Photocatalytic Performance of Electrospun Silk Fibroin/ZnO Mats to Remove Pesticide Residues from Water under Natural Sunlight
Abstract
1. Introduction
2. Results and Discussion
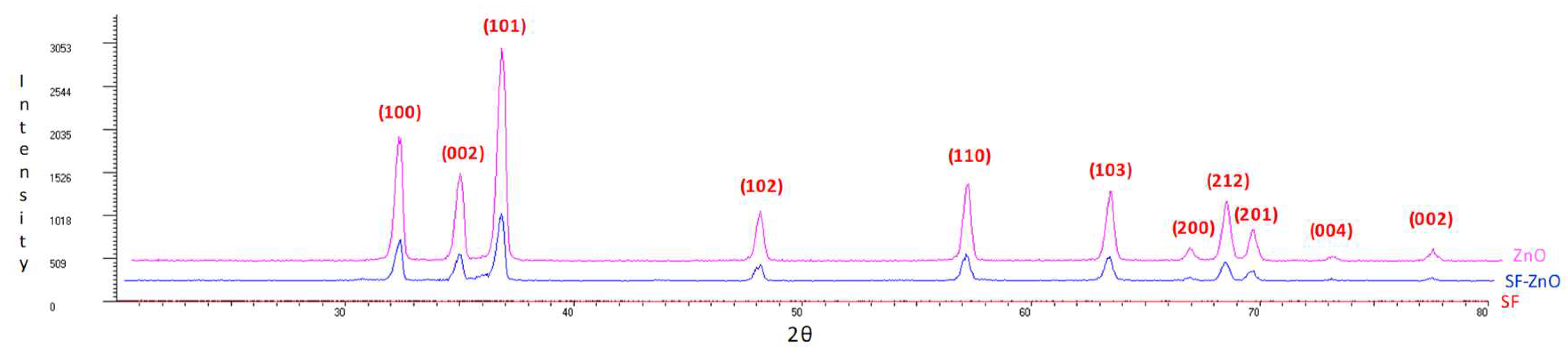
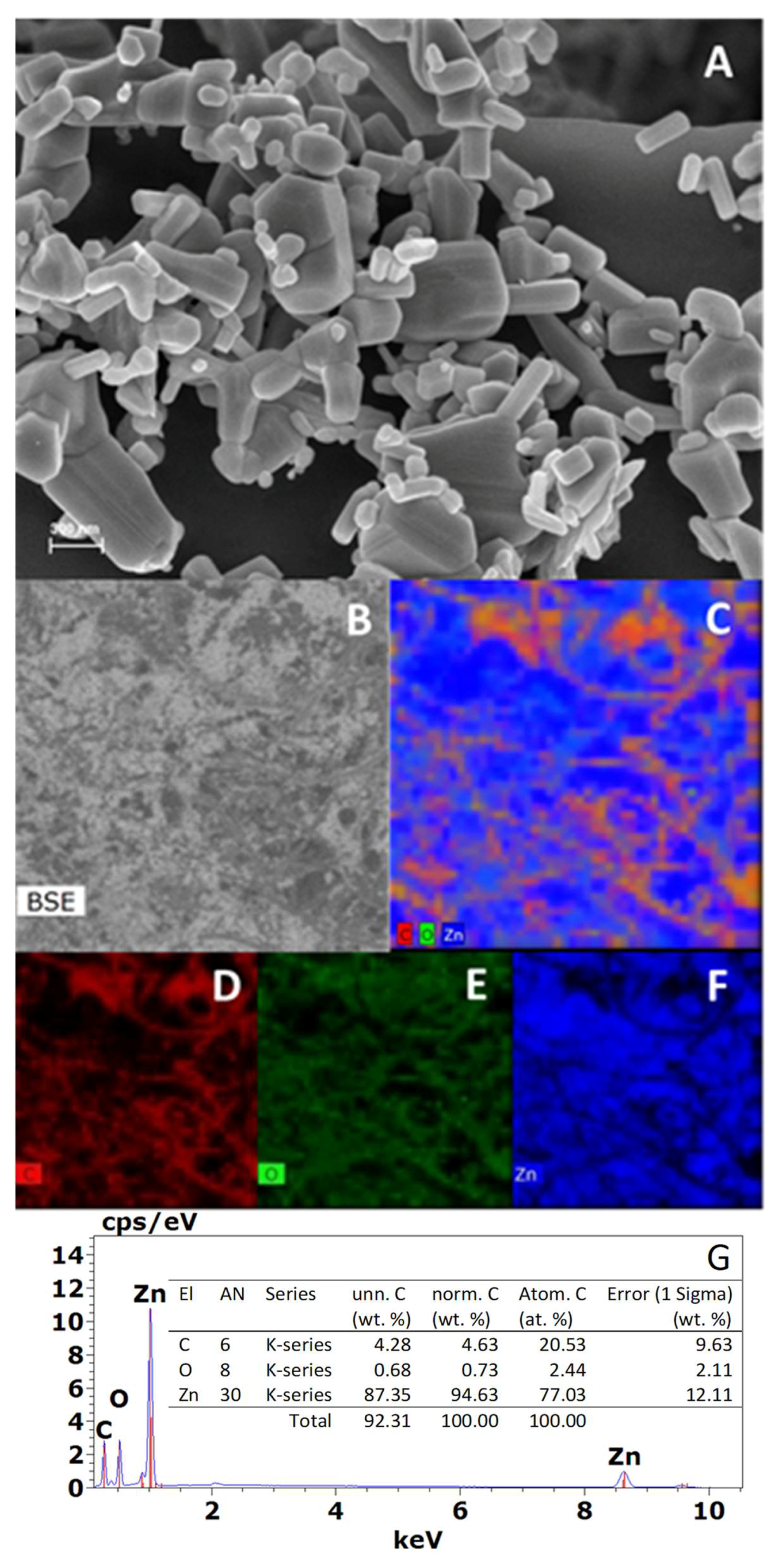
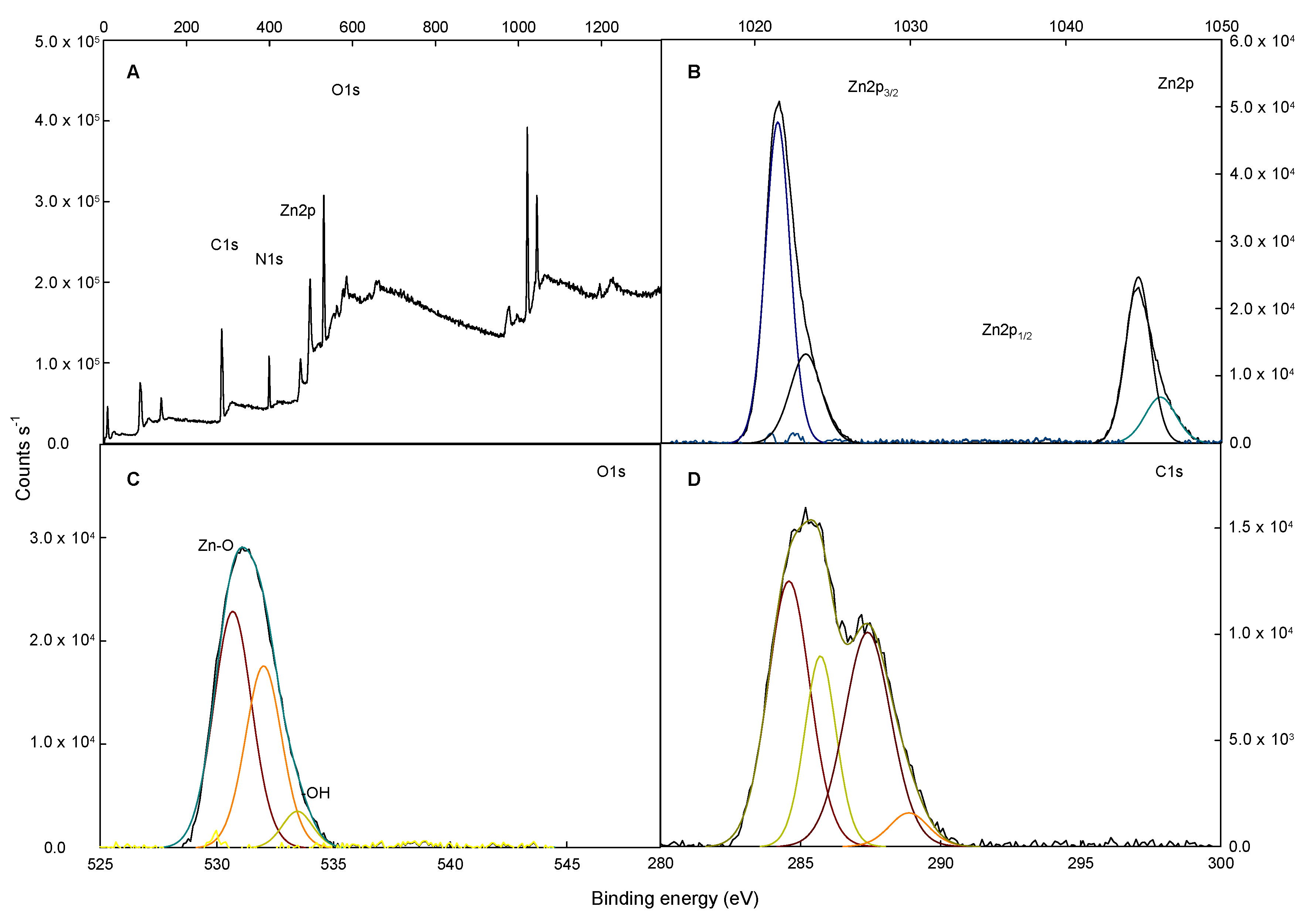
2.1. X-ray Diffraction (XRD), Field Emission Scanning Electron Microscopy (FESEM), X-ray Photoelectron Spectroscopy (XPS), and Energy Dispersive X-ray Spectroscopy Analysis (XDS) Characterization
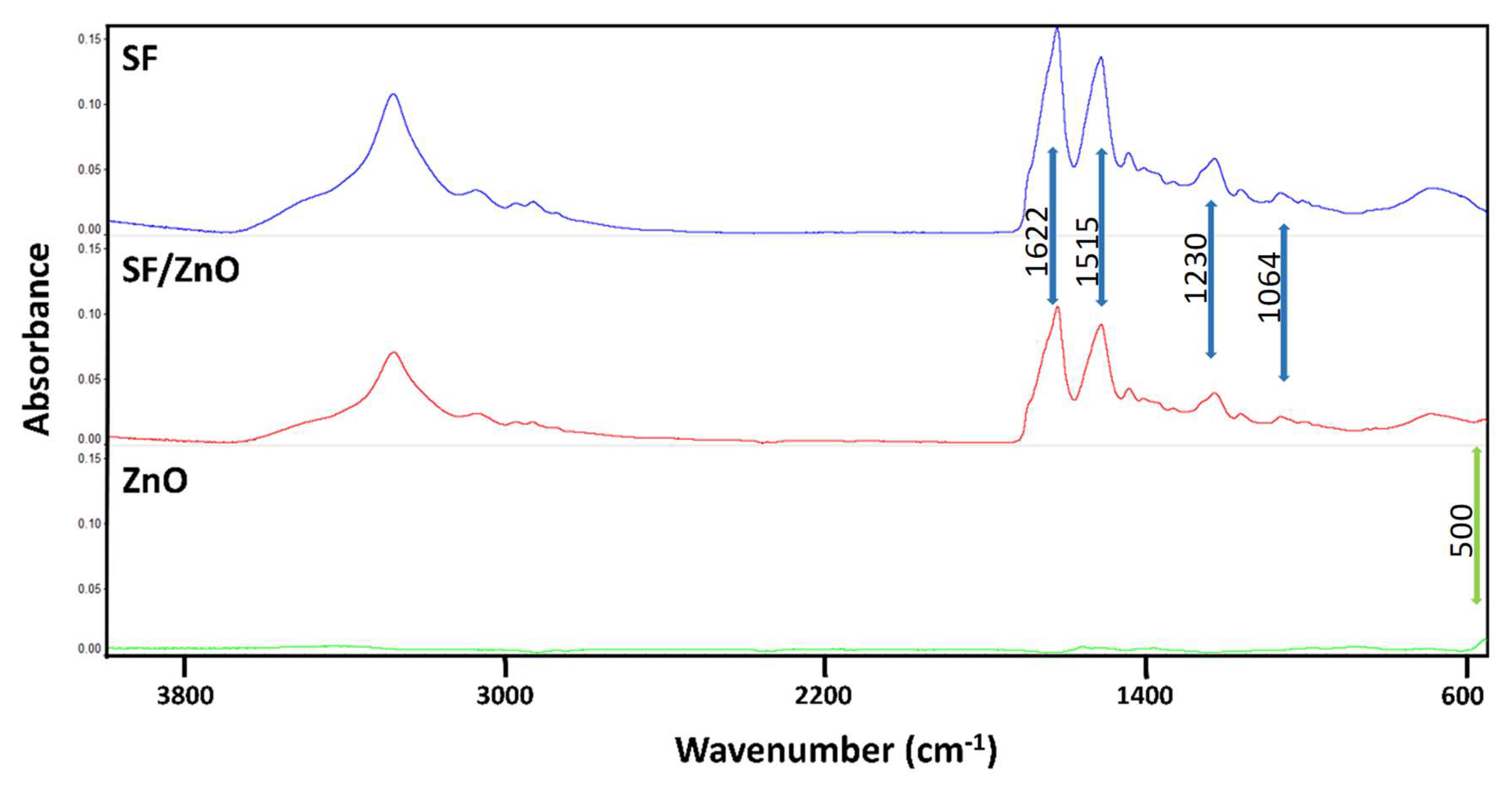
2.2. Attenuated Total Reflectance Fourier Transformed Infrared Spectroscopy (ATR-FTIR) Depiction
2.3. Mechanical Properties and Fibre Diameters
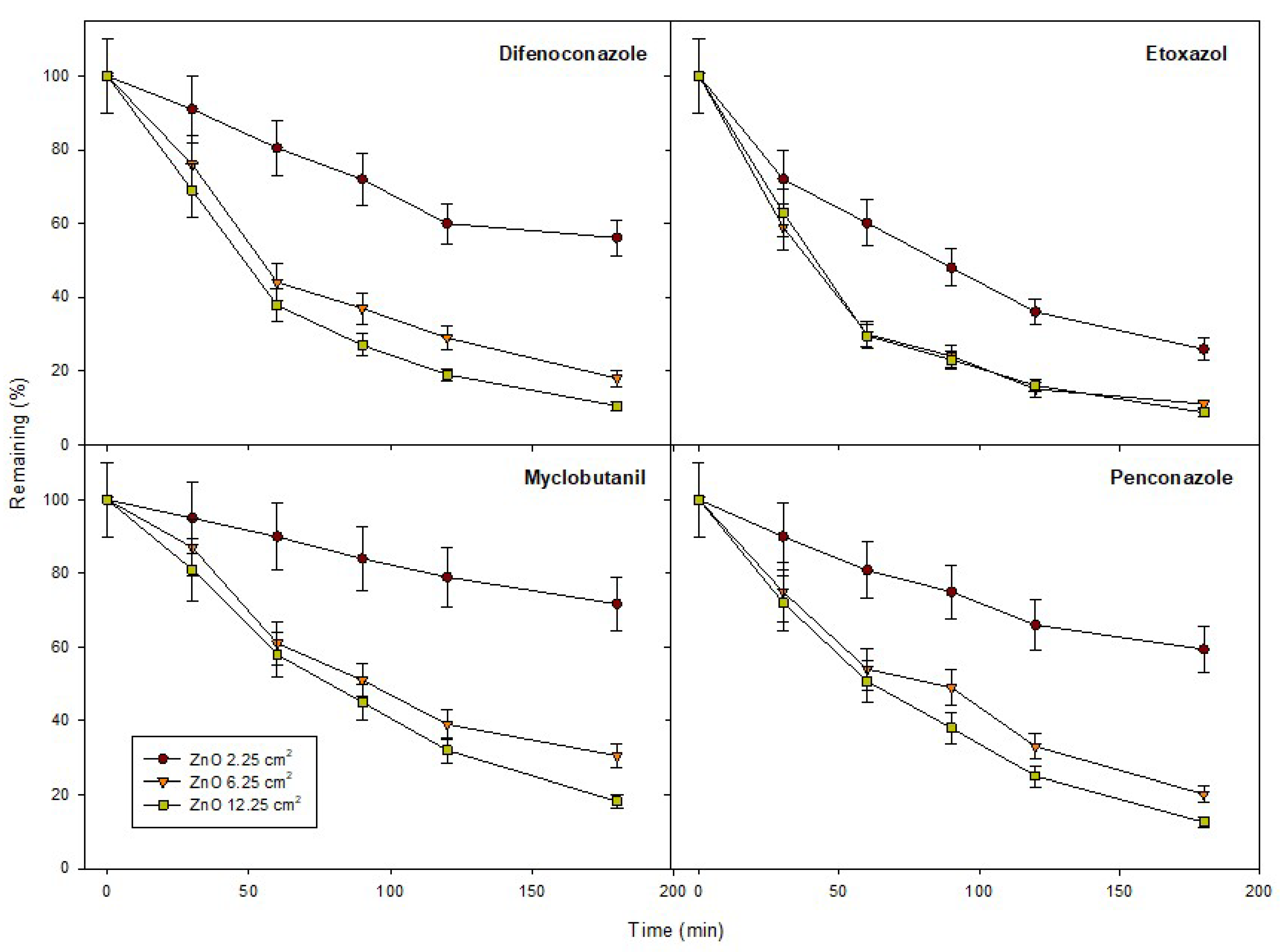
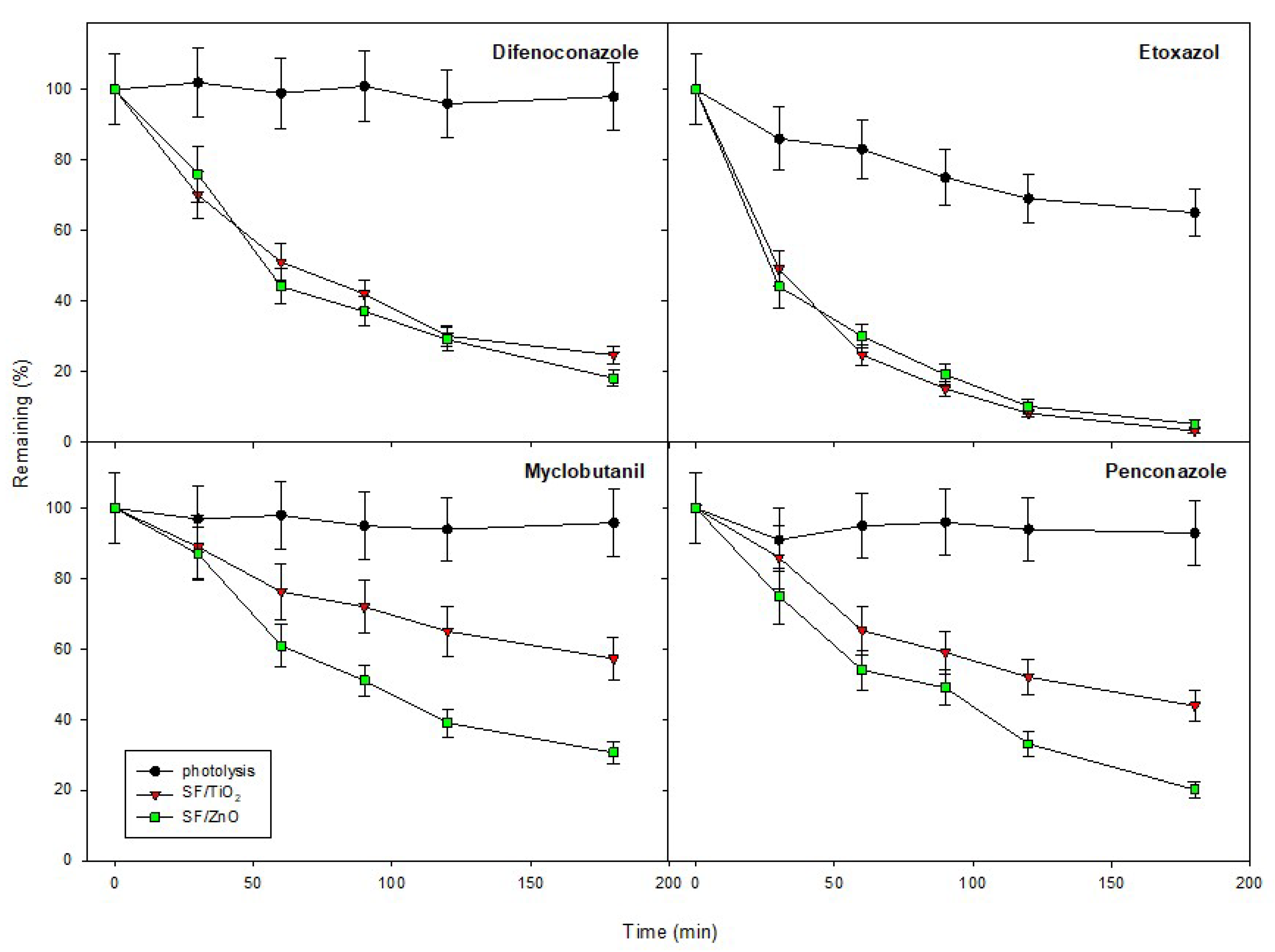
2.4. Photocatalytic Activity and Kinetics
3. Materials and Methods
3.1. Pesticides and Reagents
3.2. Silk Fibroin Processing
3.3. Electrospinning and Post-Treatment of SF/TiO2 and SF/ZnO Mats
3.4. XRD, FESEM, XPS, XDS and SBET
3.5. ATR-FTIR
3.6. Analysis of Mechanical Properties and Fibre Diameter
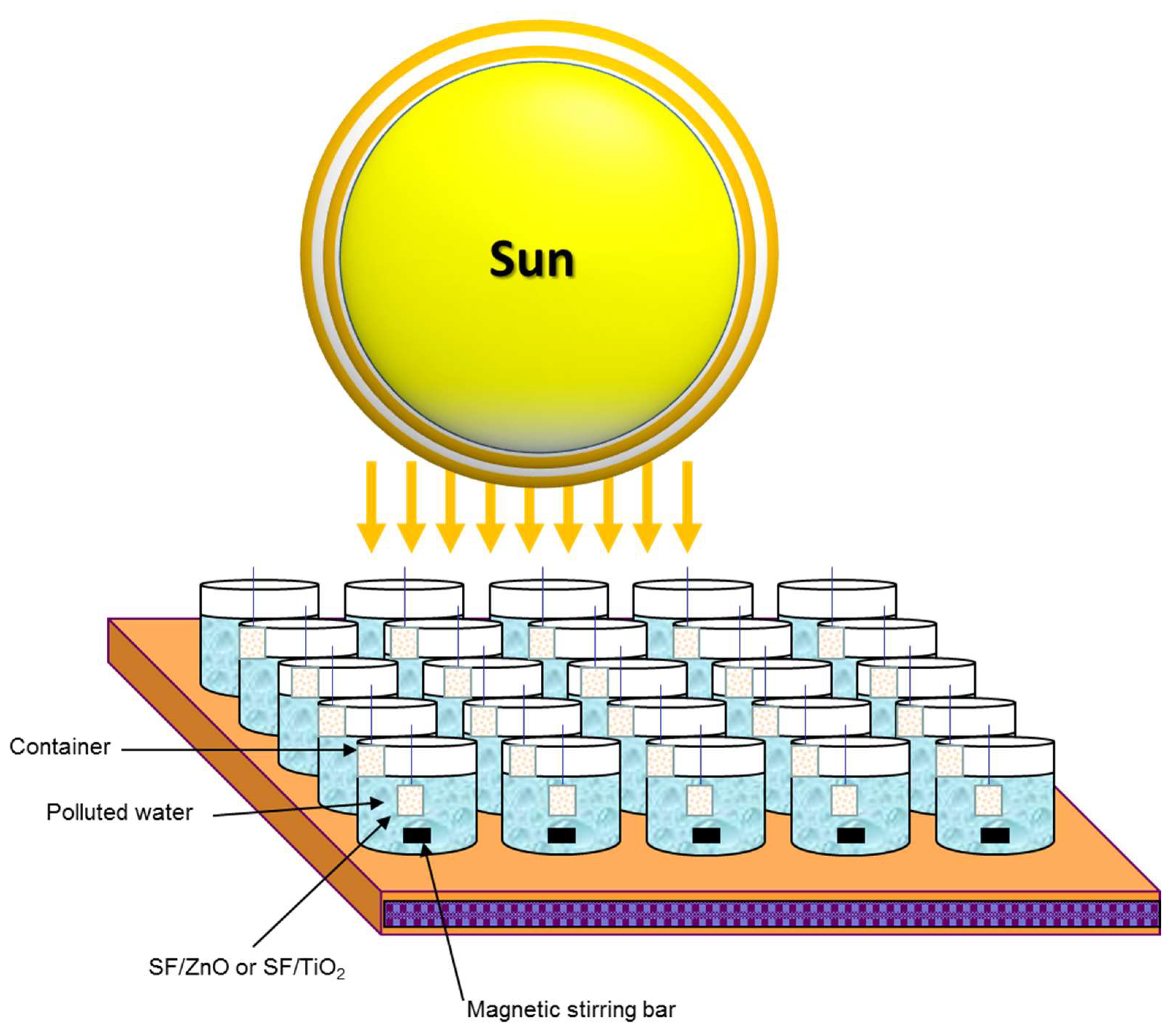
3.7. Photoreaction Setup
3.8. Analytical Determinations
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed]
- Vela, N.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Recent overview on the abatement of pesticide residues in water by photocatalytic treatment using TiO2. In Applications of Titanium Dioxide; Janus, M., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 147–177. [Google Scholar]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Fagan, R.; McCormack, E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment. A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, M.A.; Bandala, E.R.; Martínez-Huitle, C.A. Advanced Oxidation Processes (AOPs) for Removal of Pesticides from Aqueous Media. In Pesticides-Formulations, Effects, Fate; Stoytcheva, M., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 685–730. [Google Scholar]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Verma, N. Photocatalytic activity of ZnO nanoparticles with optimization of defects. Mater. Res. Bull. 2017, 95, 468–476. [Google Scholar] [CrossRef]
- Wu, M.C.; Chan, S.H.; Lin, T.H. Fabrication and photocatalytic performance of electrospun PVA/silk/TiO2 nanocomposite textile. Funct. Mater. Lett. 2015, 8, 1540013. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2 and ZnO based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Montero-Muñoz, M.; Ramos-Ibarra, J.E.; Rodríguez-Páez, J.E.; Teodoro, M.D.; Marques, G.E.; Sanabria, A.E.; Cajas, P.C.; Páez, C.A.; Heinrichs, B.; Coaquira, J.A.H. Role of defects on the enhancement of the photocatalytic response of ZnO nanostructures. Appl. Surf. Sci. 2018, 448, 646–654. [Google Scholar] [CrossRef]
- Wei, G.; Zuo, H.F.; Guo, Y.R.; Pan, Q.J. Synthesis of ZnO with enhanced photocatalytic activity: A novel approach using nanocellulose. BioResources 2016, 11, 6244–6253. [Google Scholar] [CrossRef]
- Xu, J.; Su, H.; Han, J.; Chen, Y.; Song, W.; Gu, Y.; Moon, W.J.; Zhang, D. In situ deposition of flower-like ZnO on silk fibroin fibers. Appl. Phys. A 2012, 108, 235–238. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Zhang, L.; Ye, Z.G.; Ren, W.; Xu, F.; Wang, S.; Liu, M.; Zhang, X. 3D Conformal modification of electrospun silk nanofibers with nanoscaled ZnO deposition for enhanced photocatalytic activity. ACS Biomater. Sci. Eng. 2017, 3, 2900–2906. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, J.; Garrido, I.; Cava, J.; Hellín, P.; Flores, P.; Navarro, S. Photometabolic pathways of chlorantraniliprole in aqueous slurries containing binary and ternary oxides of Zn and Ti. Chem. Eng. J. 2015, 264, 720–727. [Google Scholar] [CrossRef]
- Garrido, I.; Pastor-Belda, M.; Campillo, N.; Viñas, P.; Yañez, M.J.; Vela, N.; Navarro, S.; Fenoll, J. Photooxidation of insecticide residues by ZnO and TiO2 coated magnetic nanoparticles under natural sunlight. J. Photochem. Photobiol. A Chem. 2019, 372, 245–253. [Google Scholar] [CrossRef]
- Cai, L.; Shao, H.; Hu, X.; Zhang, Y. Reinforced and ultraviolet resistant silks from silkworms fed with titanium dioxide nanoparticles. ACS Sustain. Chem. Eng. 2015, 3, 2551–2557. [Google Scholar] [CrossRef]
- Feng, X.X.; Zhang, L.L.; Chen, J.Y.; Guo, Y.H.; Zhang, H.P.; Jia, C.I. Preparation and characterization of novel nanocomposite films formed from silk fibroin and nano-TiO2. Int. J. Biol. Macromol. 2007, 40, 105–111. [Google Scholar] [CrossRef]
- Kim, J.H.; Sheikh, F.A.; Ju, H.W.; Park, H.J.; Moon, B.M.; Lee, O.J.; Park, C.H. 3D silk fibroin scaffold incorporating titanium dioxide (TiO2) nanoparticle (NPs) for tissue engineering. Int. J. Biol. Macromol. 2014, 68, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Jao, W.C.; Yang, M.C.; Lin, C.H.; Hsu, C.C. Fabrication and characterization of electrospun silk fibroin/TiO2 nanofibrous mats for wound dressings. Polym. Adv. Technol. 2012, 23, 1066–1076. [Google Scholar] [CrossRef]
- Li, C.; Vepari, C.; Jin, H.J.; Kim, H.J.; Kaplan, D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Bhutto, M.A.; Zhu, T.; Yu, K.; Bao, J.; Morsi, Y.; El-Hamshary, H.; El-Newehy, M.; Mo, X. Fabrication and characterization of Antheraea pernyi silk fibroin-blended P(LLA-CL) nanofibrous scaffolds for peripheral nerve tissue engineering. Front. Mater. Sci. 2017, 11, 22–32. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.; Roca, M.I.; Martinez, J.G.; Meseguer-Olmo, L.; Cenis, J.L.; Moraleda, J.M.; Otero, T.F. Fabrication of conductive electrospun silk fibroin scaffolds by coating with polypyrrole for biomedical applications. Bioelectrochemistry 2012, 85, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Cervantes, S.; Pagán, A.; Martínez, J.G.; Bernabeu-Esclapez, A.; Otero, T.F.; Meseguer-Olmo, L.; Paredes, J.I.; Cenis, J.L. Electrospun silk fibroin scaffolds coated with reduced graphene promote neurite outgrowth of PC-12 cells under electrical stimulation. Mater. Sci. Eng. C 2017, 79, 315–325. [Google Scholar] [CrossRef]
- Martinez, J.G.; Aznar-Cervantes, S.; Abel Lozano-Pérez, A.; Cenis, J.L.; Otero, T.F. Graphene adsorbed on silk-fibroin meshes: Biomimetic and reversible conformational movements driven by reactions. Electrochim. Acta 2016, 209, 521–528. [Google Scholar] [CrossRef]
- Zhang, X.; Reagan, M.R.; Kaplan, D.L. Electrospun silk biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 988–1006. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.D.; Vicente-Cervantes, D.; Meseguer-Olmo, L.; Cenis, J.L.; Lozano-Pérez, A.A. Influence of the protocol used for fibroin extraction on the mechanical properties and fiber sizes of electrospun silk mats. Mater. Sci. Eng. C 2013, 33, 1945–1950. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.D.; Lozano-Pérez, A.A.; García Montalban, M.; Villora, G.; Vicente-Cervantes, D.; Cenis, J.L. Importance of refrigeration time in the electrospinning of silk fibroin aqueous solutions. J. Mater. Sci. 2015, 50, 4879–4887. [Google Scholar] [CrossRef]
- Kearns, V.; MacIntosh, A.C.; Crawford, A.; Hatton, P.V. Silk-based biomaterials for tissue engineering. Top. Tissue Eng. 2008, 4, 1–19. [Google Scholar]
- Du, G.Y.; He, S.W.; Sun, C.X.; Mi, L.D. Bone morphogenic protein-2 (rhBMP2)-loaded silk fibroin scaffolds to enhance the osteoinductivity in bone tissue engineering. Nanoscale Res. Lett. 2017, 12, 573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sahoo, S.; Toh, S.L.; Goh, J.C.H. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials 2010, 31, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Nalvuran, H.; Elçin, A.E.; Elçin, Y.M. Nanofibrous silk fibroin/reduced graphene oxide scaffolds for tissue engineering and cell culture applications. Int. J. Biol. Macromol. 2018, 114, 77–84. [Google Scholar] [CrossRef]
- López-Maya, E.; Montoro, C.; Rodríguez-Albelo, L.M.; Aznar Cervantes, S.D.; Lozano-Pérez, A.A.; Cenís, J.L.; Barea, E.; Navarro, J.A.R. Textile/metal—Organic-framework composites as self-detoxifying filters for chemical-warfare agents. Angew. Chem. Int. Ed. 2015, 54, 6790–6794. [Google Scholar] [CrossRef]
- Fenoll, J.; Garrido, I.; Pastor-Belda, M.; Camillo, N.; Viñas, P.; Yáñez, M.J.; Vela, N.; Navarro, S. Solar detoxification of water polluted with fungicide residues using ZnO coated magnetic particles. Chem. Eng. J. 2017, 330, 71–81. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.; Aliste, M.; Garrido, I.; Yáñez-Gascón, M.J.; Vela, N.; Cenis, J.L.; Navarro, S.; Fenoll, J. Electrospun silk fibroin/TiO2 mats. Preparation, characterization and efficiency for the photocatalytic solar treatment of pesticide polluted water. RSC Adv. 2020, 10, 1917–1924. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Powell, C.J.; Gaarenstroom, S.W. NIST X-Ray Photoelectron Spectroscopy Database 20, Version 4.1. 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 15 September 2019).
- Ökte, A.N. Characterization and photocatalytic activity of Ln (La, Eu, Gd, Dy and Ho) loaded ZnO nanocatalysts. Appl. Catal. A Gen. 2014, 475, 27–39. [Google Scholar] [CrossRef]
- Zhang, F.; Zuo, B.Q.; Bai, L. Study on the structure of SF fiber mats electrospun with HFIP and FA and cells behavior. J. Mater. Sci. 2009, 44, 5682–5687. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H.J.; Kaplan, D.L.; Rutledge, G.C. Mechanical Properties of Electrospun Silk Fibers. Macromolecules 2004, 37, 6856–6864. [Google Scholar] [CrossRef]
- Wang, H.; Shao, H.; Hu, X. Structure of silk fibroin fibers made by an electrospinning process from a silk fibroin aqueous solution. J. Appl. Polym. Sci. 2006, 101, 961–968. [Google Scholar] [CrossRef]
- Ochoa, Y.; Ortegón, Y.; Vargas, M.; Rodríguez Páez, J.E. Síntesis de TiO2, fase anatasa, por el método Pechini. Suplemento de la Revista Latinoamericana de Metalurgia y Materiales 2009, 1, 931–937. [Google Scholar]
- Senthilkumaar, S.; Rajendran, K.; Banerjee, S.; Chini, T.K.; Sengodan, V. Influence of Mn doping on the microstructure and optical property of ZnO. Mater. Sci. Semicond. Process. 2008, 11, 6–12. [Google Scholar] [CrossRef]
- Djaja, N.F.; Montja, D.A.; Saleh, R. The effect of Co incorporation into ZnO nanoparticles. Adv. Mater. Phys. Chem. 2013, 3, 33–41. [Google Scholar] [CrossRef]
- Millán-Rivero, J.E.; Martínez, C.M.; Romecín, P.A.; Aznar-Cervantes, S.D.; Carpes-Ruiz, M.; Cenis, J.L.; Moraleda, J.M.; Atucha, N.M.; García-Bernal, D. Silk fibroin scaffolds seeded with Wharton’s jelly mesenchymal stem cells enhance re-epithelialization and reduce formation of scar tissue after cutaneous wound healing. Stem Cell Res. Ther. 2019, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Cervantes, S.; Martínez, J.G.; Bernabeu-Esclapez, A.; Lozano-Pérez, A.A.; Meseguer-Olmo, L.; Otero, T.F.; Cenis, J.L. Fabrication of electrospun silk fibroin scaffolds coated with graphene oxide and reduced graphene for applications in biomedicine. Bioelectrochemistry 2016, 108, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Fernández-Ibañez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energ Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Salah, N.H.; Bouhelassa, M.; Bekkouche, S.; Boultif, A. Study of photocatalytic degradation of phenol. Desalination 2004, 166, 347–354. [Google Scholar] [CrossRef]
- Kitsiou, V.; Filippidis, N.; Mantzavinos, D.; Pouios, I. Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in aqueous solutions. Appl. Catal. B Eviron. 2009, 86, 27–35. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Fenoll, J.; Hellín, P.; Martínez, C.M.; Flores, P.; Navarro, S. Determination of 48 pesticides and their main metabolites in water samples by employing sonication and liquid chromatography—Tandem mass spectrometry. Talanta 2011, 85, 975–982. [Google Scholar] [CrossRef] [PubMed]
| Mats | Fiber Diameter (nm) | Tensile Strength (MPa) | Elastic Modulus (MPa) | Strain at Break (%) |
|---|---|---|---|---|
| SF | 2948 ± 748 | 0.41 ± 0.12 | 24.24 ± 8.13 | 2.28 ± 0.26 |
| SF/ZnO | 2766 ± 599 | 0.32 ± 0.10 | 20.77 ± 10.21 | 2.27 ± 0.41 |
| SF/TiO2 | 3858 ± 1167 * | 0.33 ± 0.09 | 22.77 ± 5.06 | 1.85 ± 0.27 |
| Fungicide | SF/TiO2 | SF/ZnO | ||||
|---|---|---|---|---|---|---|
| R2 | k (min−1) | t½ (min) | R2 | k (min−1) | t½ (min) | |
| Difenoconazole | 0.978 | 9.416 × 10−3 | 73.6 | 0.979 | 1.092 × 10−2 | 63.5 |
| Etoxazole | 0.998 | 2.258 × 10−2 | 30.7 | 0.983 | 2.092 × 10−2 | 33.1 |
| Myclobutanil | 0.974 | 3.276 × 10−3 | 211.6 | 0.979 | 7.446 × 10−3 | 93.1 |
| Penconazole | 0.962 | 5.183 × 10−3 | 133.7 | 0.990 | 8.909 × 10−3 | 77.8 |
| Compound | Structure | MF a | MW b | Log KOW c | VP d | WS e | GUS f |
|---|---|---|---|---|---|---|---|
| Difenoconazole | 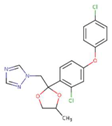 | C19H17Cl2N3O3 | 406.3 | 4.36 | 3.3 × 10−5 | 15.0 | 0.90 |
| Etoxazole | 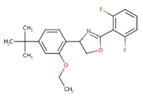 | C21H23FNO2 | 359.4 | 5.52 | 0.007 | 0.07 | 0.25 |
| Myclobutanil | 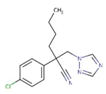 | C15H17ClN4 | 288.8 | 2.89 | 0.198 | 132 | 3.30 |
| Penconazole |  | C13H15Cl2N3 | 284.2 | 3.72 | 0.366 | 73.0 | 1.36 |
| Compound | tR (min) | SRM1 | Fragmentor1 (V) | Collision Energy1 (V) | SRM2 | Fragmentor2 (V) | Collision Energy2 (V) |
|---|---|---|---|---|---|---|---|
| Difenoconazole | 28.10 | 406→251 | 130 | 20 | 406→337 | 130 | 15 |
| Etoxazole | 32.90 | 360→141 | 120 | 30 | 360→304 | 120 | 20 |
| Myclobutanil | 25.60 | 289→70 | 130 | 15 | 289→125 | 130 | 40 |
| Penconazole | 26.90 | 284→70 | 110 | 30 | 284→159 | 110 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, I.; Aznar-Cervantes, S.; Aliste, M.; Yáñez-Gascón, M.J.; Vela, N.; Cenis, J.L.; Navarro, S.; Fenoll, J. Photocatalytic Performance of Electrospun Silk Fibroin/ZnO Mats to Remove Pesticide Residues from Water under Natural Sunlight. Catalysts 2020, 10, 110. https://doi.org/10.3390/catal10010110
Garrido I, Aznar-Cervantes S, Aliste M, Yáñez-Gascón MJ, Vela N, Cenis JL, Navarro S, Fenoll J. Photocatalytic Performance of Electrospun Silk Fibroin/ZnO Mats to Remove Pesticide Residues from Water under Natural Sunlight. Catalysts. 2020; 10(1):110. https://doi.org/10.3390/catal10010110
Chicago/Turabian StyleGarrido, Isabel, Salvador Aznar-Cervantes, Marina Aliste, María J. Yáñez-Gascón, Nuria Vela, José L. Cenis, Simón Navarro, and José Fenoll. 2020. "Photocatalytic Performance of Electrospun Silk Fibroin/ZnO Mats to Remove Pesticide Residues from Water under Natural Sunlight" Catalysts 10, no. 1: 110. https://doi.org/10.3390/catal10010110
APA StyleGarrido, I., Aznar-Cervantes, S., Aliste, M., Yáñez-Gascón, M. J., Vela, N., Cenis, J. L., Navarro, S., & Fenoll, J. (2020). Photocatalytic Performance of Electrospun Silk Fibroin/ZnO Mats to Remove Pesticide Residues from Water under Natural Sunlight. Catalysts, 10(1), 110. https://doi.org/10.3390/catal10010110







