Characteristic and Catalytic Performance of Co and Co-Mo Metal Impregnated in Sarulla Natural Zeolite Catalyst for Hydrocracking of MEFA Rubber Seed Oil into Biogasoline Fraction
Abstract
1. Introduction
2. Results and Discussion
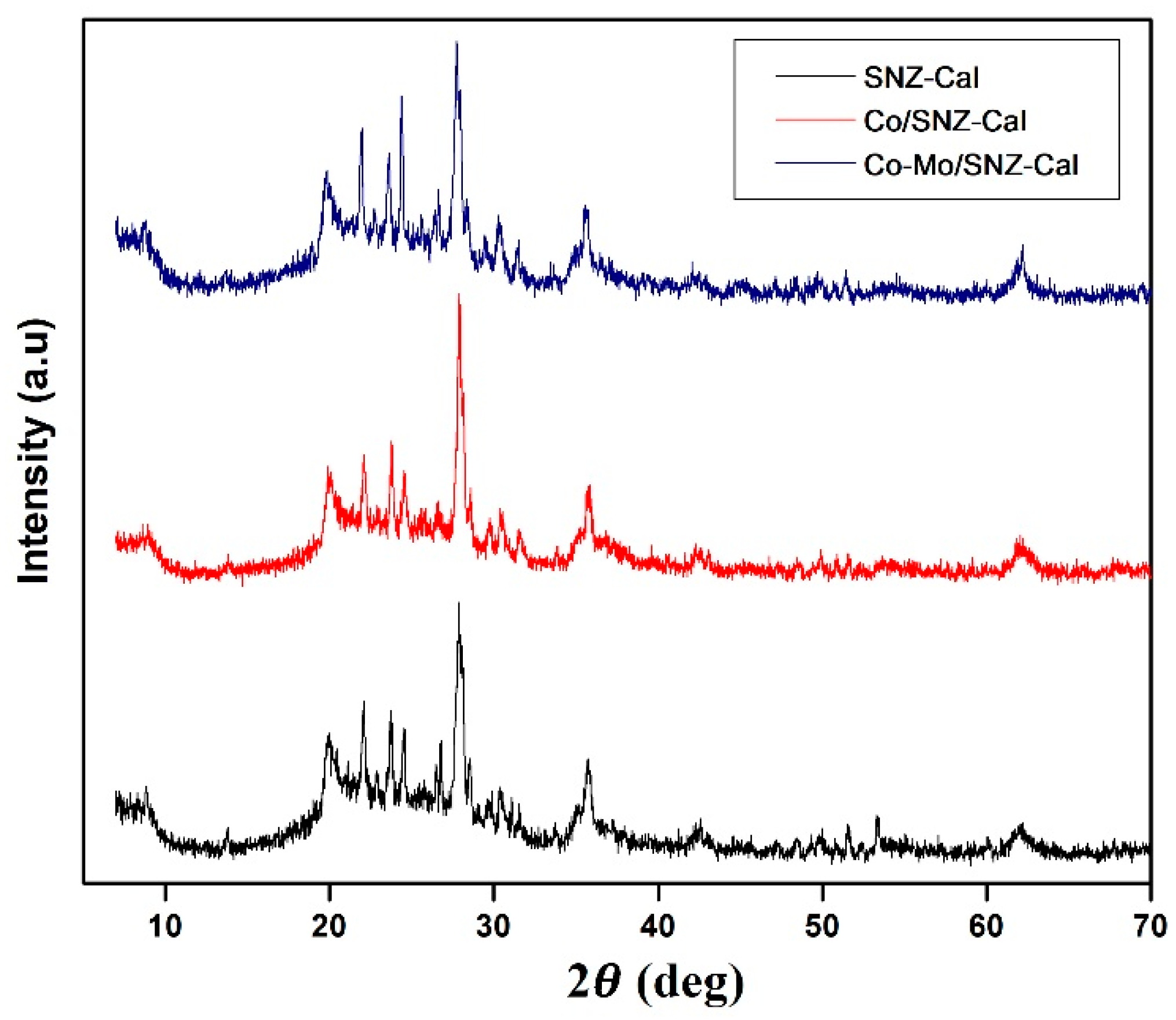
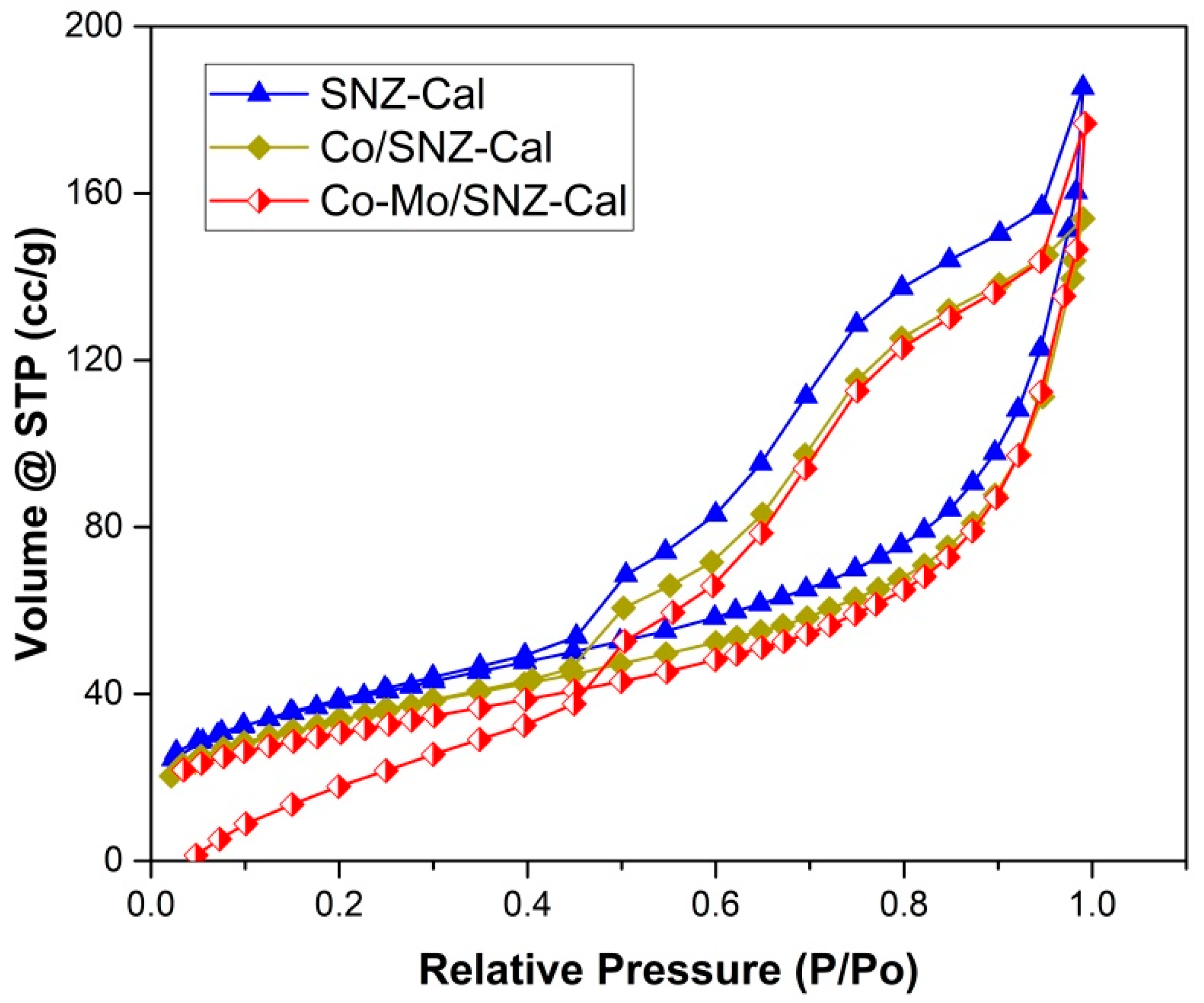
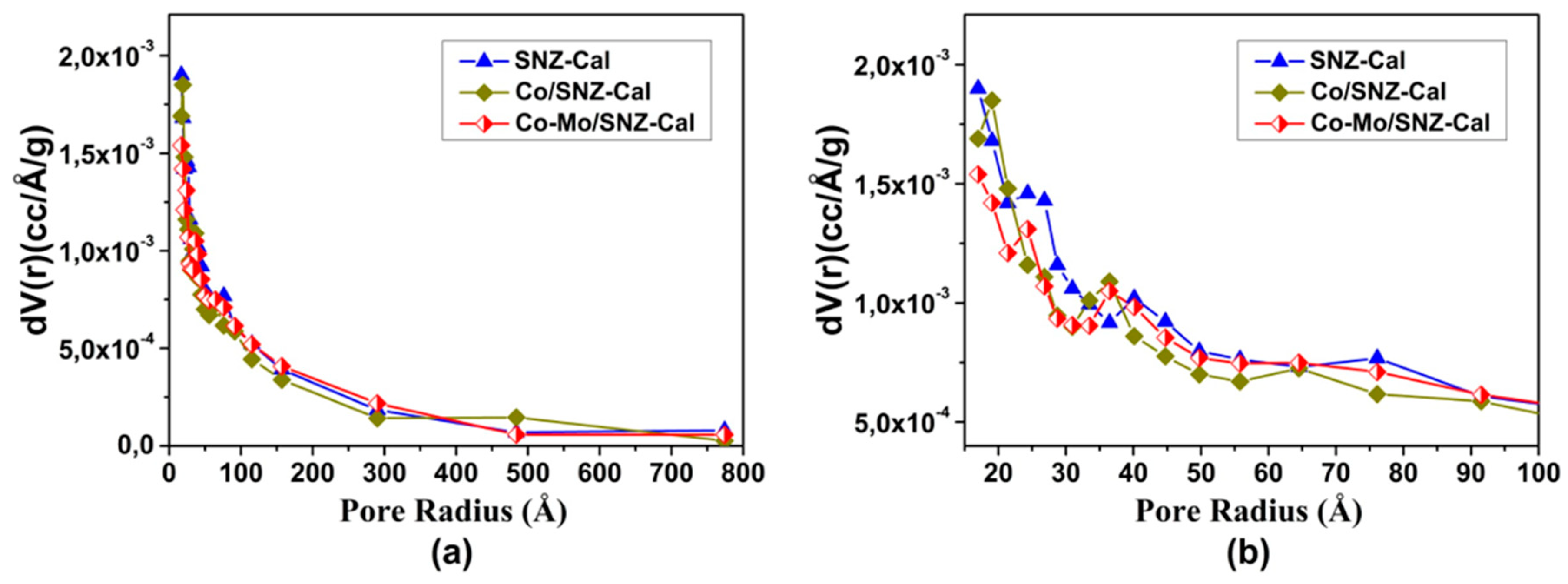
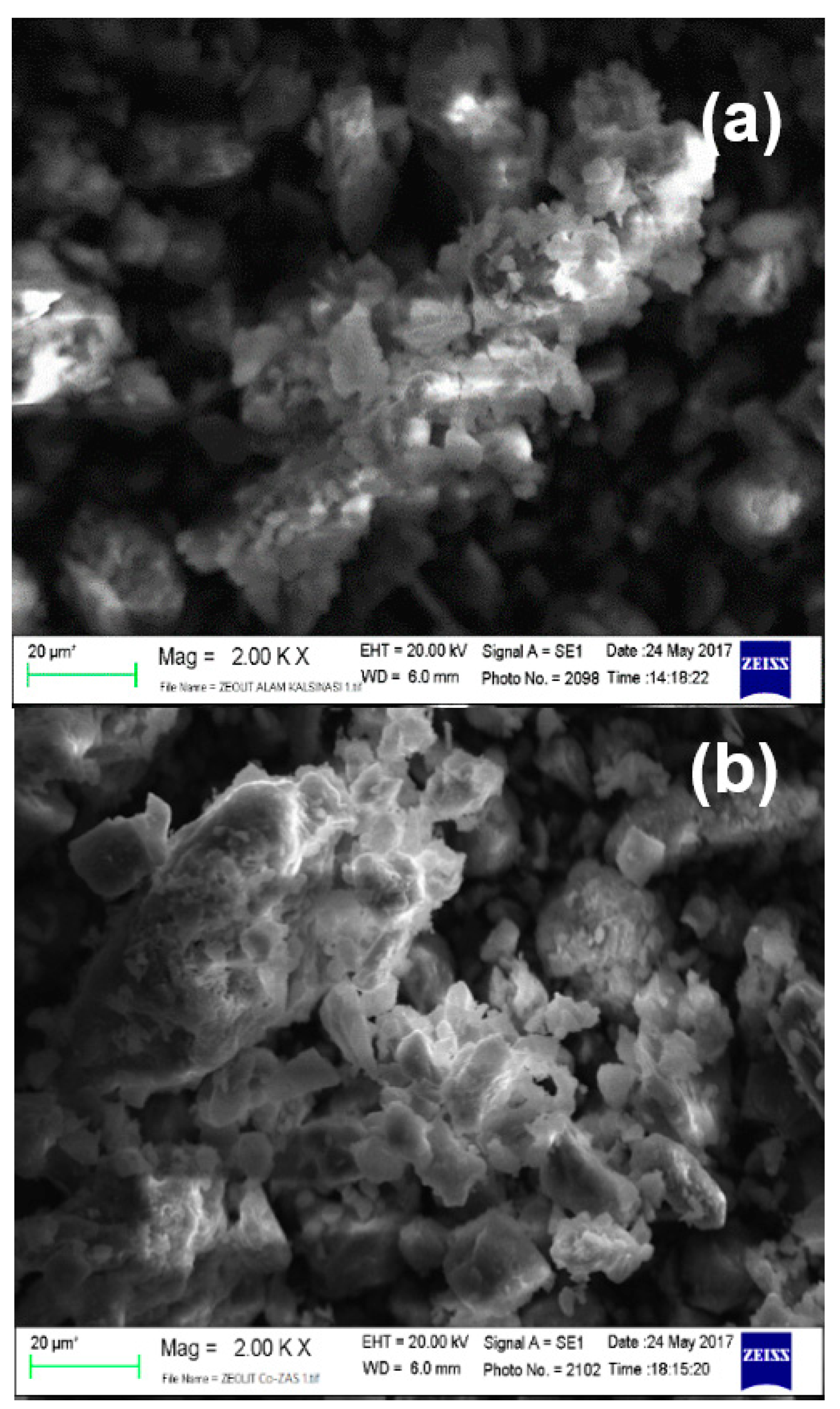
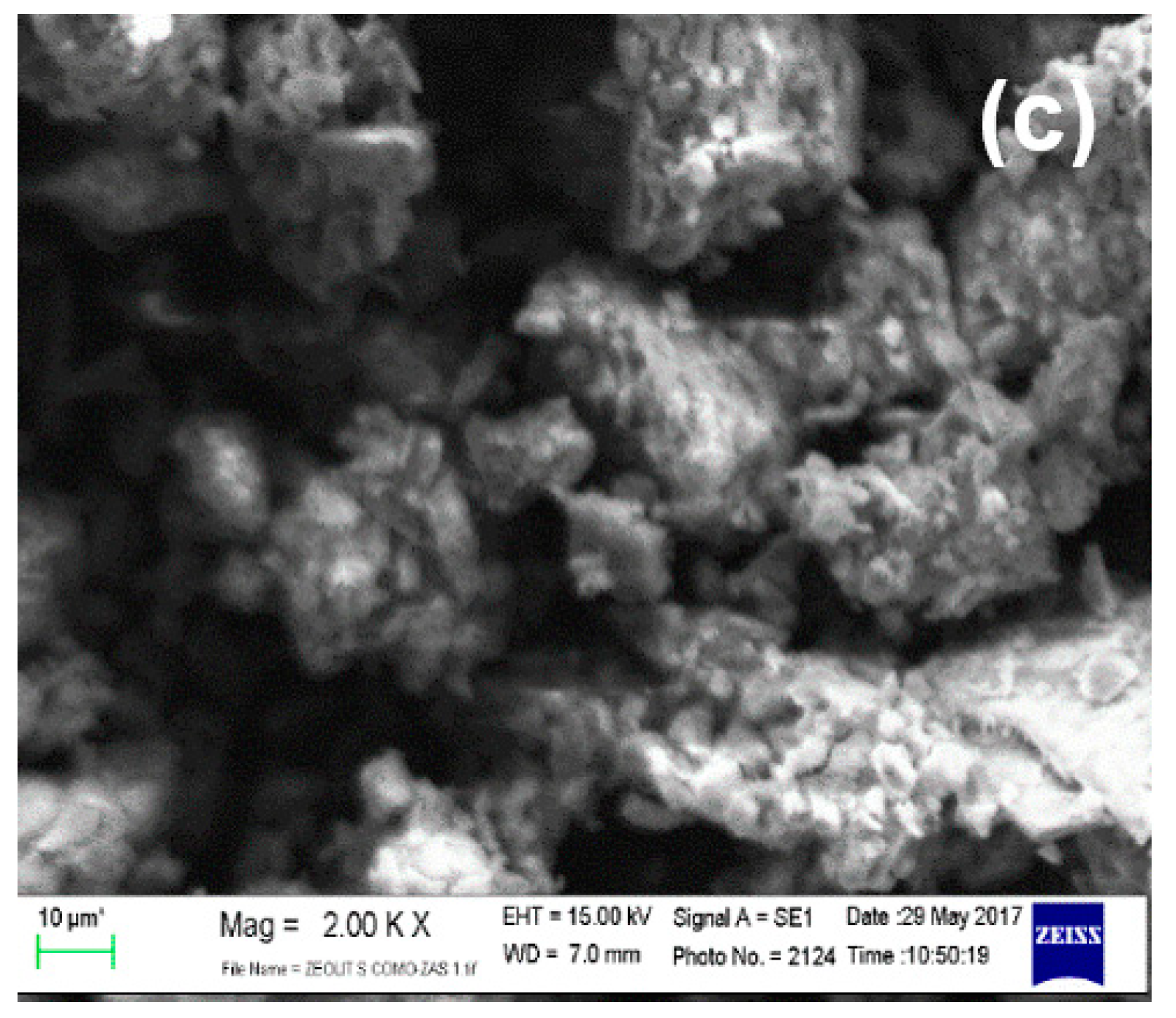
2.1. Catalysts Characteristics
- D = Average size crystal grain
- λ = X-ray wavelength (0.15406 nm)
- θ = Braag degree angle
- B = FWHM value in radii
- The result of crystal size measurements for each catalyst is described in Table 2.
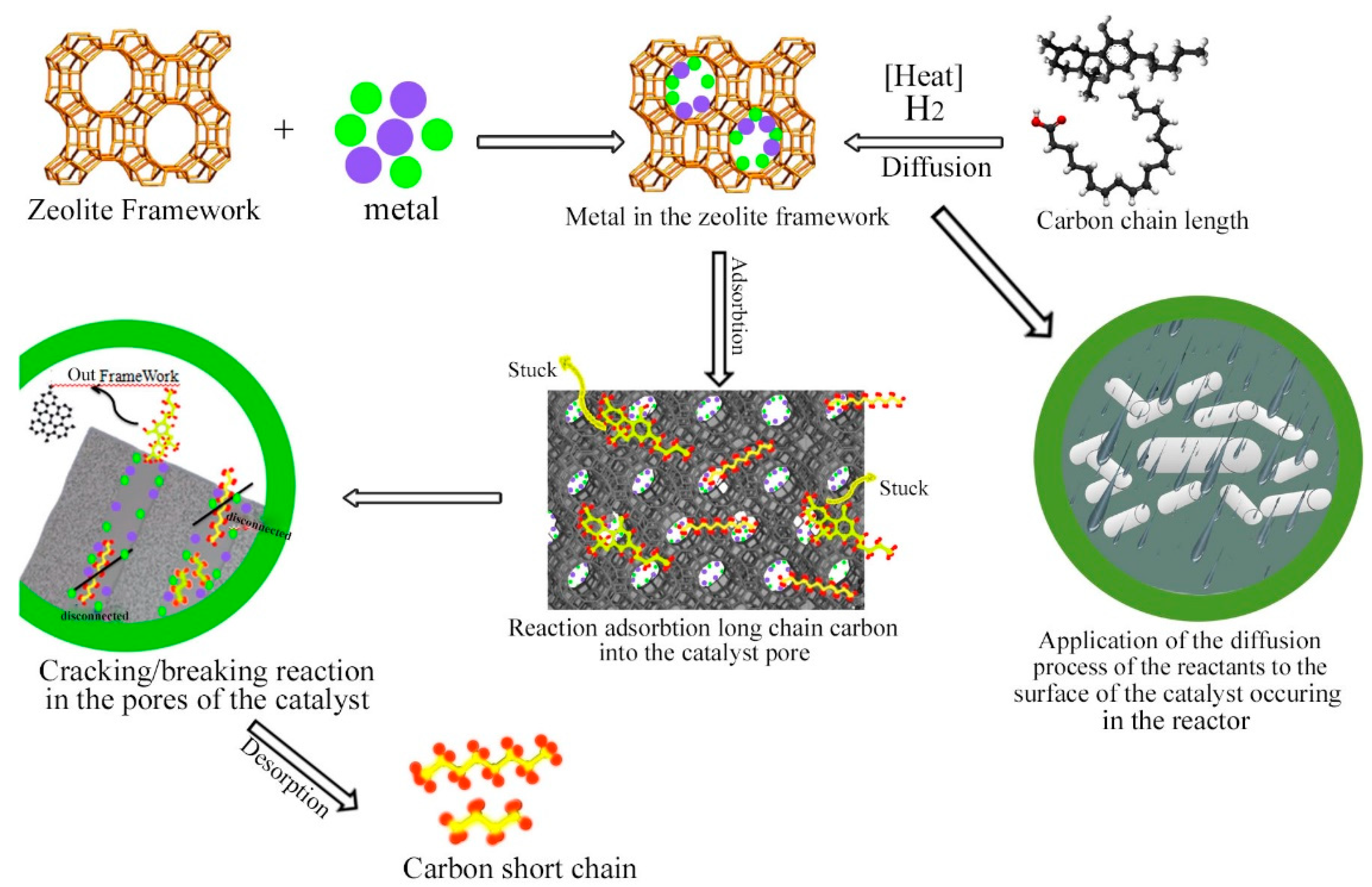
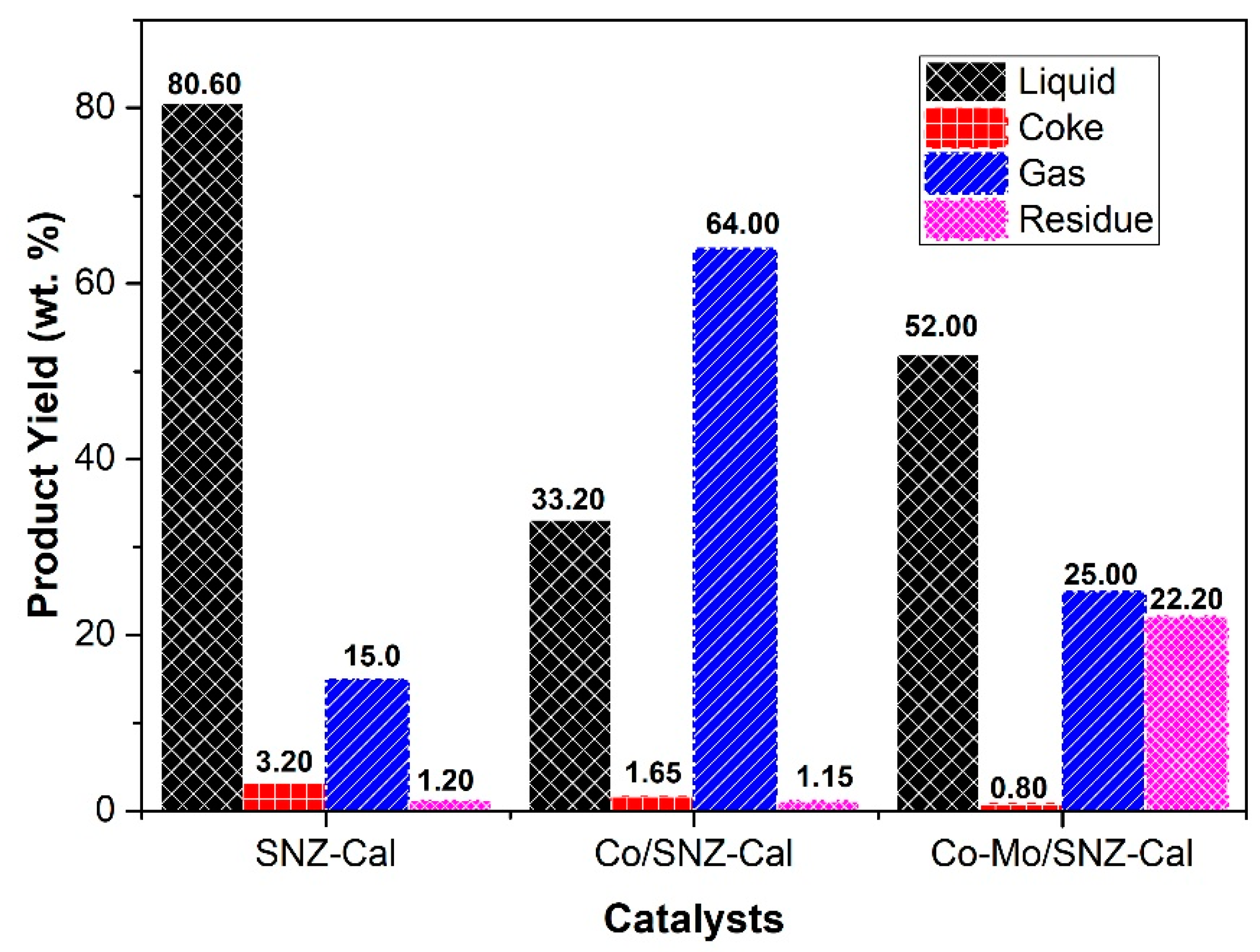
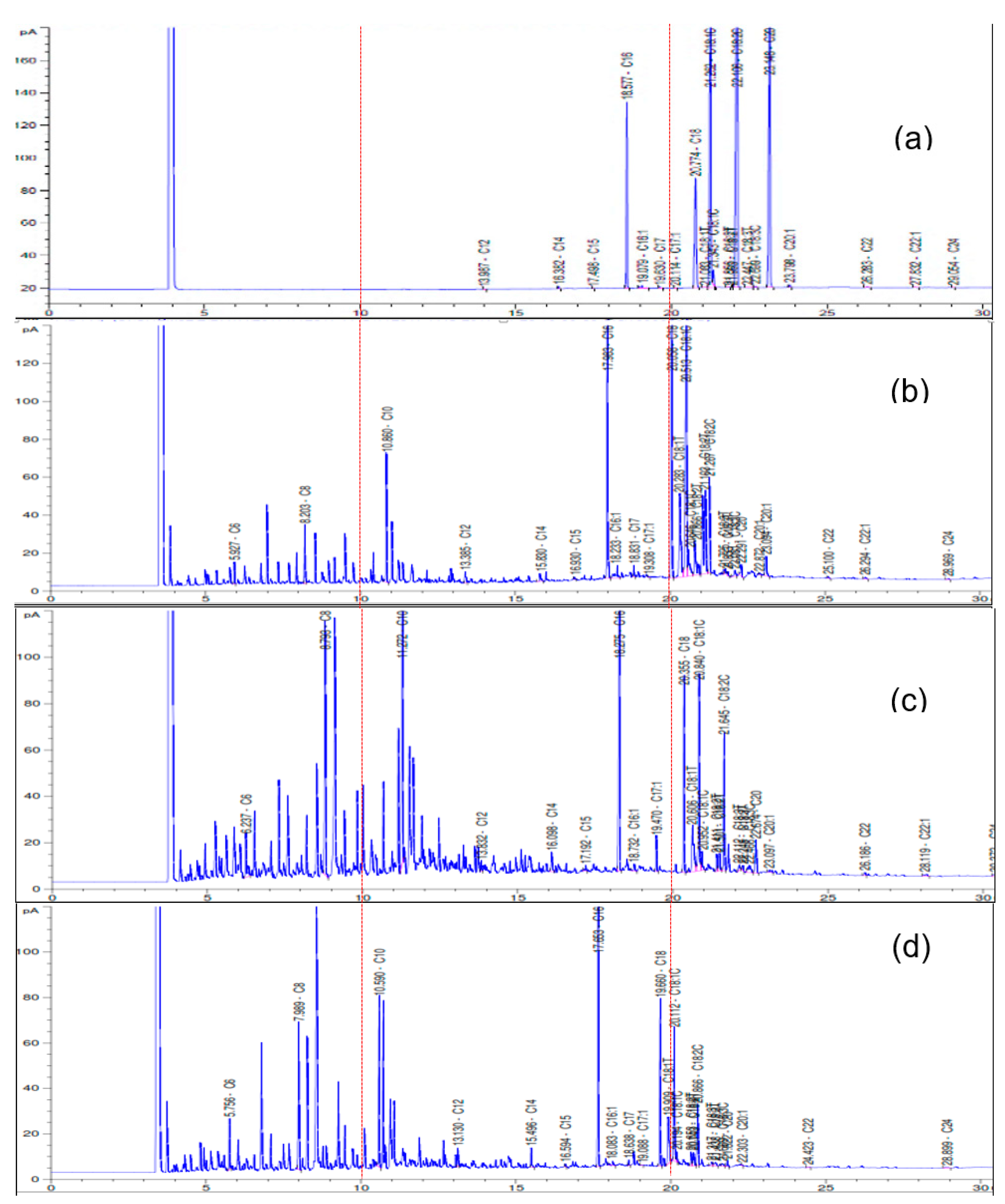
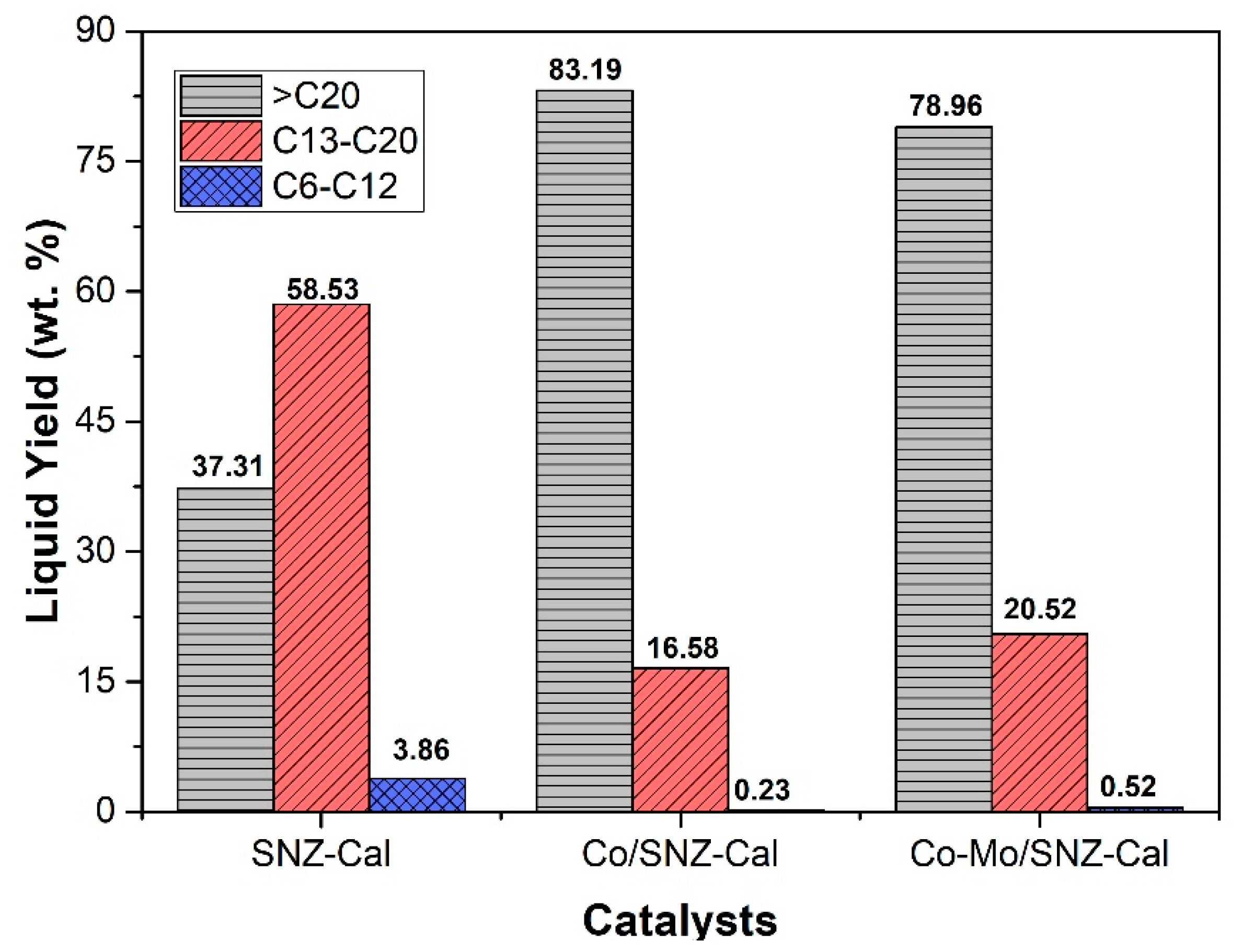
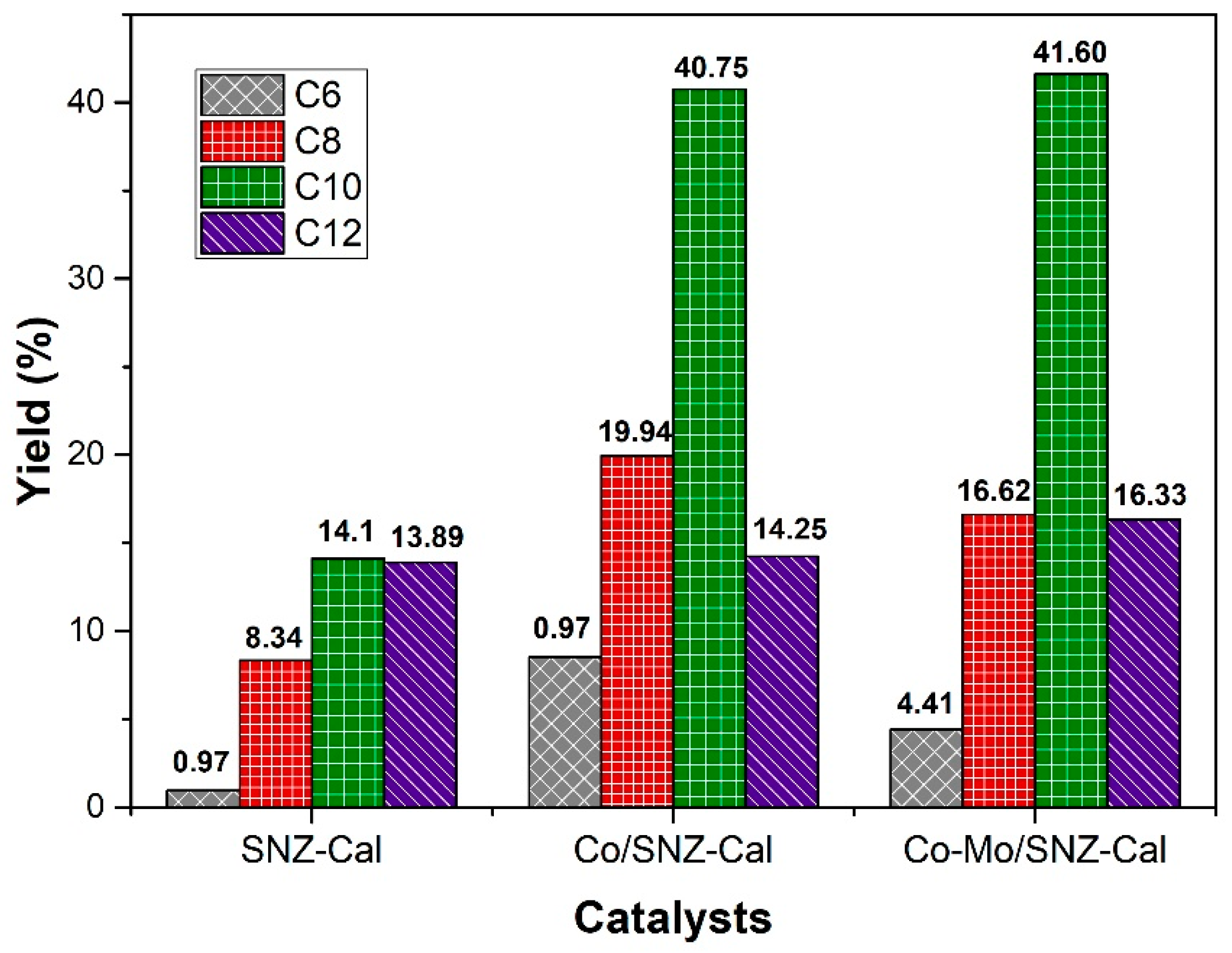
2.2. Catalytic Performance of Catalysts
3. Materials and Method
3.1. Materials
3.2. Preparation of Sarulla Natural Zeolite-Calcination (SNZ-Cal)
3.3. Preparation of Co/SNZ Catalyst
3.4. Preparation of Co-Mo/SNZ Catalyst
3.5. Characterization of Catalysts
3.6. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nam, L.T.H.; Vinh, T.Q.; Loan, N.T.T.; Tho, V.D.S.; Yang, X.-Y.; Su, B.-L. Preparation of Bio-Fuels by Catalytic Cracking Reaction of Vegetable Oil Sludge. Fuel 2011, 90, 1069–1075. [Google Scholar] [CrossRef]
- Wibowo, A.A.; Firdausyah, S.; Hajjah, S.; Dwiyanti, D.; Sihombing, J.L.; Pulungan, A.N. Study of Rubber Seed Oils Hydrocracking into Bio Gasoline and Diesel Fraction over the Combination Y-Zeolite and Ni Catalyst. In The First International Seminar on Trends in Science and Science Education; Universitas Negeri Medan: Medan, Indonesia, 2014; pp. 132–140. [Google Scholar]
- Suarez, P.A.Z.; Moser, B.R.; Sharma, B.K.; Erhan, S.Z. Comparing the Lubricity of Biofuels Obtained from Pyrolysis and Alcoholysis of Soybean Oil and Their Blends with Petroleum Diesel. Fuel 2009, 88, 1143–1147. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Ioannidou, O.; Skoulou, V. Rapeseed Residues Utilization for Energy and 2nd Generation Biofuels. Fuel 2008, 87, 1492–1502. [Google Scholar] [CrossRef]
- Fabbri, D.; Bevoni, V.; Notari, M.; Rivetti, F. Properties of a Potential Biofuel Obtained from Soybean Oil by Transmethylation with Dimethyl Carbonate. Fuel 2007, 86, 690–697. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel Production from Vegetable Oils via Catalytic and Non-Catalytic Supercritical Methanol Transesterification Methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Bezergianni, S.; Kalogianni, A.; Vasalos, I.A. Hydrocracking of Vacuum Gas Oil-Vegetable Oil Mixtures for Biofuels Production. Bioresour. Technol. 2009, 100, 3036–3042. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Chen, J.; Sun, Y. Biofuel Production from Catalytic Cracking of Woody Oils. Bioresour. Technol. 2010, 101, 5586–5591. [Google Scholar] [CrossRef]
- Sriningsih, W.; Saerodji, M.G.; Trisunaryanti, W.; Triyono; Armunanto, R.; Falah, I.I. Fuel Production from LDPE Plastic Waste over Natural Zeolite Supported Ni, Ni-Mo, Co and Co-Mo Metals. Procedia Environ. Sci. 2014, 20, 215–224. [Google Scholar] [CrossRef]
- Khowatimy, F.A.; Priastomo, Y.; Febriyanti, E.; Riyantoko, H.; Trisunaryanti, W. Study of Waste Lubricant Hydrocracking into Fuel Fraction over the Combination of Y-Zeolite and ZnO Catalyst. Procedia Environ. Sci. 2014, 20, 225–234. [Google Scholar] [CrossRef]
- Twaiq, F.A.; Mohamed, A.R.; Bhatia, S. Liquid Hydrocarbon Fuels from Palm Oil by Catalytic Cracking over Aluminosilicate Mesoporous Catalysts with Various Si/Al Ratios. Microporous Mesoporous Mater. 2003, 64, 95–107. [Google Scholar] [CrossRef]
- Twaiq, F.A.; Zabidi, N.A.M.; Mohamed, A.R.; Bhatia, S. Catalytic Conversion of Palm Oil over Mesoporous Aluminosilicate MCM-41 for the Production of Liquid Hydrocarbon Fuels. Fuel Process. Technol. 2003, 84, 105–120. [Google Scholar] [CrossRef]
- Sang, O.Y. Biofuel Production from Catalytic Cracking of Palm Oil. Energy Sources 2003, 25, 859–869. [Google Scholar] [CrossRef]
- Degnan, T.F., Jr. Applications of Zeolites in Petroleum Refining. Top. Catal. 2000, 13, 349–356. [Google Scholar] [CrossRef]
- Nagim, I.A.; Kulkarni, K.S.; Kulkarni, A.D. Impact of Zeolites in Petroleum Industries. J. Eng. Res. Stud. 2011, 2, 272–275. [Google Scholar]
- Kusdarto, K. Potency of Zeolite in Indonesia. J. Zeolit Indones. 2008, 7, 78–87. [Google Scholar]
- Sihombing, J.L.; Gea, S.; Pulungan, A.N.; Agusnar, H.; Wirjosentono, B.; Hutapea, Y.A. The Characterization of Sarulla Natural Zeolite Crystal and Its Morphological Structure. AIP Conf. Proc. 2018, 2049, 020062. [Google Scholar] [CrossRef]
- Sihombing, J.L.; Gea, S.; Kembaren, A.; Sabani; Pulungan, A.N.; Wibowo, A.A.; Wirjosentono, B. Activity Assays of Calcinated Sarulla Natural Zeolite (Snz-Cal) in Catalytic Hydrocracking Rubber Seed Oil. J. Phys. Conf. Ser. 2018, 1116, 042035. [Google Scholar] [CrossRef]
- Parkhomchuk, E.V.; Lysikov, A.I.; Okunev, A.G.; Parunin, P.D.; Semeikina, V.S.; Ayupov, A.B.; Trunova, V.A.; Parmon, V.N. Meso/Macroporous CoMo Alumina Pellets for Hydrotreating of Heavy Oil. Ind. Eng. Chem. Res. 2013, 52, 17117–17125. [Google Scholar] [CrossRef]
- Pashigreva, A.V.; Bukhtiyarova, G.A.; Klimov, O.V.; Chesalov, Y.A.; Litvak, G.S.; Noskov, A.S. Activity and Sulfidation Behavior of the CoMo/Al2O3 Hydrotreating Catalyst: The Effect of Drying Conditions. Catal. Today 2010, 149, 19–27. [Google Scholar] [CrossRef]
- Semeykina, V.S.; Parkhomchuk, E.V.; Polukhin, A.V.; Parunin, P.D.; Lysikov, A.I.; Ayupov, A.B.; Cherepanova, S.V.; Kanazhevskiy, V.V.; Kaichev, V.V.; Glazneva, T.S.; et al. CoMoNi Catalyst Texture and Surface Properties in Heavy Oil Processing. Part I: Hierarchical Macro/Mesoporous Alumina Support. Ind. Eng. Chem. Res. 2016, 55, 3535–3545. [Google Scholar] [CrossRef]
- Trisunaryanti, W.; Syoufian, A.; Purwono, S. Characterization and Modification of Indonesian Natural Zeolite for Hydrocracking of Waste Lubricant Oil into Liquid Fuel Fraction. J. Chem. Chem. Eng. 2013, 7, 175. [Google Scholar]
- Gultom, F.; Wirjosentono, B.; Thamrin; Nainggolan, H.; Eddiyanto. Preparation and Characterization of North Sumatera Natural Zeolite Polyurethane Nanocomposite Foams for Light-Weight Engineering Materials. Procedia Chem. 2016, 19, 1007–1013. [Google Scholar] [CrossRef]
- Sadek, R.; Chalupka, K.A.; Mierczynski, P.; Rynkowski, J.; Gurgul, J.; Dzwigaj, S. Cobalt Based Catalysts Supported on Two Kinds of Beta Zeolite for Application in Fischer-Tropsch Synthesis. Catalysts 2019, 9, 497. [Google Scholar] [CrossRef]
- Shen, H.; Yao, B.; Zhang, J.; Zhu, X.; Xiang, X.; Zhou, X.; Zu, X. Effect of Thickness of Molybdenum Nano-Interlayer on Cohesion between Molybdenum/Titanium Multilayer Film and Silicon Substrate. Nanomaterials 2019, 9, 616. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, S.; García, G.; Calvillo, L.; Celorrio, V.; Pastor, E.; Lázaro, M.J. Carbon-Supported Fe Catalysts for CO2 Electroreduction to High-Added Value Products: A DEMS Study: Effect of the Functionalization of the Support. Int. J. Electrochem. 2011, 2011. [Google Scholar] [CrossRef]
- Kumar, K.B.; Raji, P. Synthesis and Characterization of Nano Zinc Oxide by Sol Gel Spin Coating. Recent Res. Sci. Technol. 2011, 3, 48–52. [Google Scholar]
- Zhang, A.M.; Han, D.C.; Zhu, Z.Q.; Lee, J.W.; Rhee, H.K. Synthesis and Catalytic Application of Ni-Supported Carbon Nanotubes for n-Heptane Cracking. Korean J. Chem. Eng. 2003, 20, 649–652. [Google Scholar] [CrossRef]
- Ding, L.; Zheng, Y.; Yang, H.; Parviz, R. LCO Hydrotreating with Mo-Ni and W-Ni Supported on Nano- and Micro-Sized Zeolite Beta. Appl. Catal. A Gen. 2009, 353, 17–23. [Google Scholar] [CrossRef]
- Looi, P.Y.; Tye, C.T.; Mohamed, A.R. Reactions of Used Motor Oil and Residual Oil Using Mesoporous Mo/Al2O3 Catalyst. J. Inst. Eng. Malays. 2013, 74, 21–27. [Google Scholar]
- Looi, P.Y.; Mohamed, A.R.; Tye, C.T. Hydrocracking of Residual Oil Using Molybdenum Supported over Mesoporous Alumina as a Catalyst. Chem. Eng. J. 2012, 181–182, 717–724. [Google Scholar] [CrossRef]
- Ghosh, U.; Kulkarni, K.; Kulkarni, A.D.; Chaudhari, P.L. Review—Hydrocracking Using Different Catalysts. Chem. Process Eng. Res. 2015, 34, 51–55. [Google Scholar]
| Catalysts | % Metal (wt.) * | |
|---|---|---|
| Co | Mo | |
| SNZ-Cal | - | - |
| Co/SNZ-Cal | 0.76 | - |
| Co-Mo/SNZ-Cal | 0.58 | 0.002 |
| SNZ-Cal | Co/SNZ-Cal | Co-Mo/SNZ-Cal | |||
|---|---|---|---|---|---|
| (Degree) | D (nm) | D (nm) | (Degree) | D (nm) | |
| 27.88 | 16.103 | 27.92 | 19.038 | 27.76 | 17.482 |
| 23.70 | 22.944 | 23.76 | 29.288 | 23.57 | 23.275 |
| 22.04 | 21.149 | 22.08 | 24.661 | 21.90 | 24.203 |
| 20.10 | 9.028 | 20.05 | 11.160 | 19.90 | 9.909 |
| Catalysts | Surface Area (m2/g) * | Pore Volume (cc/g) * | Pore Diameter (nm) * |
|---|---|---|---|
| SNZ-Cal | 107 | 0.23 | 4.27 |
| Co/SNZ-Cal | 96 | 0.19 | 3.96 |
| Co-Mo/SNZ-Cal | 86 | 0.22 | 5.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sihombing, J.L.; Gea, S.; Wirjosentono, B.; Agusnar, H.; Pulungan, A.N.; Herlinawati, H.; Yusuf, M.; Hutapea, Y.A. Characteristic and Catalytic Performance of Co and Co-Mo Metal Impregnated in Sarulla Natural Zeolite Catalyst for Hydrocracking of MEFA Rubber Seed Oil into Biogasoline Fraction. Catalysts 2020, 10, 121. https://doi.org/10.3390/catal10010121
Sihombing JL, Gea S, Wirjosentono B, Agusnar H, Pulungan AN, Herlinawati H, Yusuf M, Hutapea YA. Characteristic and Catalytic Performance of Co and Co-Mo Metal Impregnated in Sarulla Natural Zeolite Catalyst for Hydrocracking of MEFA Rubber Seed Oil into Biogasoline Fraction. Catalysts. 2020; 10(1):121. https://doi.org/10.3390/catal10010121
Chicago/Turabian StyleSihombing, Junifa Layla, Saharman Gea, Basuki Wirjosentono, Harry Agusnar, Ahmad Nasir Pulungan, Herlinawati Herlinawati, Muhammad Yusuf, and Yasir Arafat Hutapea. 2020. "Characteristic and Catalytic Performance of Co and Co-Mo Metal Impregnated in Sarulla Natural Zeolite Catalyst for Hydrocracking of MEFA Rubber Seed Oil into Biogasoline Fraction" Catalysts 10, no. 1: 121. https://doi.org/10.3390/catal10010121
APA StyleSihombing, J. L., Gea, S., Wirjosentono, B., Agusnar, H., Pulungan, A. N., Herlinawati, H., Yusuf, M., & Hutapea, Y. A. (2020). Characteristic and Catalytic Performance of Co and Co-Mo Metal Impregnated in Sarulla Natural Zeolite Catalyst for Hydrocracking of MEFA Rubber Seed Oil into Biogasoline Fraction. Catalysts, 10(1), 121. https://doi.org/10.3390/catal10010121





