Optimal Vaccination Strategies to Reduce Endemic Levels of Meningitis in Africa
Abstract
1. Introduction
2. Methods
3. Results
3.1. Optimal Vaccination Strategies
3.2. Uncertainty and Sensitivity Analysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Acosta-Alonzo, C., Erovenko, I., Lancaster, A., Oh, H., Rychtář, J., & Taylor, D. (2020). High endemic levels of typhoid fever in rural areas of Ghana may stem from optimal voluntary vaccination behaviour. Proceedings of the Royal Society A, 476(2241), 20200354. [Google Scholar] [CrossRef]
- Agusto, F., Erovenko, I., Fulk, A., Abu-Saymeh, Q., Romero-Alvarez, D., Ponce, J., Sindi, S., Ortega, O., Saint Onge, J., & Peterson, A. T. (2022). To isolate or not to isolate: The impact of changing behavior on COVID-19 transmission. BMC Public Health, 22, 138. [Google Scholar]
- Agusto, F., Erovenko, I., & Gribovskii, G. (n.d.). A game-theoretic analysis of COVID-19 dynamics with self-isolation and vaccination behavior. in preparation.
- Angina, J., Bachhu, A., Talati, E., Talati, R., Rychtář, J., & Taylor, D. (2022). Game-theoretical model of the voluntary use of insect repellents to prevent Zika fever. Dynamic Games and Applications, 12, 133–146. [Google Scholar] [CrossRef]
- Augsburger, I., Galanthay, G., Tarosky, J., Rychtář, J., & Taylor, D. (2022). Voluntary vaccination may not stop monkeypox outbreak: A game-theoretic model. PLoS Neglected Tropical Diseases, 16(12), e0010970. [Google Scholar] [CrossRef]
- Augsburger, I., Galanthay, G., Tarosky, J., Rychtář, J., & Taylor, D. (2023). Imperfect vaccine can yield multiple Nash equilibria in vaccination games. Mathematical Biosciences, 356, 108967. [Google Scholar] [CrossRef]
- Bankuru, S., Kossol, S., Hou, W., Mahmoudi, P., Rychtář, J., & Taylor, D. (2020). A game-theoretic model of Monkeypox to assess vaccination strategies. PeerJ, 8, e9272. [Google Scholar] [CrossRef]
- Barazanji, M., Ngo, J., Powe, J., Schneider, K. P., Rychtář, J., & Taylor, D. (2023). Modeling the “F” in “SAFE”: The dynamic game of facial cleanliness in trachoma prevention. PLoS ONE, 18(6), e0287464. [Google Scholar] [CrossRef]
- Bauch, C., & Earn, D. (2004). Vaccination and the theory of games. Proceedings of the National Academy of Sciences of the United States of America, 101(36), 13391–13394. [Google Scholar] [CrossRef]
- Bauch, C., Galvani, A., & Earn, D. (2003). Group interest versus self-interest in smallpox vaccination policy. Proceedings of the National Academy of Sciences of the United States of America, 100(18), 10564–10567. [Google Scholar] [CrossRef]
- Brettin, A., Rossi-Goldthorpe, R., Weishaar, K., & Erovenko, I. (2018). Ebola could be eradicated through voluntary vaccination. Royal Society Open Science, 5(1), 171591. [Google Scholar] [CrossRef]
- Broom, M., & Rychtář, J. (2013). Game-theoretical models in biology. Chapman and Hall/CRC. [Google Scholar]
- Broom, M., Rychtář, J., & Spears-Gill, T. (2016). The game-theoretical model of using insecticide-treated bed-nets to fight malaria. Applied Mathematics, 7, 852–860. [Google Scholar] [CrossRef]
- Caasi, J., Joseph, B., Kodiyamplakkal, H., Manibusan, J., Aquino, L., Oh, H., Rychtář, J., & Taylor, D. (2022). A game-theoretic model of voluntary yellow fever vaccination to prevent urban outbreaks. Games, 13, 55. [Google Scholar] [CrossRef]
- Campo, V., Palacios, J., Nagahashi, H., Oh, H., Rychtář, J., & Taylor, D. (2022). A game-theoretic model of rabies in domestic dogs with multiple voluntary preventive measures. Journal of Mathematical Biology, 85, 57. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. (n.d.-a). Bacterial meningitis. Available online: https://www.cdc.gov/meningitis/about/bacterial-meningitis.html (accessed on 29 October 2018).
- Centers for Disease Control and Prevention. (n.d.-b). Immunology and vaccine-preventable diseases—Pink book—Meningitis. Available online: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-14-meningococcal-disease.html (accessed on 31 October 2018).
- Centers for Disease Control and Prevention. (n.d.-c). Meningitis. Available online: https://www.cdc.gov/meningitis/index.html (accessed on 29 October 2018).
- Centers for Disease Control and Prevention. (n.d.-d). Meningococcal disease. Available online: https://www.cdc.gov/meningococcal/index.html (accessed on 31 October 2018).
- Centers for Disease Control and Prevention. (n.d.-e). Meningococcal disease: Causes and spread to others. Available online: https://www.cdc.gov/meningococcal/about/causes-transmission.html (accessed on 31 October 2018).
- Centers for Disease Control and Prevention. (n.d.-f). Meningococcal disease: Diagnosis, treatment, and complications. Available online: https://www.cdc.gov/meningococcal/about/diagnosis-treatment.html (accessed on 31 October 2018).
- Centers for Disease Control and Prevention. (n.d.-g). Meningococcal disease in other countries. Available online: https://www.cdc.gov/meningococcal/php/global/index.html (accessed on 2 November 2018).
- Centers for Disease Control and Prevention. (n.d.-h). Meningococcal disease: Prevention. Available online: https://www.cdc.gov/meningococcal/about/prevention.html (accessed on 31 October 2018).
- Chen, X., & Fu, F. (2019). Imperfect vaccine and hysteresis. Proceedings of the Royal Society B, 286, 20182406. [Google Scholar] [CrossRef]
- Cheng, E., Gambhirrao, N., Patel, R., Zhowandai, A., Rychtář, J., & Taylor, D. (2020). A game-theoretical analysis of Poliomyelitis vaccination. Journal of Theoretical Biology, 499, 110298. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, A., Maiwand, S., Ngo, M., Putalapattu, V., Rychtář, J., & Taylor, D. (2020). Game-theoretical model of retroactive Hepatitis B vaccination in China. Bulletin of Mathematical Biology, 82(6), 80. [Google Scholar] [CrossRef]
- Crawford, K., Lancaster, A., Oh, H., & Rychtář, J. (2015). A voluntary use of insecticide-treated cattle can eliminate African sleeping sickness. Letters in Biomathematics, 2(1), 91–101. [Google Scholar] [CrossRef]
- Cressman, R. (2003). Evolutionary dynamics and extensive form games. The MIT Press. [Google Scholar]
- Djatcha Yaleu, G., Bowong, S., Houpa Danga, E., & Kurths, J. (2017). Mathematical analysis of the dynamical transmission of Neisseria meningitidis serogroup A. International Journal of Computer Mathematics, 94(12), 2409–2434. [Google Scholar] [CrossRef]
- Dorsett, C., Oh, H., Paulemond, M., & Rychtář, J. (2016). Optimal repellent usage to combat dengue fever. Bulletin of Mathematical Biology, 78(5), 916–922. [Google Scholar] [CrossRef]
- Fefferman, N., & Naumova, E. (2015). Dangers of vaccine refusal near the herd immunity threshold: A modelling study. The Lancet Infectious Diseases, 15(8), 922–926. [Google Scholar] [CrossRef]
- Fortunato, A., Glasser, C., Watson, J., Lu, Y., Rychtář, J., & Taylor, D. (2021). Mathematical modelling of the use of insecticide-treated nets for elimination of visceral leishmaniasis in Bihar, India. Royal Society Open Science, 8(6), 201960. [Google Scholar] [CrossRef]
- Galvani, A., Reluga, T., & Chapman, G. (2007). Long-standing influenza vaccination policy is in accord with individual self-interest but not with the utilitarian optimum. Proceedings of the National Academy of Sciences of the United States of America, 104(13), 5692–5697. [Google Scholar] [CrossRef]
- Geoffard, P.-Y., & Philipson, T. (1997). Disease eradication: Private versus public vaccination. American Economic Review, 87(1), 222–230. [Google Scholar]
- Gribovskii, G., & Erovenko, I. (n.d.). A game-theoretical model of optimal condom usage to prevent chlamydia infection. Journal of Dynamics and Games. in press.
- Han, C., Issa, H., Rychtář, J., Taylor, D., & Umana, N. (2020). A voluntary use of insecticide treated nets can stop the vector transmission of Chagas disease. PLoS Neglected Tropical Diseases, 14(11), e0008833. [Google Scholar] [CrossRef]
- Hassan, A., Tapp, Z., Tran, D., Rychtář, J., & Taylor, D. (2024). Mathematical model of rabies vaccination in the United States. Theoretical Population Biology, 157, 47–54. [Google Scholar] [CrossRef]
- Hausken, K., & Ncube, M. (2017). Policy makers, the international community and the population in the prevention and treatment of diseases: Case study on HIV/AIDS. Health Economics Review, 7(1), 5. [Google Scholar] [CrossRef]
- Hausken, K., & Ncube, M. (2018). Policy makers, the international community and people living with HIV/AIDS: The need for new commitment mechanisms. International Journal of Public Policy, 14(3–4), 275–301. [Google Scholar] [CrossRef]
- Hausken, K., & Ncube, M. (2020). Game theoretic analysis of persons, the pharmaceutical industry, and donors in disease contraction and recovery. Humanities and Social Sciences Communications, 7(1), 150. [Google Scholar] [CrossRef]
- Hausken, K., & Ncube, M. (2021). Decisions of persons, the pharmaceutical industry, and donors in disease contraction and recovery assuming virus mutation. Health Economics Review, 11(1), 26. [Google Scholar] [CrossRef]
- Hausken, K., & Ncube, M. (2022). A game theoretic analysis of competition between vaccine and drug companies during disease contraction and recovery. Medical Decision Making, 42(5), 571–586. [Google Scholar] [CrossRef]
- Hofbauer, J., & Sigmund, K. (1998). Evolutionary games and population dynamics. Cambridge University Press. [Google Scholar]
- Irving, T., Blyuss, K., Colijn, C., & Trotter, C. (2012). Modelling meningococcal meningitis in the African meningitis belt. Epidemiology and Infection, 140(5), 897–905. [Google Scholar] [CrossRef]
- Klein, S., Foster, A., Feagins, D., Rowell, J., & Erovenko, I. (2020). Optimal voluntary and mandatory insect repellent usage and emigration strategies to control the chikungunya outbreak on Reunion Island. PeerJ, 8, e10151. [Google Scholar] [CrossRef]
- Kobe, J., Pritchard, N., Short, Z., Erovenko, I., Rychtář, J., & Rowell, J. (2018). A game-theoretic model of cholera with optimal personal protection strategies. Bulletin of Mathematical Biology, 80(10), 2580–2599. [Google Scholar] [CrossRef]
- Liu, J., Kochin, B., Tekle, Y., & Galvani, A. (2012). Epidemiological game-theory dynamics of chickenpox vaccination in the USA and Israel. Journal of the Royal Society Interface, 9(66), 68–76. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S., Majid, S., Syed, R., Rychtář, J., & Taylor, D. (2024). Mathematical model of voluntary vaccination against Schistosomiasis. PeerJ, 12, e16869. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, P., & D’Onofrio, A. (2013). Modeling the interplay between human behavior and the spread of infectious diseases. Springer. [Google Scholar] [CrossRef]
- Marino, S., Hogue, I., Ray, C., & Kirschner, D. (2008). A methodology for performing global uncertainty and sensitivity analysis in systems biology. Journal of Theoretical Biology, 254(1), 178–196. [Google Scholar] [CrossRef]
- Marquez, R., Minas, M., Santos, J., Yoon, K., Campo, V., Oh, H., Rychtář, J., & Taylor, D. (2024). Game-theoretical model of COVID-19 vaccination in the endemic equilibrium. Journal of Biological Systems, 32(2), 349–370. [Google Scholar] [CrossRef]
- Maynard Smith, J. (1982). Evolution and the theory of games. Cambridge University Press. [Google Scholar]
- Onifade, A., Rychtář, J., & Taylor, D. (2024). A dynamic game of lymphatic filariasis prevention by voluntary use of insecticide treated nets. Journal of Theoretical Biology, 585, 111796. [Google Scholar] [CrossRef] [PubMed]
- Rychtář, J., & Taylor, D. (2022). A game-theoretic model of lymphatic filariasis prevention. PLoS Neglected Tropical Diseases, 16(9), e0010765. [Google Scholar] [CrossRef]
- Scheckelhoff, K., Ejaz, A., & Erovenko, I. (2025). A game-theoretic model of optimal clean equipment usage to prevent hepatitis C among injecting drug users. Mathematics, 13(14), 2270. [Google Scholar] [CrossRef]
- Scheckelhoff, K., Ejaz, A., Erovenko, I., Rychtář, J., & Taylor, D. (2021). Optimal voluntary vaccination of adults and adolescents can help eradicate hepatitis B in China. Games, 12(4), 82. [Google Scholar] [CrossRef]
- Shim, E., Chapman, G., Townsend, J., & Galvani, A. (2012). The influence of altruism on influenza vaccination decisions. Journal of the Royal Society Interface, 9(74), 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Shim, E., Grefenstette, J., Albert, S., Cakouros, B., & Burke, D. (2012). A game dynamic model for vaccine skeptics and vaccine believers: Measles as an example. Journal of Theoretical Biology, 295, 194–203. [Google Scholar] [CrossRef]
- Shim, E., Kochin, B., & Galvani, A. (2009). Insights from epidemiological game theory into gender-specific vaccination against rubella. Mathematical Biosciences and Engineering, 6(4), 839–854. [Google Scholar] [CrossRef]
- Sykes, D., & Rychtář, J. (2015). A game-theoretic approach to valuating toxoplasmosis vaccination strategies. Theoretical Population Biology, 105, 33–38. [Google Scholar] [CrossRef]
- van den Driessche, P., & Watmough, J. (2002). Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Mathematical Biosciences, 180, 29–48. [Google Scholar] [CrossRef]
- Vincent, T., & Brown, J. (2005). Evolutionary game theory, natural selection, and darwinian dynamics. Cambridge University Press. [Google Scholar] [CrossRef]
- von Neumann, J., & Morgenstern, O. (1944). Theory of games and economic behavior. Princeton University Press. [Google Scholar]
- Wang, Z., Bauch, C., Bhattacharyya, S., d’Onofrio, A., Manfredi, P., Perc, M., Perra, N., Salathé, M., & Zhao, D. (2016). Statistical physics of vaccination. Physics Reports, 664, 1–113. [Google Scholar] [CrossRef]
- World Health Organization. (n.d.-a). Defeating bacterial meningitis by 2030. Available online: https://www.who.int/initiatives/defeating-meningitis-by-2030 (accessed on 5 November 2018).
- World Health Organization. (n.d.-b). Meningococcal meningitis. Available online: http://www.who.int/emergencies/diseases/meningitis/en/ (accessed on 2 November 2018).
- World Health Organization. (n.d.-c). Meningococcal meningitis. Available online: http://www.who.int/news-room/fact-sheets/detail/ (accessed on 2 November 2018).
- World Health Organization. (2018a). Epidemic meningitis control in countries of the African meningitis belt, 2017. Weekly Epidemiological Record, 93(14), 173–184. [Google Scholar]
- World Health Organization. (2018b). Global health estimates 2016: Disease burden by cause, age, sex, by country and by region, 2000–2016. World Health Organization. [Google Scholar]
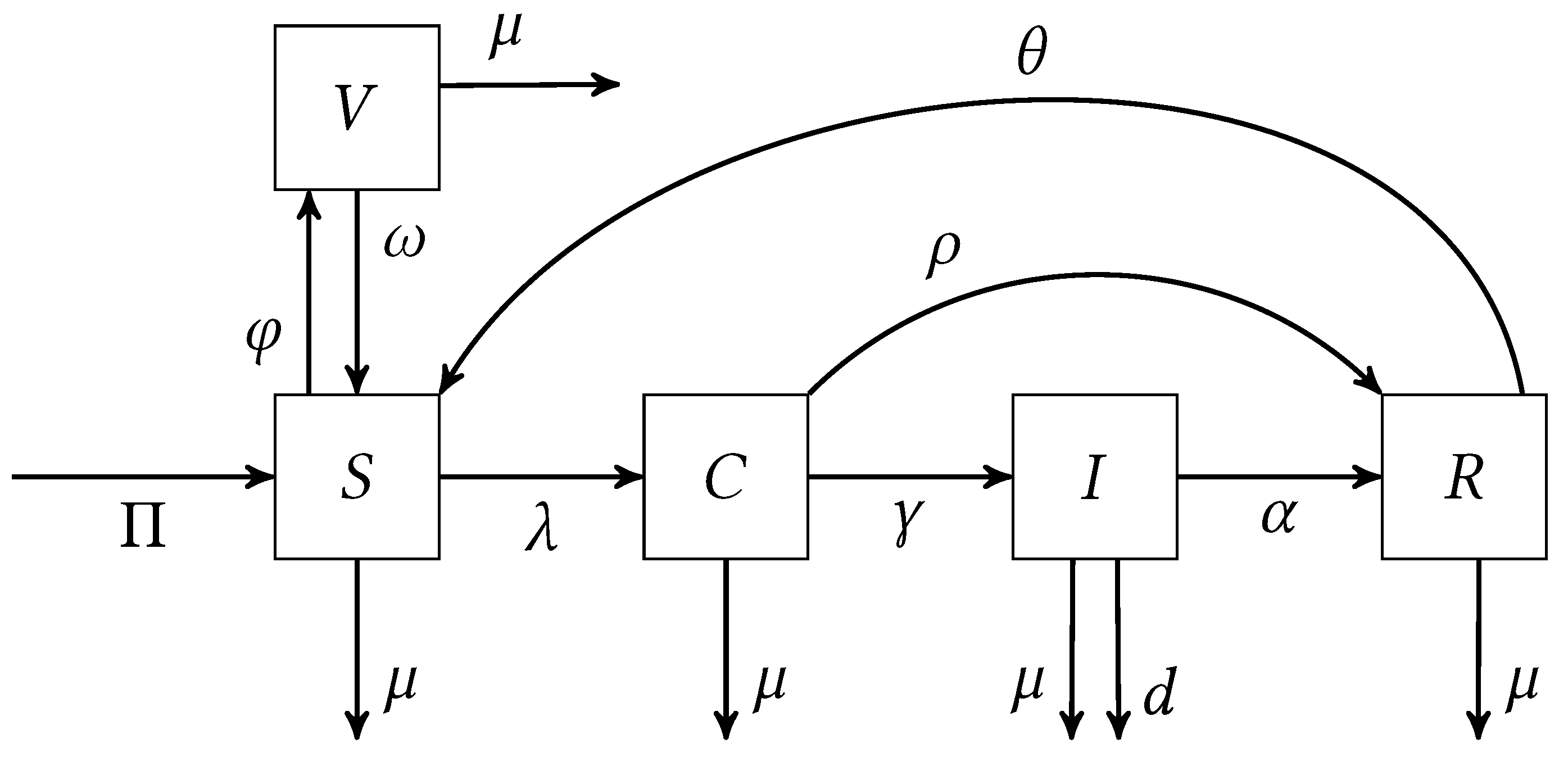





| Symbol | Meaning |
|---|---|
| Population recruitment rate | |
| Natural death rate | |
| Vaccination rate | |
| Vaccine wear-off rate | |
| Rate of carriers developing invasive disease | |
| Recovery rate of carriers | |
| Recovery rate of infected individuals | |
| d | Rate of mortality due to invasive disease |
| Rate of immunity loss in recovered individuals | |
| Transmission rate | |
| Modification parameter for infectiousness of infected individuals |
| Symbol | Baseline Value | Range of Values | Unit | Source |
|---|---|---|---|---|
| 5 | none | 1/day | Assumed | |
| 1/day | Irving et al. (2012) | |||
| varies | 1/day | Inferred | ||
| 1/day | Assumed | |||
| 1/day | Irving et al. (2012) | |||
| 1/day | Irving et al. (2012) | |||
| 1/day | Irving et al. (2012) | |||
| d | 1/day | Irving et al. (2012) | ||
| 1/day | Irving et al. (2012) | |||
| 1/day | Inferred | |||
| none | Djatcha Yaleu et al. (2017) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, A.; Machado, J.; Sanchez, E.; Erovenko, I.V. Optimal Vaccination Strategies to Reduce Endemic Levels of Meningitis in Africa. Games 2025, 16, 45. https://doi.org/10.3390/g16050045
Martinez A, Machado J, Sanchez E, Erovenko IV. Optimal Vaccination Strategies to Reduce Endemic Levels of Meningitis in Africa. Games. 2025; 16(5):45. https://doi.org/10.3390/g16050045
Chicago/Turabian StyleMartinez, Alfredo, Jonathan Machado, Eric Sanchez, and Igor V. Erovenko. 2025. "Optimal Vaccination Strategies to Reduce Endemic Levels of Meningitis in Africa" Games 16, no. 5: 45. https://doi.org/10.3390/g16050045
APA StyleMartinez, A., Machado, J., Sanchez, E., & Erovenko, I. V. (2025). Optimal Vaccination Strategies to Reduce Endemic Levels of Meningitis in Africa. Games, 16(5), 45. https://doi.org/10.3390/g16050045






