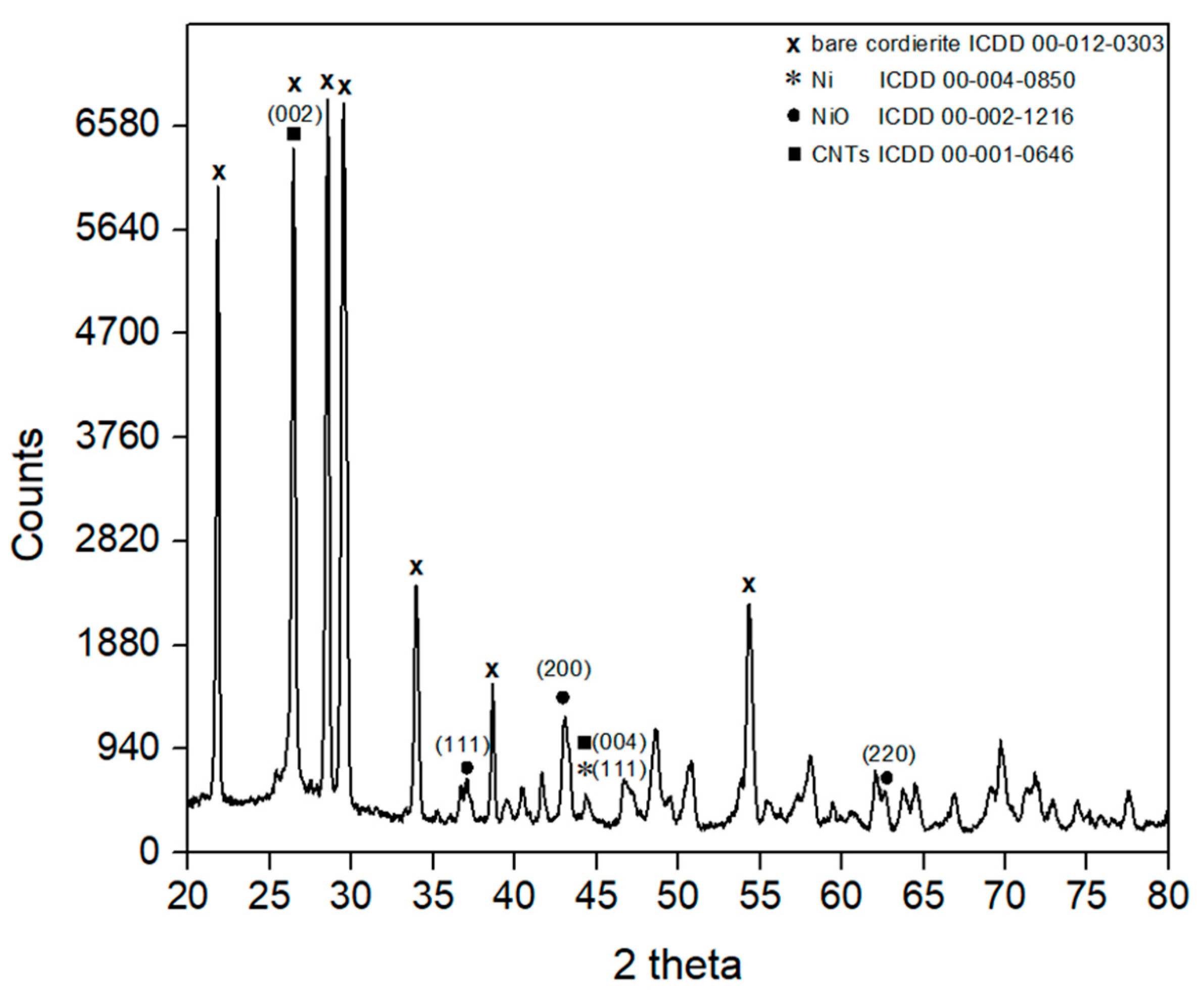
2.1. Analysis of the CNTs’ Growth on Monolith
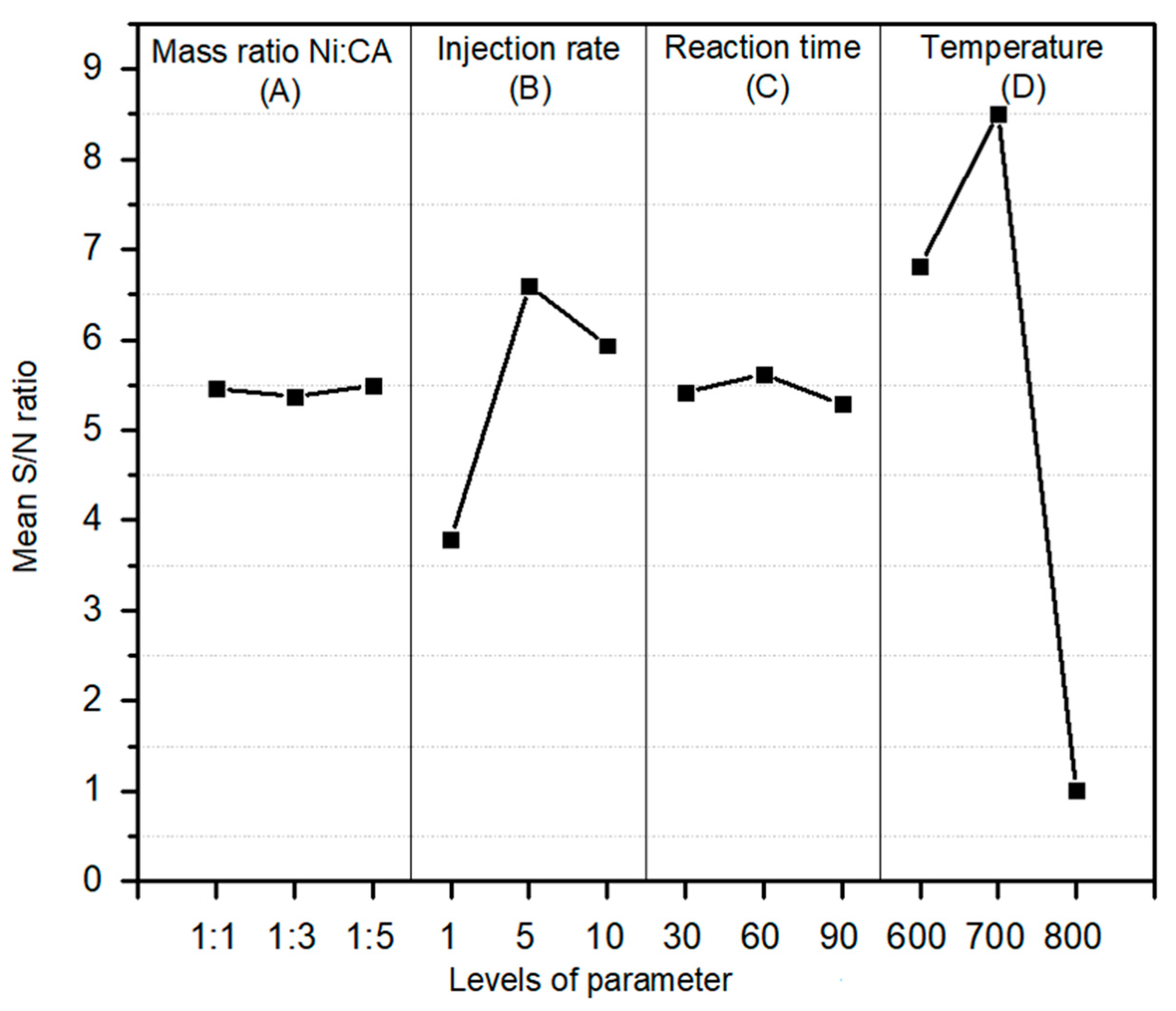
2.2. Response and Variance Analysis
2.3. Confirmation Test at Optimum Conditions
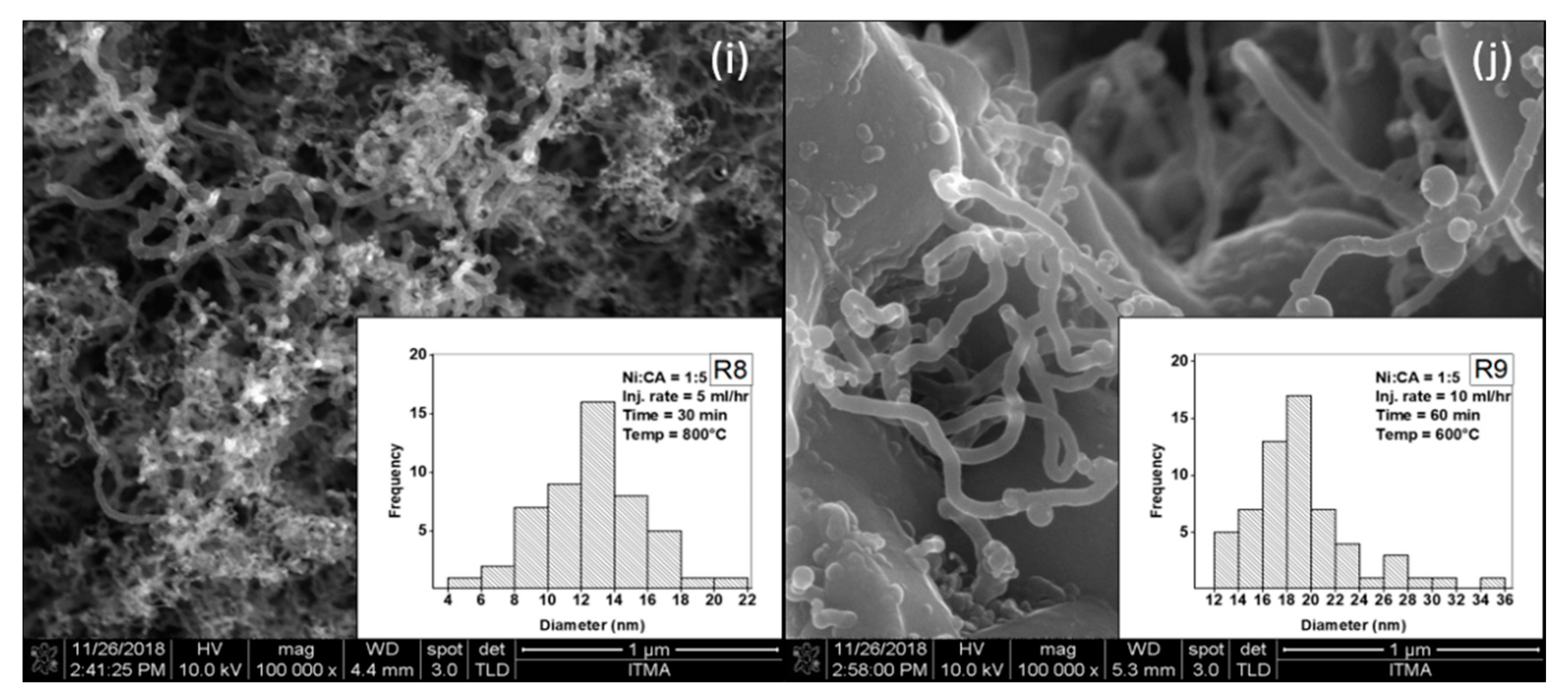
2.4. Characterization of CNT Monolith at Optimum Conditions
3. Materials and Methods
3.1. Materials
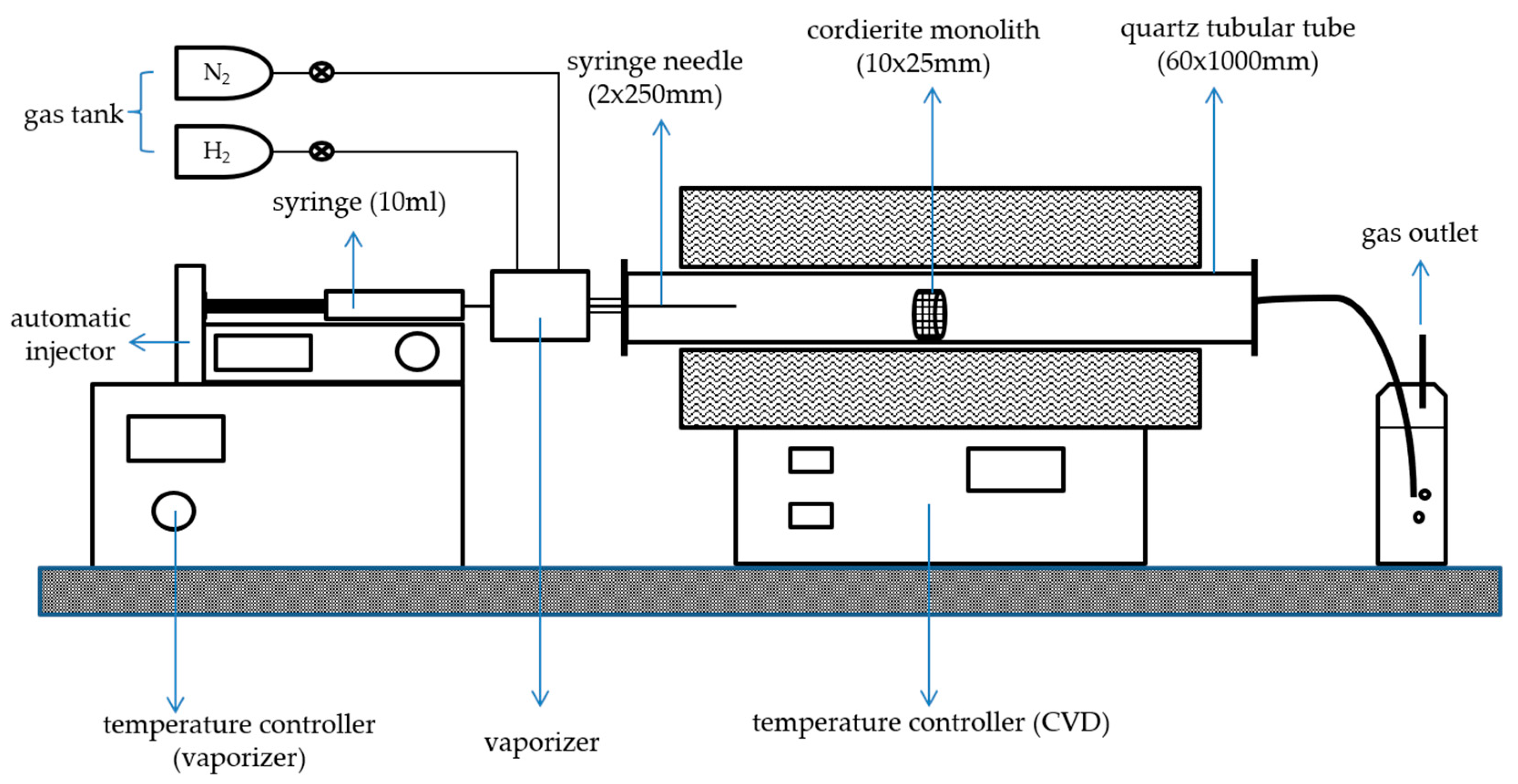
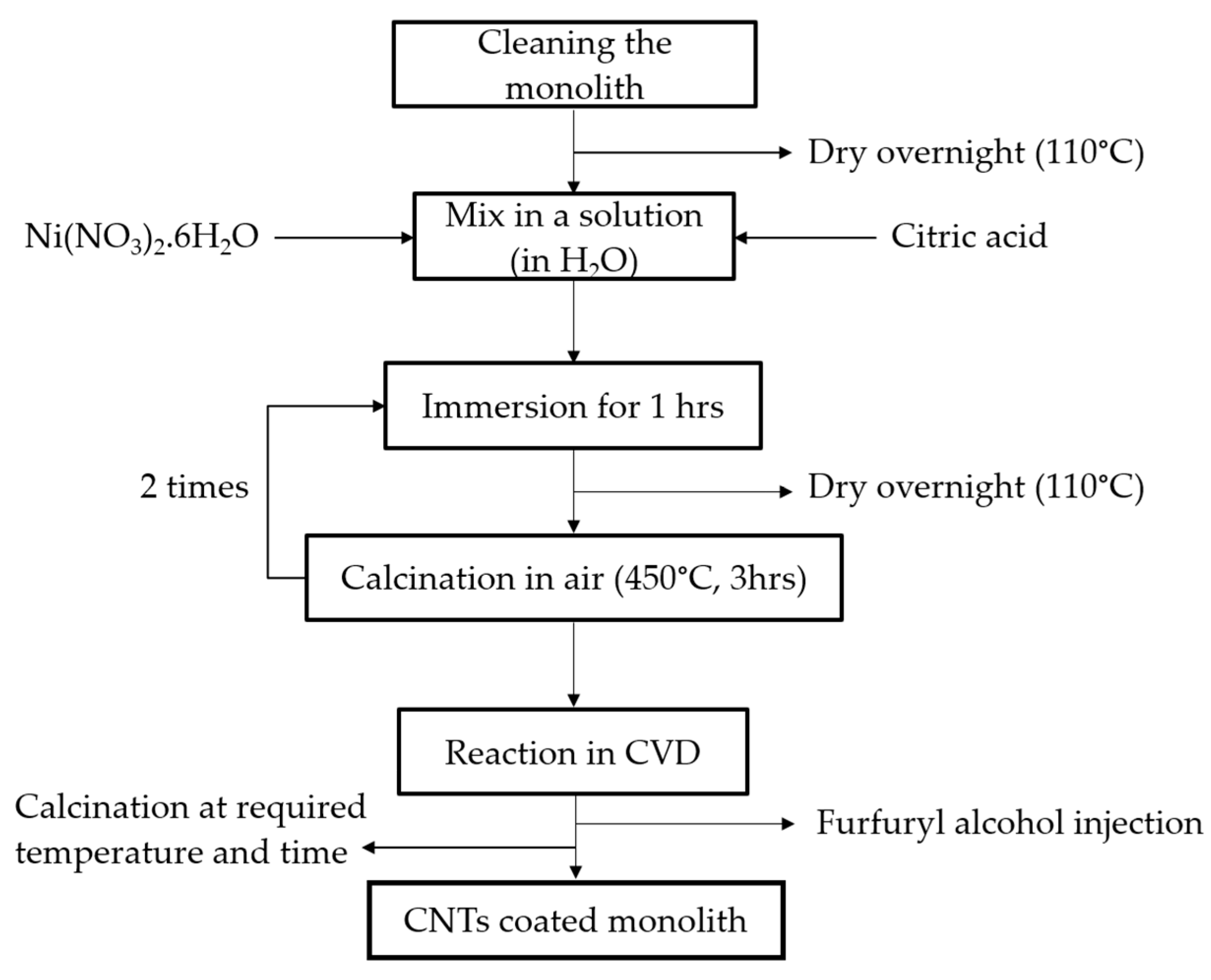
3.2. Preparation of the CNT-Coated Monolith
3.3. Taguchi Method of CNT-Coated Monolith
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Liu, W.W.; Chai, S.P.; Mohamed, A.R.; Hashim, U. Synthesis and characterization of graphene and carbon nanotubes: A review on the past and recent developments. J. Ind. Eng. Chem. 2014, 20, 1171–1185. [Google Scholar] [CrossRef]
- Ibrahim, K.S. Carbon nanotubes-properties and applications: A review. Carbon Lett. 2013, 14, 131–144. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Dongil, A.B.; Benito, N.; Espinoza-González, R.; Escalona, N.; Gracia, F. CO2 methanation over nickel-ZrO2 catalyst supported on carbon nanotubes: A comparison between two impregnation strategies. Appl. Catal. B Environ. 2018, 237, 817–825. [Google Scholar] [CrossRef]
- Sivakumar, V.M.; Mohamed, A.R.; Abdullah, A.Z.; Chai, S.P. Role of reaction and factors of carbon nanotubes growth in chemical vapour decomposition process using methane: A highlight. J. Nanomater. 2010, 2010, 11. [Google Scholar]
- Iijima, S. Synthesis of carbon nanotubes. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; De Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603. [Google Scholar] [CrossRef]
- Peretz, S.; Regev, O. Carbon nanotubes as nanocarriers in medicine. Curr. Opin. Coll. Interf. Sci. 2012, 17, 360–368. [Google Scholar] [CrossRef]
- Wong, B.S.; Yoong, S.L.; Jagusiak, A.; Panczyk, T.; Ho, H.K.; Ang, W.H.; Pastorin, G. Carbon nanotubes for delivery of small molecule drugs. Adv. Drug Deliv. Rev. 2013, 65, 1964–2015. [Google Scholar] [CrossRef]
- Xiang, L.; Zhang, H.; Hu, Y.; Peng, L.M. Carbon nanotube-based flexible electronics. J. Mater. Chem. C 2018, 6, 7714–7727. [Google Scholar] [CrossRef]
- Farrera, C.; Torres Andon, F.; Feliu, N. Carbon nanotubes as optical sensors in biomedicine. ACS Nano 2017, 11, 10637–10643. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Multiwalled carbon nanotubes reinforced polypropylene composite material. J. Nanomater. 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Sui, X.; Wagner, H.D. Tough nanocomposites: The role of carbon nanotube type. Nano Lett. 2009, 9, 1423–1426. [Google Scholar] [CrossRef]
- Minett, D.R.; O’Byrne, J.P.; Jones, M.D.; Ting, V.P.; Mays, T.J.; Mattia, D. One-step production of monolith-supported long carbon nanotube arrays. Carbon 2013, 51, 327–334. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Bian, B.; Fan, J.; Yang, J. Cobalt doped Ni based ordered mesoporous catalysts for CO2 methanation with enhanced catalytic performance. Int. J. Hydrogen Energy 2018, 43, 4893–4901. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, T.W.; Lee, S.H.; Park, E.D. Effects of Na content in Na/Ni/SiO2 and Na/Ni/CeO2 catalysts for CO and CO2 methanation. Catal. Today 2018, 303, 159–167. [Google Scholar] [CrossRef]
- Mihet, M.; Lazar, M.D. Methanation of CO2 on Ni/γ-Al2O3: Influence of Pt, Pd or Rh promotion. Catal. Today 2018, 306, 294–299. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, Y.; Gu, D.; Chen, C.; Jiang, L.; Takehira, K. Ni/Al2O3-ZrO2 catalyst for CO2 methanation: The role of γ-(Al, Zr)2O3 formation. Appl. Surf. Sci. 2018, 459, 74–79. [Google Scholar] [CrossRef]
- Dos Santos, P.A.M.; Zanella, I.; Costa, T.M.H.; da Silva, P.R.; Gallas, M.R. Preparation of carbon nanotube monoliths by high-pressure compaction. New Carbon Mater. 2014, 29, 193–202. [Google Scholar] [CrossRef]
- Sollier, B.M.; Gómez, L.E.; Boix, A.V.; Miró, E.E. Oxidative coupling of methane on cordierite monoliths coated with Sr/La2O3 catalysts. Influence of honeycomb structure and catalyst-cordierite chemical interactions on the catalytic behavior. Appl. Catal. A Gen. 2018, 550, 113–121. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths: A review of the basics, preparation methods and their relevance to oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Kapteijn, F.; Moulijn, J.A. Preparation and characterisation aspects of carbon-coated monoliths. Catal. Today 2001, 69, 357–363. [Google Scholar] [CrossRef]
- Jarrah, N.A.; van Ommen, J.G.; Lefferts, L. Mechanistic aspects of the formation of carbon-nanofibers on the surface of Ni foam: A new microstructured catalyst support. J. Catal. 2006, 239, 460–469. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Beer, A.E.W.; Vergunst, T.; Hoek, I.; Kapteinjn, F.; Moulijn, J.A. Preparation of monolithic catalysts. Catal. Rev. 2001, 43, 345–380. [Google Scholar] [CrossRef]
- Marconi, E.; Tuti, S.; Luisetto, I. Structure-Sensitivity of CO2 Methanation over nanostructured Ni supported on CeO2 nanorods. Catalysts 2019, 9, 375. [Google Scholar] [CrossRef]
- Zhu, H.; Razzaq, R.; Li, C.; Muhmmad, Y.; Zhang, S. Catalytic methanation of carbon dioxide by active oxygen material CexZr1− xO2 supported Ni-Co bimetallic nanocatalysts. AIChE J. 2013, 59, 2567–2576. [Google Scholar] [CrossRef]
- Du, C.; Pan, N. CVD growth of carbon nanotubes directly on nickel substrate. Mater. Lett. 2005, 59, 1678–1682. [Google Scholar] [CrossRef]
- Noda, L.K.; Gonçalves, N.S.; Valentini, A.; Probst, L.F.D.; de Almeida, R.M. Effect of Ni loading and reaction temperature on the formation of carbon nanotubes from methane catalytic decomposition over Ni/SiO2. J. Mater. Sci. 2007, 42, 914–922. [Google Scholar] [CrossRef]
- Hoyos-Palacio, L.M.; García, A.G.; Pérez-Robles, J.F.; González, J.; Martínez-Tejada, H.V. Catalytic effect of Fe, Ni, Co and Mo on the CNTs production. IOP Conf. Ser. Mater. Sci. Eng. 2014, 59, 012005. [Google Scholar] [CrossRef]
- Xiao, S.; Sun, W.; Du, J.; Li, G. Application of CFD, Taguchi method, and ANOVA technique to optimize combustion and emissions in a light duty diesel engine. Math. Probl. Eng. 2014, 2014, 502902. [Google Scholar] [CrossRef]
- Anjalin, F.M. Synthesis and characterization of MWCNTs/PVDF nanocomposite and its electrical studies. Der Pharma Chem. 2014, 6, 354–359. [Google Scholar]
- Soghrati, E.; Kazemeini, M.; Rashidi, A.M.; Jozani, K.J. Development of a structured monolithic support with a CNT washcoat for the naphtha HDS process. J. Taiwan Inst. Chem. Eng. 2014, 45, 887–895. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Kapteijn, F.; Moulijn, J. Preparation and characterisation of carbon-coated monoliths for catalyst supports. Carbon 2002, 40, 1079–1088. [Google Scholar] [CrossRef]
- Abate, S.; Barbera, K.; Giglio, E.; Deorsola, F.; Bensaid, S.; Perathoner, S.; Pirone, R.; Centi, G. Synthesis, characterization, and activity pattern of Ni–Al hydrotalcite catalysts in CO2 methanation. Ind. Eng. Chem. Res. 2016, 55, 8299–8308. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Li, P.; Deng, G.; Guo, X.; Liu, H.; Jiang, W.; Li, F. Preparation of nickel and Ni3Sn nanoparticles via extension of conventional citric acid and ethylene diamine tetraacetic acid mediated sol–gel method. J. Alloys Compd. 2016, 668, 159–168. [Google Scholar] [CrossRef]
- Esquenazi, G.; Brinson, B.; Barron, A. Catalytic growth of carbon nanotubes by direct liquid injection CVD using the nanocluster [HxPMo12O40⊂H4Mo72Fe30 (O2CMe)15O254(H2O)98-y(EtOH)y]. J. Carbon Res. 2018, 4, 17. [Google Scholar] [CrossRef]
- Mittal, G.; Rhee, K.Y. Chemical vapor deposition-based grafting of CNTs onto basalt fabric and their reinforcement in epoxy-based composites. Compos. Sci. Technol. 2018, 165, 84–94. [Google Scholar] [CrossRef]
- Ping, D.; Wang, C.; Dong, X.; Dong, Y. Co-production of hydrogen and carbon nanotubes on nickel foam via methane catalytic decomposition. Appl. Surf. Sci. 2016, 369, 299–307. [Google Scholar] [CrossRef]
- Moonngam, S.; Tunjina, P.; Deesom, D.; Banjongprasert, C. Fe-Cr/CNTs nanocomposite feedstock powders produced by chemical vapor deposition for thermal spray coatings. Surf. Coat. Technol. 2016, 306, 323–327. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.J.; Ulrich, S.; Araujo, P.T.; Bakker, M.G. Catalytic graphitization in nanocast carbon monoliths by iron, cobalt and nickel nanoparticles. Carbon 2018, 134, 452–463. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Y.; Zhang, H.; Lin, Q.; Zhu, B. Synthesis and characterization of in-situ CNTs reinforced TiB2-based composite by CVD using Ni catalysts. Ceram. Int. 2018, 44, 2042–2047. [Google Scholar] [CrossRef]
- Tripathi, N.; Mishra, P.; Joshi, B.; Islam, S.S. Catalyst free, excellent quality and narrow diameter of CNT growth on Al2O3 by a thermal CVD technique. Phys. E Low Dimens. Syst. Nanostruct. 2014, 62, 43–47. [Google Scholar] [CrossRef]
- Pham, Q.N.; Larkin, L.S.; Lisboa, C.C.; Saltonstall, C.B.; Qiu, L.; Schuler, J.D.; Rupert, T.J.; Norris, P.M. Effect of growth temperature on the synthesis of carbon nanotube arrays and amorphous carbon for thermal applications. Phys. Status Solidi A 2017, 214, 1600852. [Google Scholar] [CrossRef]
- Taleshi, F. Evaluation of new processes to achieve a high yield of carbon nanotubes by CVD method. Int. Nano Lett. 2012, 2, 23. [Google Scholar] [CrossRef]
- Ross, P.J. Taguchi Techniques for Quality Engineering: Loss Function, Orthogonal Experiments, Parameter and Tolerance Design; McGraw-Hill: New York, NY, USA, 1988; pp. 208–212. [Google Scholar]




| Parameters | * Carbon Yield (CNTs Growth) | Response | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Run | A | B | C | D | Yield 1 | Yield 2 | Average Yield | Standard error | ** S/N ratio |
| mL/h | min | °C | % | % | % | dB | |||
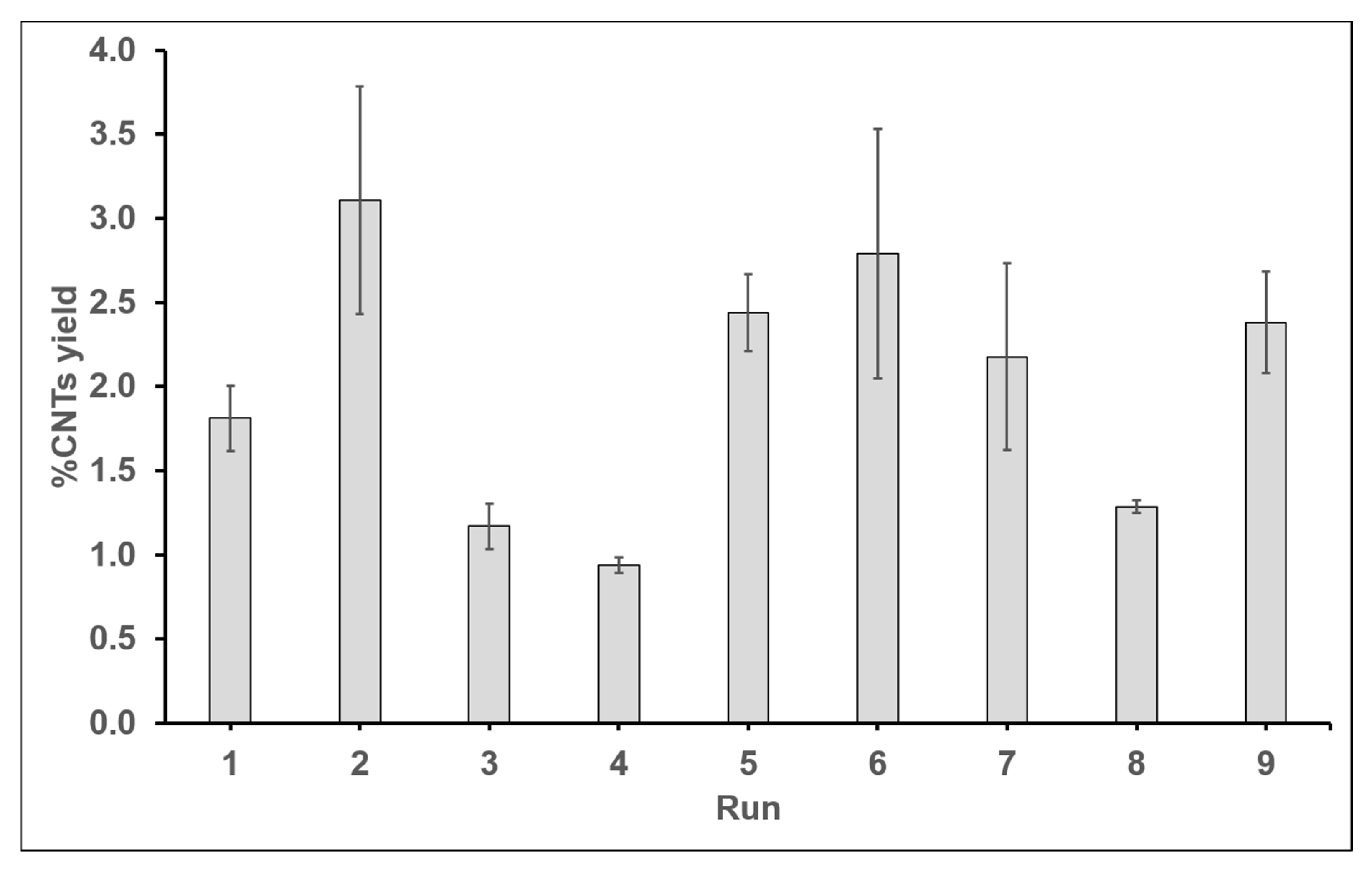
| 1 | 1:1 | 1 | 30 | 600 | 2.005 | 1.617 | 1.811 | 0.194 | 5.158 |
| 2 | 1:1 | 5 | 60 | 700 | 3.785 | 2.433 | 3.109 | 0.676 | 9.852 |
| 3 | 1:1 | 10 | 90 | 800 | 1.304 | 1.036 | 1.170 | 0.134 | 1.360 |
| 4 | 1:3 | 1 | 60 | 800 | 0.986 | 0.894 | 0.940 | 0.046 | −0.538 |
| 5 | 1:3 | 5 | 90 | 600 | 2.670 | 2.211 | 2.440 | 0.229 | 7.749 |
| 6 | 1:3 | 10 | 30 | 700 | 2.046 | 3.533 | 2.789 | 0.743 | 8.910 |
| 7 | 1:5 | 1 | 90 | 700 | 1.620 | 2.732 | 2.176 | 0.556 | 6.753 |
| 8 | 1:5 | 5 | 30 | 800 | 1.325 | 1.248 | 1.287 | 0.038 | 2.189 |
| 9 | 1:5 | 10 | 60 | 600 | 2.079 | 2.684 | 2.381 | 0.303 | 7.536 |
| Mass Ratio of Ni:CA | Injection Rate of Carbon | Reaction Time | Operating Temperature | |
|---|---|---|---|---|
| Level | A | B | C | D |
| 1 | 5.457 | 3.791 | 5.419 | 6.814 |
| 2 | 5.374 | 6.597 | 5.617 | 8.505 |
| 3 | 5.493 | 5.935 | 5.287 | 1.004 |
| Delta | 0.119 | 2.806 | 0.329 | 7.501 |
| Rank | 4 | 2 | 3 | 1 |
| Degree of Freedom (DF) | Sum of Squares (SS) | Adjusted Mean Square (Adj. SS) | Percentage Contribution (%) | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| A | 2 | 0.022 | 0.011 | 0.02 | ||
| B | 2 | 12.910 | 6.450 | 12.17 | 138.060 | 0.0002 |
| C | 2 | 0.160 | 0.082 | 0.16 | ||
| D | 2 | 92.900 | 46.450 | 87.65 | 993.520 | <0.0001 |
| Model (B and D) | 4 | 105.810 | 26.450 | 565.790 | <0.0001 | |
| Total (A, B, C, D) | 8 | 105.640 | 52.993 | 100.00 | ||
| R-Squared | 0.9982 (significant) | |||||
| Adj. R-Squared | 0.9965 | |||||
| Pred-R-Squared | 0.9911 | |||||
| Adeq Precision | 63.957 | |||||
| Std. Dev. | 0.22 | |||||
| Optimization Prediction | Optimization Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | S/N Ratio Predicted | Yield CNTs Growth | S/N Ratio Actual | Yield CNTs Growth | ||||
| Number | A | B | C | D | dB | % | dB | % |
| 1 | 1:1 | 5 | 30 | 700 | 9.6609 | 3.0415 | 9.7502 | 3.0726 |
| 1 | 1:1 | 5 | 30 | 700 | 9.6609 | 3.0415 | 9.3195 | 2.9240 |
| Average | 9.6609 | 3.0415 | 9.5349 | 2.9983 | ||||
| Standard error | 0.1758 | 0.0607 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qistina, O.; Salmiaton, A.; Choong, T.S.Y.; Taufiq-Yap, Y.H.; Izhar, S. Optimization of Carbon Nanotube-Coated Monolith by Direct Liquid Injection Chemical Vapor Deposition Based on Taguchi Method. Catalysts 2020, 10, 67. https://doi.org/10.3390/catal10010067
Qistina O, Salmiaton A, Choong TSY, Taufiq-Yap YH, Izhar S. Optimization of Carbon Nanotube-Coated Monolith by Direct Liquid Injection Chemical Vapor Deposition Based on Taguchi Method. Catalysts. 2020; 10(1):67. https://doi.org/10.3390/catal10010067
Chicago/Turabian StyleQistina, Omar, Ali Salmiaton, Thomas S.Y. Choong, Yun Hin Taufiq-Yap, and Shamsul Izhar. 2020. "Optimization of Carbon Nanotube-Coated Monolith by Direct Liquid Injection Chemical Vapor Deposition Based on Taguchi Method" Catalysts 10, no. 1: 67. https://doi.org/10.3390/catal10010067
APA StyleQistina, O., Salmiaton, A., Choong, T. S. Y., Taufiq-Yap, Y. H., & Izhar, S. (2020). Optimization of Carbon Nanotube-Coated Monolith by Direct Liquid Injection Chemical Vapor Deposition Based on Taguchi Method. Catalysts, 10(1), 67. https://doi.org/10.3390/catal10010067








