Low-Temperature Catalytic CO Oxidation Over Non-Noble, Efficient Chromia in Reduced Graphene Oxide and Graphene Oxide Nanocomposites
Abstract
1. Introduction
2. Results and Discussion
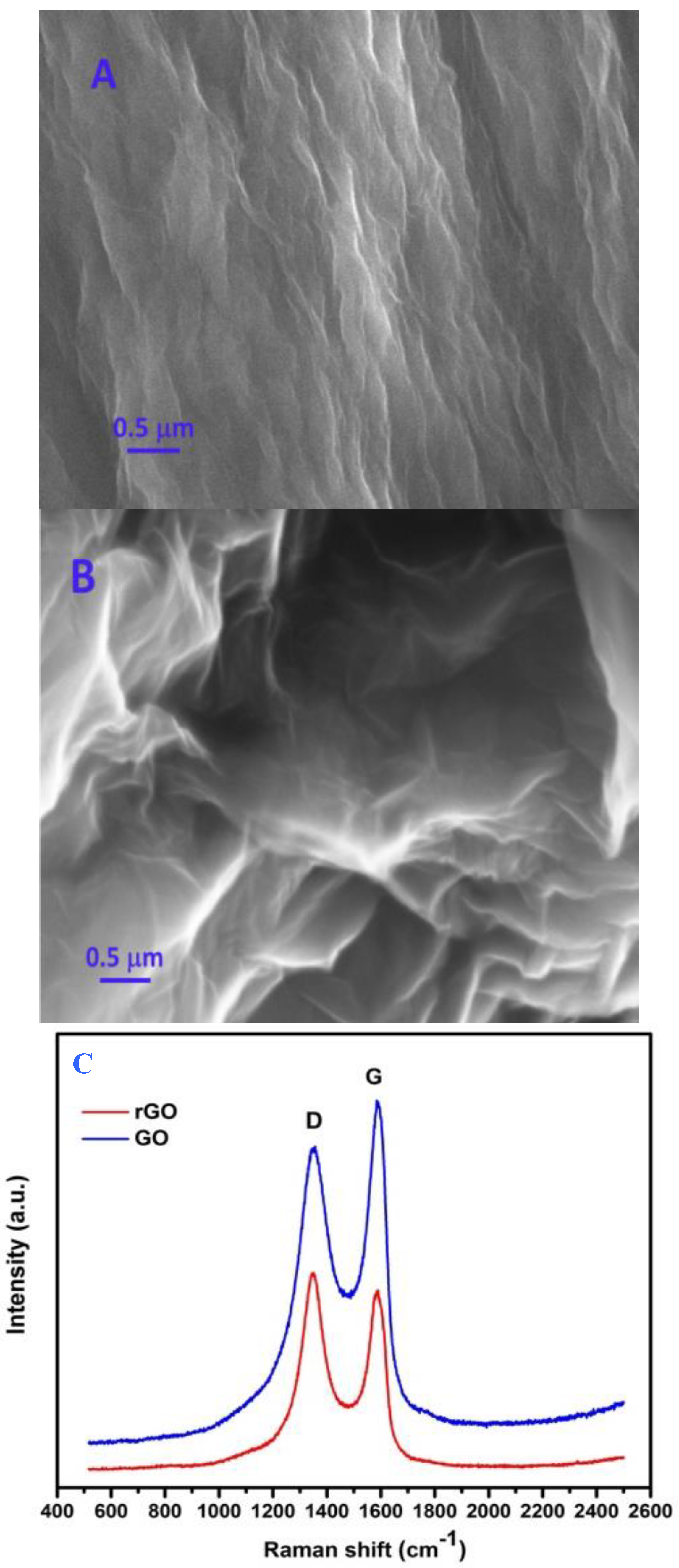
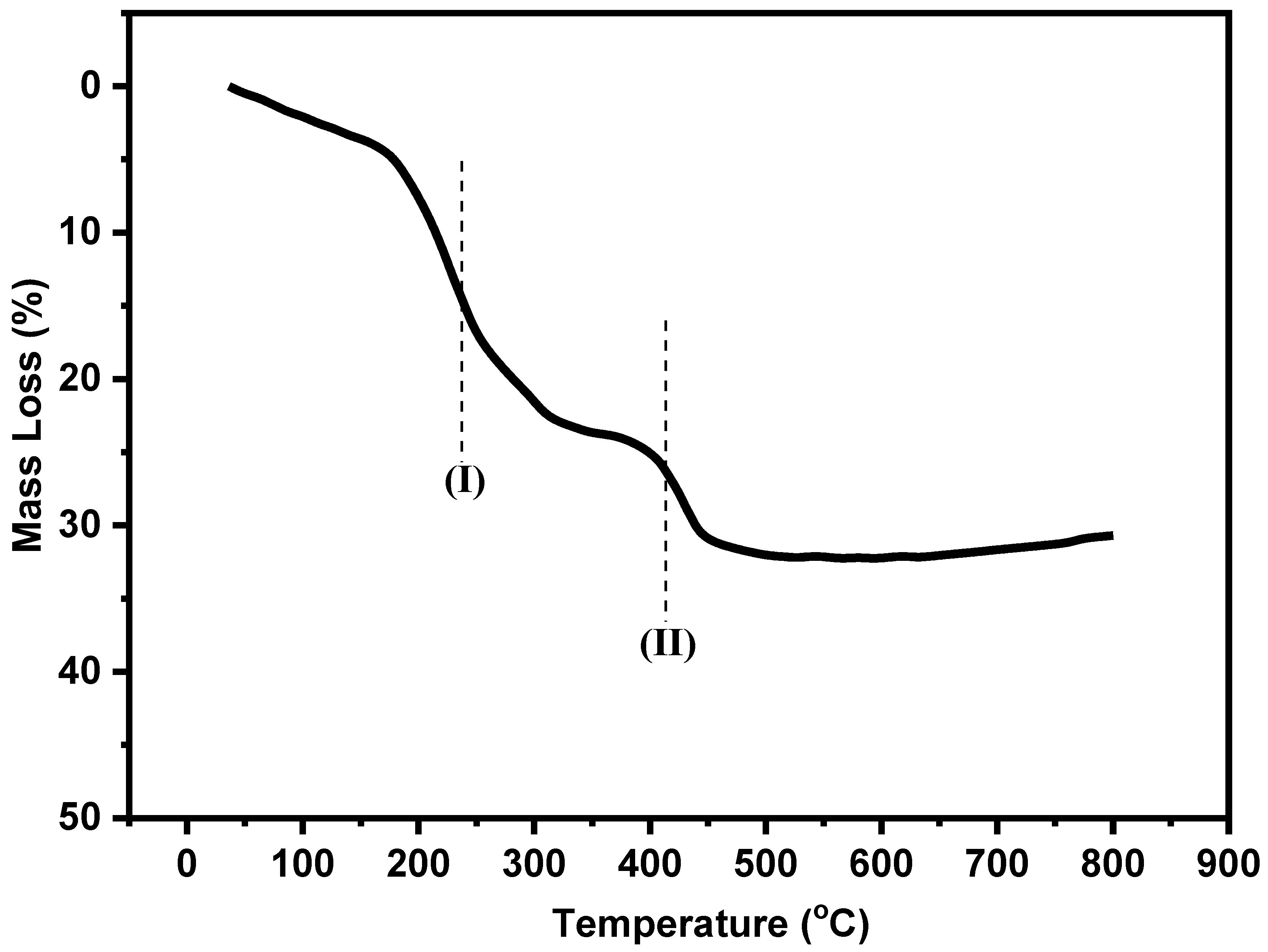
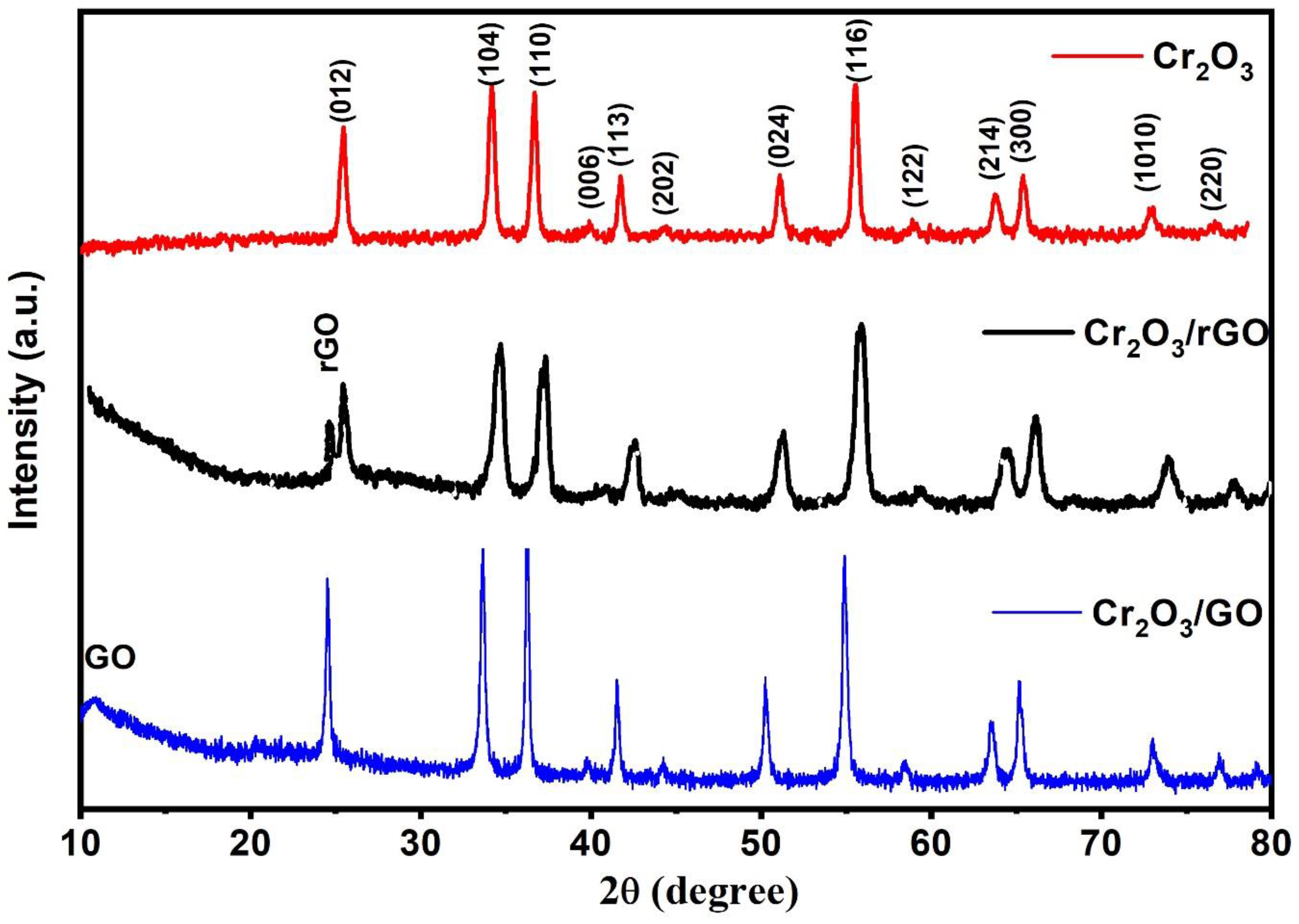
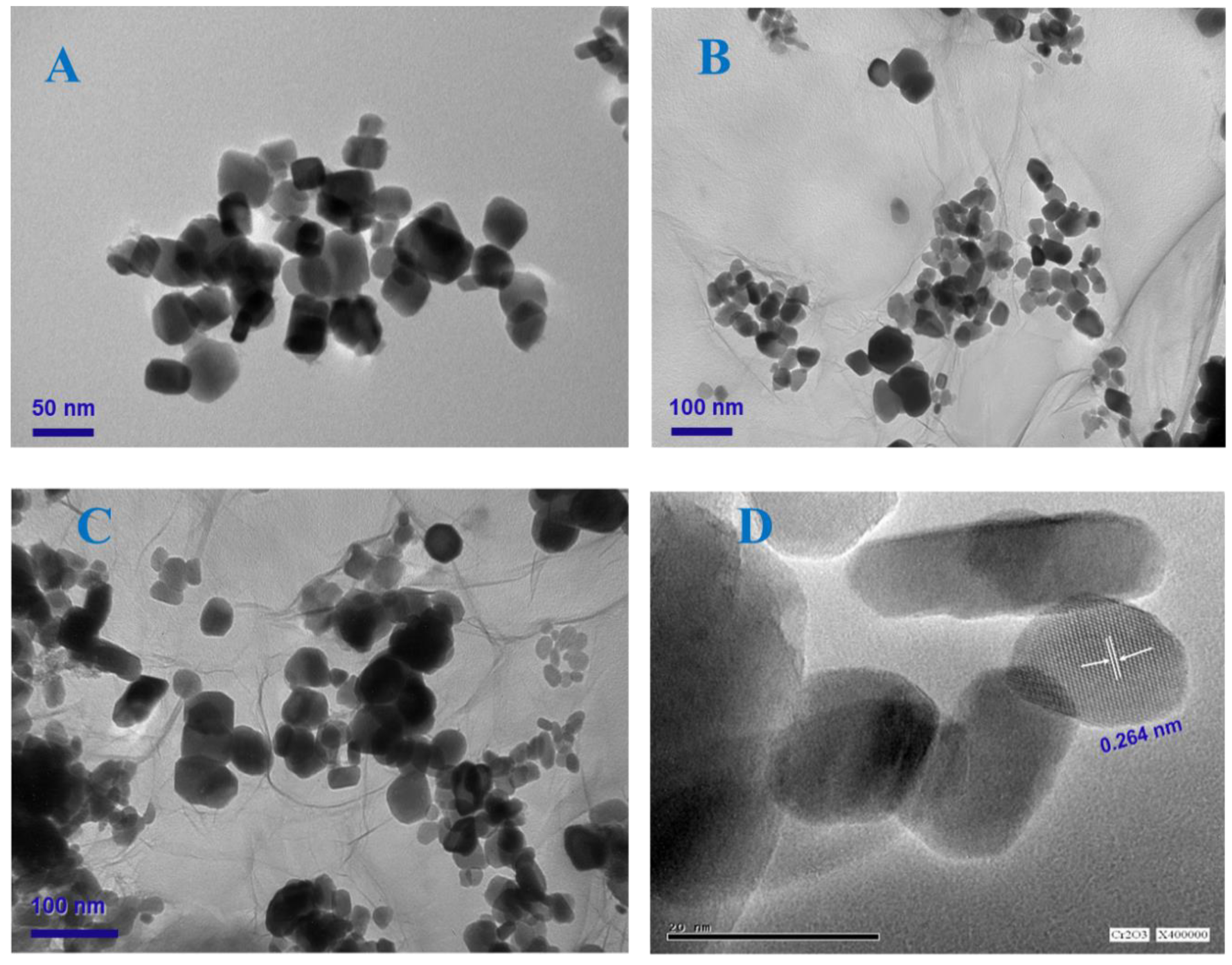
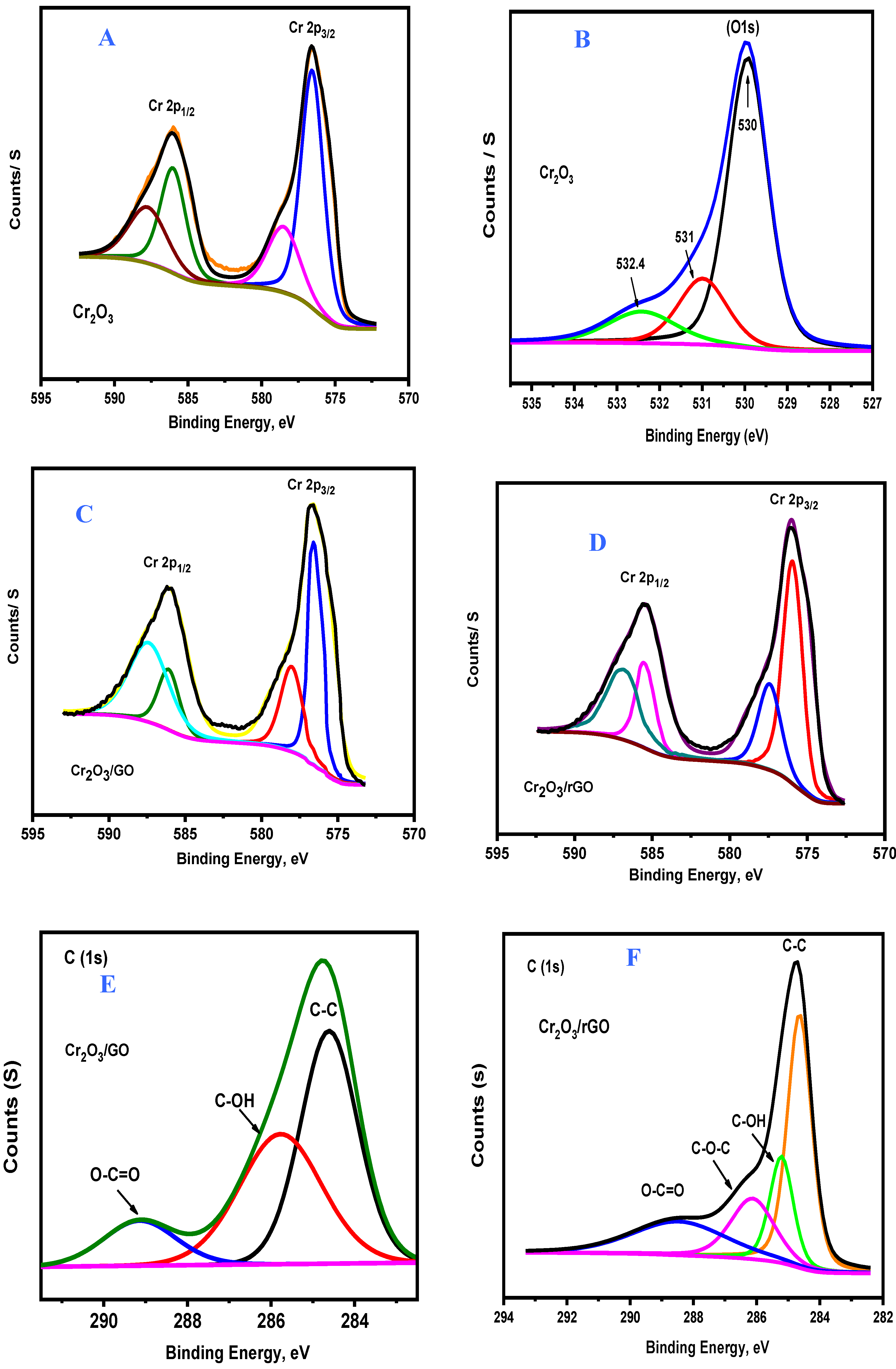
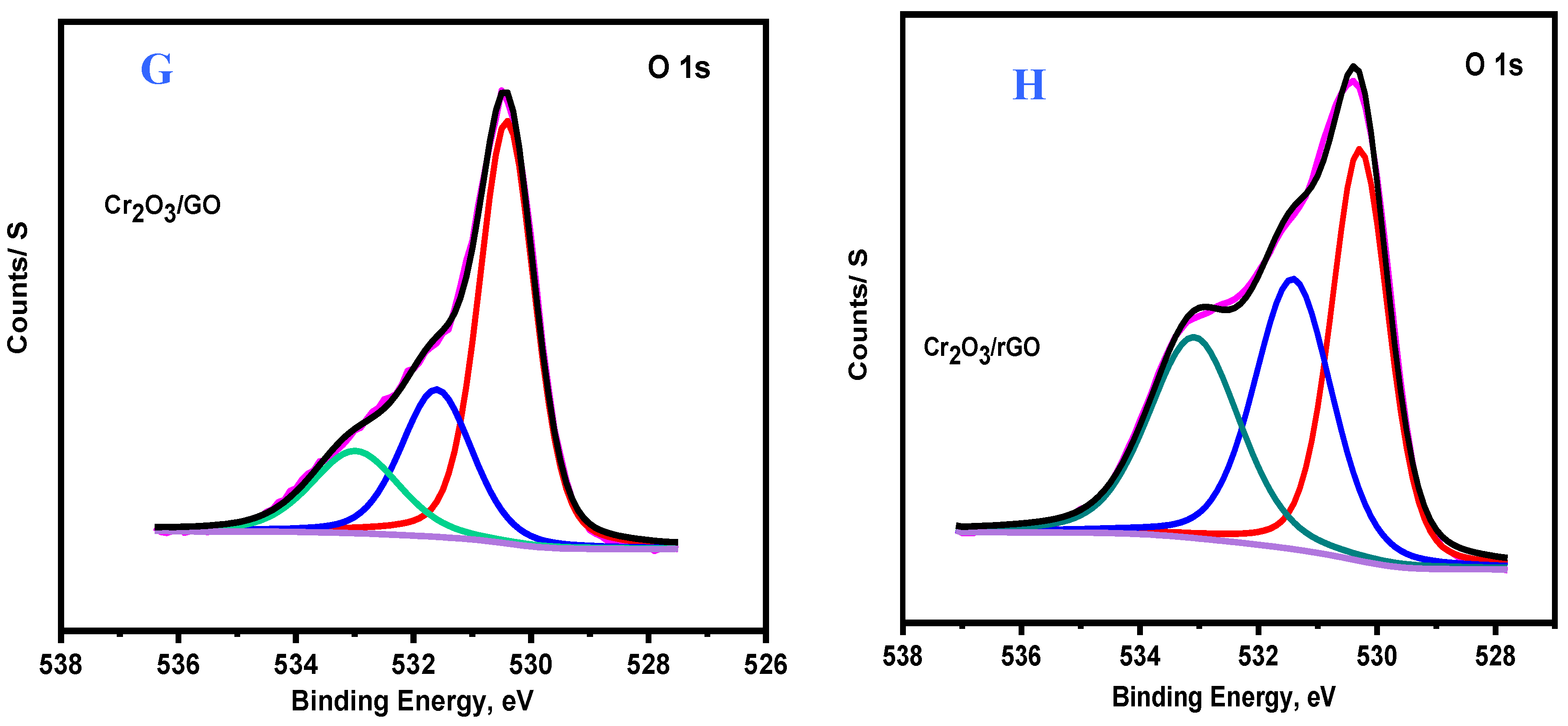
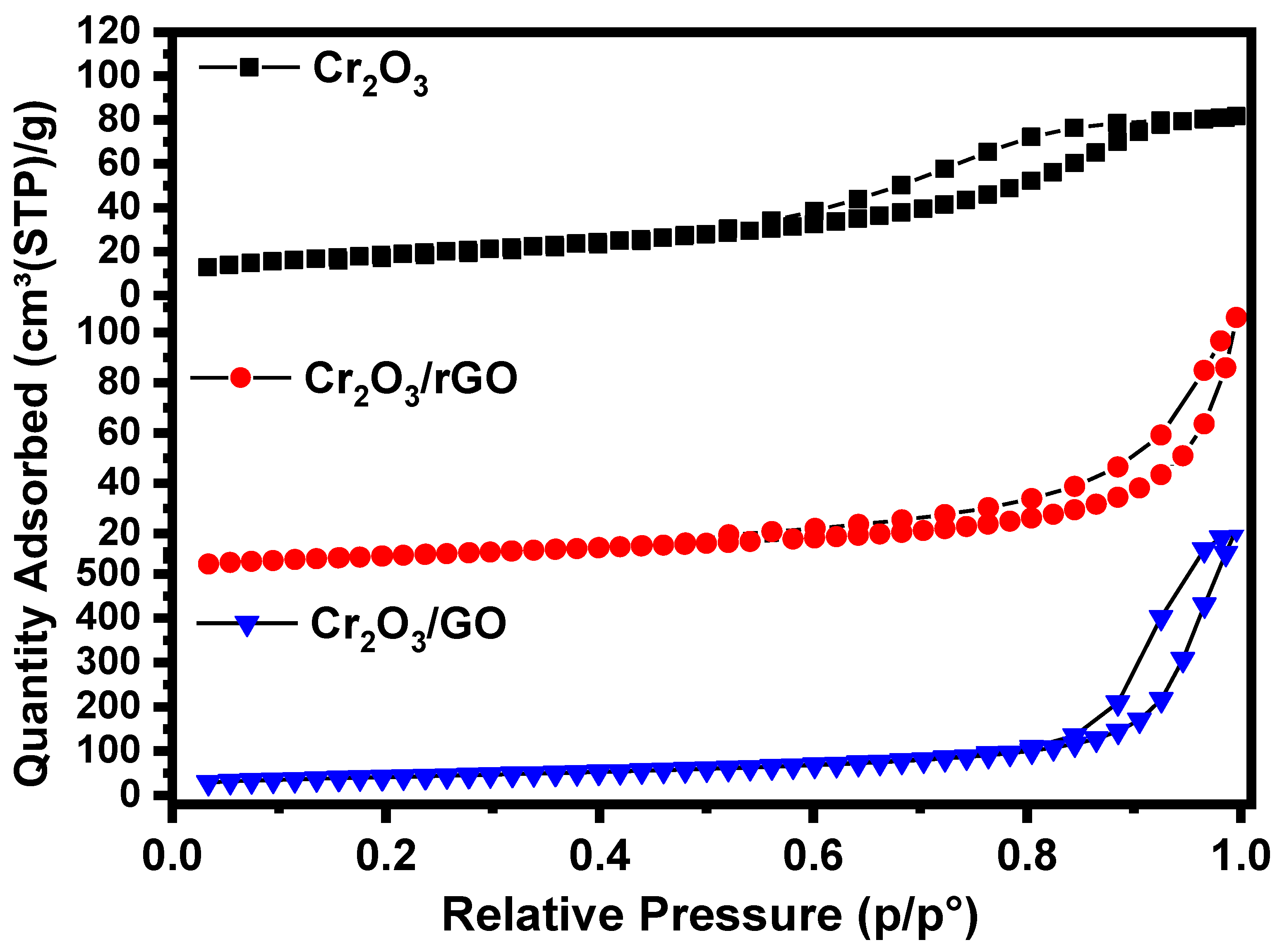
2.1. Characterization of As-Prepared Samples
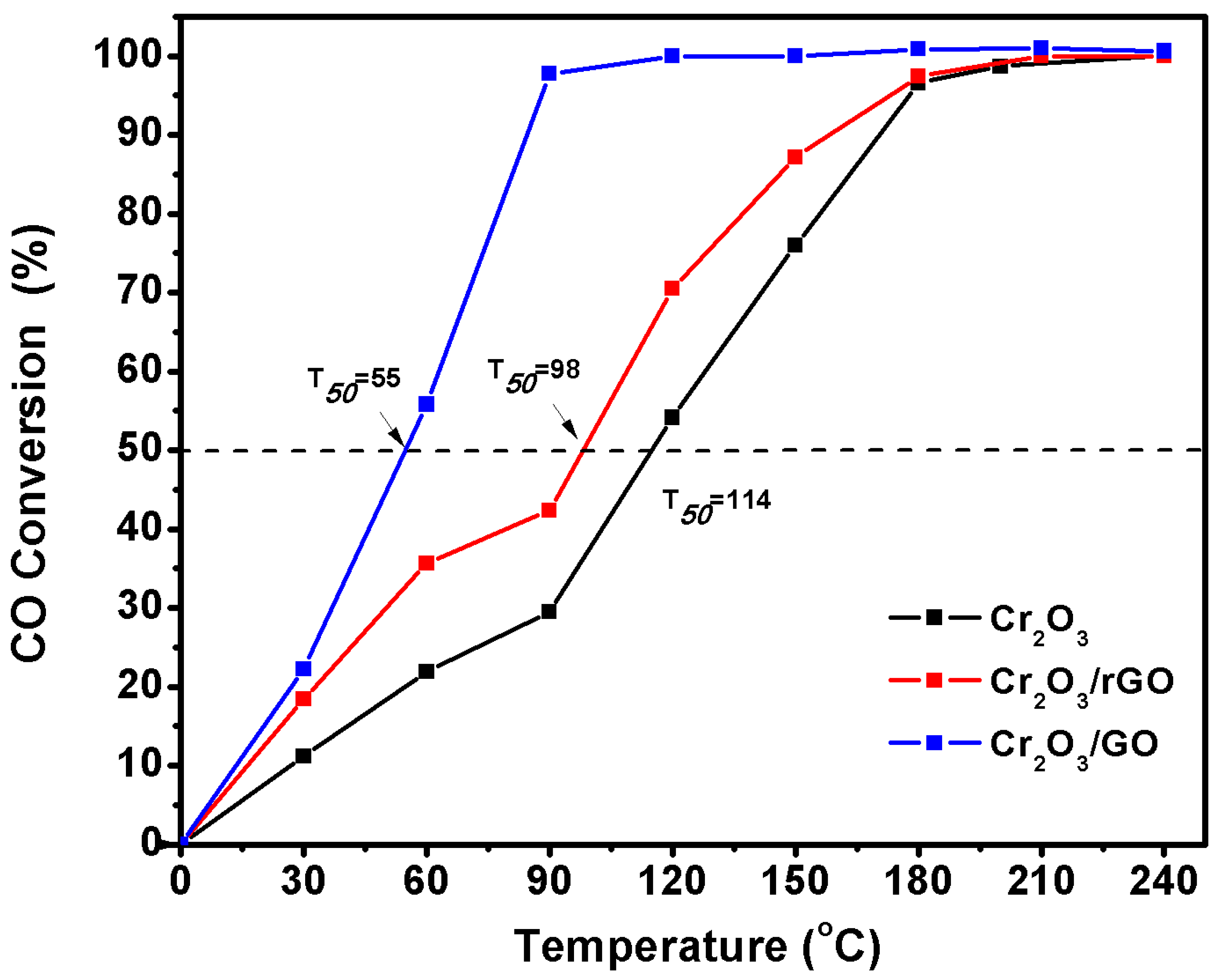
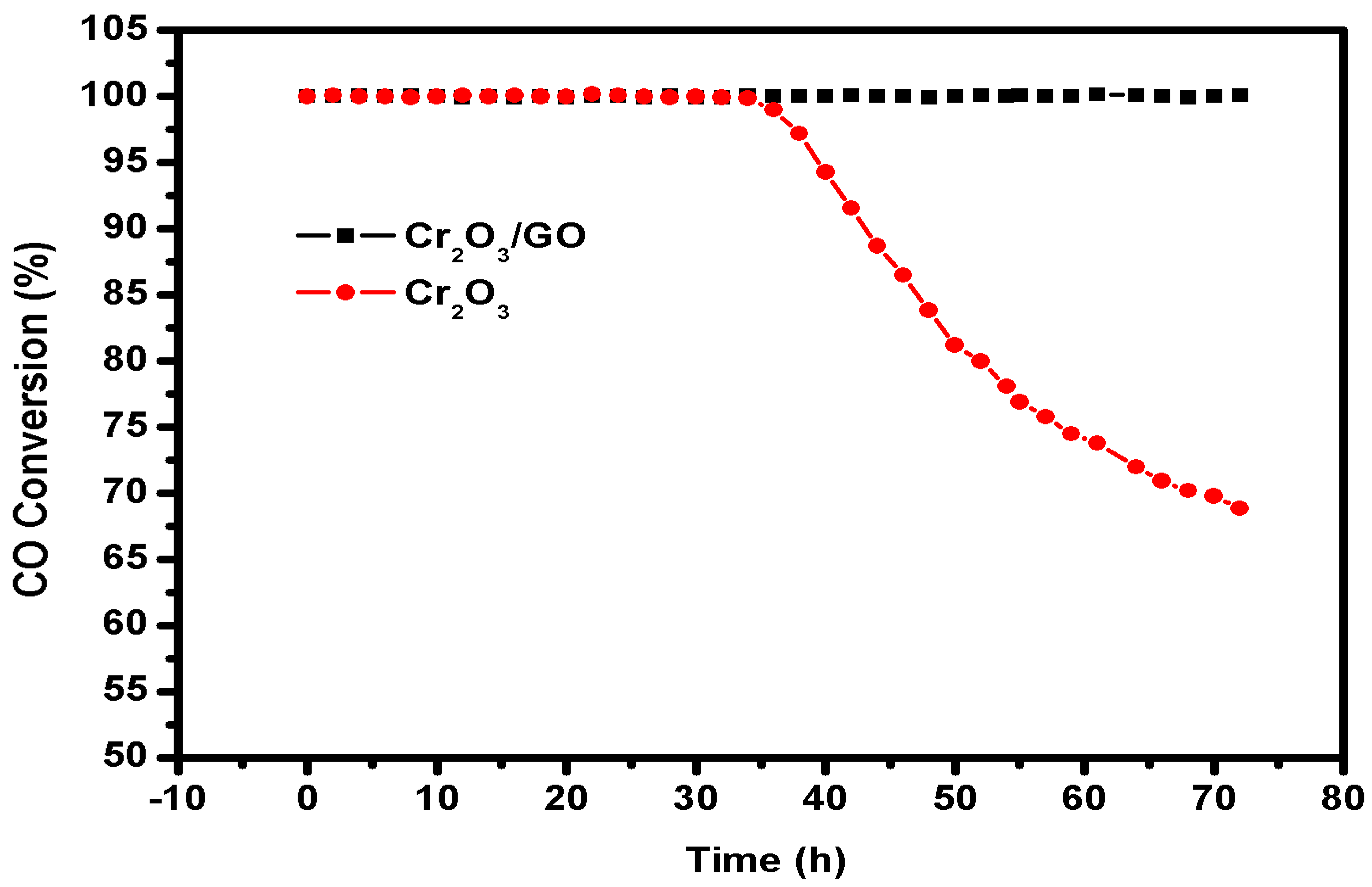
2.2. Catalytic Activity
3. Experimental Section
3.1. Materials
3.2. Preparation of GO and rGO
3.3. Preparation of Bare Chromia Nanoparticles (Cr2O3 NPs)
3.4. Preparation of the Hybrid Catalysts (Cr2O3/GO and Cr2O3/rGO)
3.5. Characterization of Chromia NPs and Hybrid Catalysts
3.6. Catalytic Activity Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, N.; Pandey, A.; Satpati, B.; Tomar, M.; Gupta, V.; Mohapatra, S. Enhanced CO gas sensing properties of Cu doped SnO2 nanostructures prepared by a facile wet chemical method. Phys. Chem. Chem. Phys. 2016, 18, 18846–18854. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Chen, J.; Li, G.; Li, S.; Gao, Y.; Tang, Z. Facile synthesis of core-shell Au@CeO2 nanocomposites with remarkably enhanced catalytic activity for CO oxidation. Energy Environ. Sci. 2012, 5, 8937–8941. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Liu, Z.-Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, H.; Xu, X.; Peng, Y.; Wang, X. Facile preparation of mesoporous Cu-Sn solid solutions as active catalysts for CO oxidation. RSC Adv. 2015, 5, 25755–25764. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Wojcieszak, R.; Wender, H.; Sato, C.; Dias, B.; Vono, L.L.R.; Eberhardt, D.; Teixeira, S.R.; Rossi, L.M. Easy Access to Metallic Copper Nanoparticles with High Activity and Stability for CO Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 7987–7994. [Google Scholar] [CrossRef]
- Chen, H.; Fu, J.; Zhang, P.; Peng, H.; Abney, C.W.; Jie, K.; Liu, X.; Chi, M.; Dai, S. Entropy-stabilized metal oxide solid solutions as CO oxidation catalysts with high-temperature stability. J. Mater. Chem. A 2018, 6, 11129–11133. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Tang, Q.; Zhang, B.; Su, D.S.; Wang, S.; Sun, G. Coupling Effect Between Cobalt Oxides and Carbon For Oxygen Reduction Reaction. ChemSusChem 2012, 5, 2315–2318. [Google Scholar] [CrossRef]
- Mao, S.; Wen, Z.; Huang, T.; Hou, Y.; Chen, J. High-performance bi-functional electrocatalysts of 3D crumpled graphene-cobalt oxide nanohybrids for oxygen reduction and evolution reactions. Energy Environ. Sci. 2014, 7, 609–616. [Google Scholar] [CrossRef]
- Ye, Y.; Kuai, L.; Geng, B. A template-free route to a Fe3O4-Co3O4 yolk-shell nanostructure as a noble-metal free electrocatalyst for ORR in alkaline media. J. Mater. Chem. 2012, 22, 19132–19138. [Google Scholar] [CrossRef]
- Horie, M.; Nishio, K.; Endoh, S.; Kato, H.; Fujita, K.; Miyauchi, A.; Nakamura, A.; Kinugasa, S.; Yamamoto, K.; Niki, E.; et al. Chromium(III) oxide nanoparticles induced remarkable oxidative stress and apoptosis on culture cells. Environ. Toxicol. 2013, 28, 61–75. [Google Scholar] [CrossRef]
- Borello, E.; Zecchina, A.; Coluccia, S.; Cerruti, L. Infrared study of surface properties of alpha-chromia. II. Oxygen chemisorption. J. Phys. Chem. 1971, 75, 2783–2790. [Google Scholar] [CrossRef]
- Fahim, R.B.; Gabr, R.M.; Zaki, M.I.; Mansour, S.A.A. Nonstoichiometry and surface characterization of chromia gel. J. Colloid Interface Sci. 1981, 81, 468–476. [Google Scholar] [CrossRef]
- Fahim, R.B.; Zaki, M.I.; Yacoub, N.H. Water sorption in relation to surface defect structure of calcined chromia gel. J. Colloid Interface Sci. 1982, 88, 502–511. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, Z.; Qian, L.; Dai, S.; He, H.; Bruce, P.G. Ordered Crystalline Mesoporous Oxides as Catalysts for CO Oxidation. Catal. Lett. 2009, 131, 146–154. [Google Scholar] [CrossRef]
- Fahim, R.B.; Zaki, M.I.; Gabr, R.M. Heterogeneous and/or homogeneous chromia-catalysed decomposition of hydrogen peroxide. Surf. Technol. 1981, 12, 317–326. [Google Scholar] [CrossRef]
- Lin-hua, H.; Ke-qiang, S.; Qing, P.; Bo-qing, X.; Ya-dong, L. Surface active sites on Co3O4 nanobelt and nanocube model catalysts for CO oxidation. Nano Res. 2010, 3, 363–368. [Google Scholar]
- Liu, H.; Wang, J.; Feng, Z.; Lin, Y.; Zhang, L.; Su, D. Facile Synthesis of Au Nanoparticles Embedded in an Ultrathin Hollow Graphene Nanoshell with Robust Catalytic Performance. Small 2015, 11, 5059–5064. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhou, Z.; Yu, G.T.; Chen, W.; Chen, Z.F. CO Catalytic Oxidation on Iron-Embedded Graphene: Computational Quest for Low-Cost Nanocatalysts. J. Phys. Chem. C 2010, 114, 6250–6254. [Google Scholar] [CrossRef]
- Kumar, G.M.; Sampath, S.; Jeena, V.S.; Anjali, R. Carbon Monoxide Pollution Levels at Environmentally Different Sites. J. Ind. Geophys. Union 2008, 12, 31–40. [Google Scholar]
- Li, Y.; Yu, Y.; Wang, J.-G.; Song, J.; Li, Q.; Dong, M.; Liu, C.-J. CO oxidation over graphene supported palladium catalyst. Appl. Catal. B Environ. 2012, 125, 189–196. [Google Scholar] [CrossRef]
- Vats, T.; Dutt, S.; Kumar, R.; Siril, P.F. Facile synthesis of pristine graphene-palladium nanocomposites with extraordinary catalytic activities using swollen liquid crystals. Sci. Rep. 2016, 6, 33053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.-H.; Huang, J.; Li, G.-J.; Cao, J.-L.; Zhang, B.; Chen, X.-Y.; Zhang, H.-L.; Jia, L. Preparation and catalytic behavior of reduced graphene oxide supported cobalt oxide hybrid nanocatalysts for CO oxidation. Trans. Nonferrous Met. Soc. China 2018, 28, 2265–2273. [Google Scholar] [CrossRef]
- Wen, C.; Gao, X.; Huang, T.; Wu, X.; Xu, L.; Yu, J.; Zhang, H.; Zhang, Z.; Han, J.; Ren, H. Reduced graphene oxide supported chromium oxide hybrid as high efficient catalyst for oxygen reduction reaction. Int. J. Hydrog. Energy 2016, 41, 11099–11107. [Google Scholar] [CrossRef]
- Xia, L.; Li, B.; Zhang, Y.; Zhang, R.; Ji, L.; Chen, H.; Cui, G.; Zheng, H.; Sun, X.; Xie, F.; et al. Cr2O3 Nanoparticle-Reduced Graphene Oxide Hybrid: A Highly Active Electrocatalyst for N2 Reduction at Ambient Conditions. Inorg. Chem. 2019, 58, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, H.-L.; Zhang, Q.; Peng, J.; Li, J.; Zhai, M.; Yu, Z.-Z. Facile synthesis of well-dispersed graphene by g-ray induced reduction of graphene oxide. J. Mater. Chem. 2012, 22, 13064. [Google Scholar] [CrossRef]
- Pham, V.H.; Cuong, T.V.; Hur, S.H.; Oh, E.; Kim, E.J.; Shin, E.W.; Chung, J.S. Chemical functionalization of graphene sheets by solvothermal reduction of a graphene oxide suspension in N-methyl-2-pyrrolidone. J. Mater. Chem. 2011, 21, 3371. [Google Scholar] [CrossRef]
- Ma, Z.; Xiao, Z.; van Bokhoven, J.A.; Liang, C. A non-alkoxide sol-gel route to highly active and selective Cu-Cr catalysts for glycerol conversion. J. Mater. Chem. 2010, 20, 755–760. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Kanan, V.; Joshi, S.; Visoly-Fisher, I. Role of oxygen functional groups in reduced graphene oxide for lubrication. Sci. Rep. 2017, 7, 45030. [Google Scholar] [CrossRef]
- Gupta, P.; Bhargava, R.; Das, R.; Poddar, P. Static and dynamic magnetic properties and effect of surface chemistry on the morphology and crystallinity of DyCrO3 nanoplatelets. RSC Adv. 2013, 3, 26427–26432. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Rooney, D.W.; Halawy, S.A.; Mohamed, M.A.; Abdelkader, A. Effect of precursor on the performance of alumina for the dehydration of methanol to dimethyl ether. Appl. Catal. B Environ. 2012, 127, 307–315. [Google Scholar] [CrossRef]
- Kadari, A.; Schemme, T.; Kadri, D.; Wollschläger, J. XPS and morphological properties of Cr2O3 thin films grown by thermal evaporation method. Results Phys. 2017, 7, 3124–3129. [Google Scholar] [CrossRef]
- Bumajdad, A.; Al-Ghareeb, S.; Madkour, M.; al Sagheer, F. Non-noble, efficient catalyst of unsupported α-Cr2O3 nanoparticles for low temperature CO Oxidation. Sci. Rep. 2017, 7, 14788. [Google Scholar] [CrossRef] [PubMed]
- Lecloux, A.J. Catalysis-Science and Engineering; Anderson, J.R., Boudart, M., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 1981; Volume 2, pp. 171–229. [Google Scholar]
- Shelef, M.; Otto, K.; Gandhi, H. The oxidation of CO by O2 and by NO on supported chromium oxide and other metal oxide catalysts. J. Catal. 1968, 12, 361–375. [Google Scholar] [CrossRef]
- Pantaleo, G.; Liotta, L.F.; Venezia, A.M.; Deganello, G.; Ezzo, E.M.; El Kherbawi, M.A.; Atia, H. Support effect on the structure and CO oxidation activity of Cu-Cr mixed oxides over Al2O3 and SiO2. Mater. Chem. Phys. 2009, 114, 604–611. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Madkour, M.; Ali, A.A.; Nazeer, A.A.; al Sagheer, F.; Belver, C. A novel natural sunlight active photocatalyst of CdS/SWCNT/CeO2 heterostructure: In depth mechanistic insights for the catalyst reactivity and dye mineralization. Appl. Surf. Sci. 2020, 499, 143988. [Google Scholar] [CrossRef]
| Material | Experimental Conditions | T100, °C | T50, °C | Ref. |
|---|---|---|---|---|
| Cr2O3 | 45–46 g (80 cm3) of catalyst, gas composition, CO~l.2%, O2~1.2%, balance He; gas rate—1400 cm3/min. | 340 | 265 | [34] |
| CuO/Cr2O3 supported on silica | The feed composition was 2% CO, 2% O2 in He. 0.1 g of catalyst at a flow rate of 100 mL min−1 | ≈500 | 213 | [35] |
| CuO/Cr2O3 supported on Alumina | The feed composition was 2% CO, 2% O2 in He. 0.1 g of catalyst at a flow rate of 100 mL min−1 | ≈320 | 233 | [35] |
| Cr2O3-4 | The dose of examined material: 250–300 mg The mass ratio 1CO to 3O2 | 200 | 98 | [32] |
| Cr2O3-6 | The dose of examined material: 250–300 mg The mass ratio 1CO to 3O2 | 248 | 115 | [32] |
| Cr2O3-8 | The dose of examined material: 250–300 mg The mass ratio 1CO to 3O2 | 304 | 132 | [32] |
| Cr2O3 | The dose of examined material: 300 mg The mass ratio 1CO to 3O2 | 240 | 114 | Present |
| Cr2O3/rGO | The dose of examined material: 300 mg The mass ratio 1CO to 3O2 | 210 | 98 | Present |
| Cr2O3/GO | The dose of examined material: 300 mg The mass ratio 1CO to 3O2 | 120 | 55 | Present |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.A.; Madkour, M.; Sagheer, F.A.; Zaki, M.I.; Abdel Nazeer, A. Low-Temperature Catalytic CO Oxidation Over Non-Noble, Efficient Chromia in Reduced Graphene Oxide and Graphene Oxide Nanocomposites. Catalysts 2020, 10, 105. https://doi.org/10.3390/catal10010105
Ali AA, Madkour M, Sagheer FA, Zaki MI, Abdel Nazeer A. Low-Temperature Catalytic CO Oxidation Over Non-Noble, Efficient Chromia in Reduced Graphene Oxide and Graphene Oxide Nanocomposites. Catalysts. 2020; 10(1):105. https://doi.org/10.3390/catal10010105
Chicago/Turabian StyleAli, Asma A., Metwally Madkour, Fakhreia Al Sagheer, Mohamed I. Zaki, and Ahmed Abdel Nazeer. 2020. "Low-Temperature Catalytic CO Oxidation Over Non-Noble, Efficient Chromia in Reduced Graphene Oxide and Graphene Oxide Nanocomposites" Catalysts 10, no. 1: 105. https://doi.org/10.3390/catal10010105
APA StyleAli, A. A., Madkour, M., Sagheer, F. A., Zaki, M. I., & Abdel Nazeer, A. (2020). Low-Temperature Catalytic CO Oxidation Over Non-Noble, Efficient Chromia in Reduced Graphene Oxide and Graphene Oxide Nanocomposites. Catalysts, 10(1), 105. https://doi.org/10.3390/catal10010105





