Abstract
Yellow fever is a vector-borne acute viral hemorrhagic disease. It is endemic in tropical areas of Africa and Latin America but demonstrated the potential for international spread during the 2016 outbreak in Luanda, Angola. Yellow fever can be prevented by vaccination, vector control, and avoiding mosquito bites. To account for human behavior in disease dynamics, we add a game-theoretic component to a recent compartmental model of yellow fever transmission. The self-interested individuals evaluate the risks of contracting yellow fever and choose to vaccinate or avoid the bites to minimize the overall costs. We find the Nash equilibria, the optimal levels of vaccination and bite protections if the individuals can decide on the use of only one of the prevention methods as well as when they can decide on the use of both of them. In the later case, we show that vaccination is the preferred method of protection from the individual standpoint and, in the Nash equilibrium, individuals use vaccination only. Our model predicts the vaccination coverage in Angola to be around 65%, which is in reasonable agreement with the empirical value of 68%. We also study whether voluntary prevention can lead to the elimination of the disease in endemic areas. We show that voluntary vaccination alone is not enough to mitigate the risks of outbreaks, suggesting that a mandatory vaccination policy is necessary.
1. Introduction
Yellow fever is a life-threatening acute viral hemorrhagic disease that is endemic in tropical areas of Africa and Latin America and difficult to distinguish from dengue and other hemorrhagic fevers [1]. It is transmitted by the bites of infected female mosquitoes Aedes aegypti [2].
Symptoms, including fever, muscle pain, headache, and nausea or vomiting, usually appear 3 to 6 days after the bite. For most patients, the symptoms disappear after 3 to 4 days. However, 15% of patients enter a second, more toxic phase affecting several body systems, including the kidneys [1]. There is no specific drug to treat yellow fever [3] but good and early supportive treatments for dehydration, liver and kidney failure, and fever improve survival rates [4].
Yellow fever can be prevented by vaccination, vector control, and by avoiding mosquito bites [4]. Vaccination is the most important preventive measure against yellow fever. The single dose vaccine is affordable (costs about $2), 99% effective (within 30 days), and offers life-long protection [1,5].
Climate changes and shifting mosquito habitats may be behind the recent rise in yellow fever and other Aedes-borne infections [6,7,8]. After the 2016 outbreak of yellow fever in Luanda, Angola and a linked outbreak in Kinshasa, Democratic Republic of the Congo [9], the Eliminate Yellow Fever Epidemics (EYE) strategy was developed to respond to the increased threat of urban outbreaks with international spread [10]. The strategy is guided by three strategic objectives: (1) protect at-risk populations, (2) prevent international spread of yellow fever, and (3) contain outbreaks rapidly.
Mathematical modeling is now a standard tool for modeling epidemics and disease elimination efforts [11,12]. There used to be very few mathematical models of yellow fever [13,14] although the 2016 outbreak sparked an increased modeling activity [15,16,17,18,19]. Several modeling studies further estimated the basic reproduction number [20,21,22].
The previous models neglect the impact that human behavior can have on infectious disease dynamics. Ref. [23] introduced vaccination games and incorporated voluntary disease prevention into standard epidemics modeling. These new types of models study complex scenarios in which self-interested individuals take actions based on the decisions of the rest of the population. As argued in [24], by incorporating human behavior, mathematical models provide more insight and better predictions. Thus, it is not surprising that the vaccination game theory is now a vibrant and growing field [25]. The game-theoretical models are now predictive tools in populations for extracting an optimal decision-making strategy [26]. They have been applied to study the prevention and elimination of many different diseases, including Ebola [27], COVID-19 [28,29,30], monkeypox [31], chikungunya [32], Hepatitis B [33] or cholera [34].
In this paper, we adapt the mathematical model from [18] that was developed and calibrated based on the 2016 outbreak in Angola. We incorporate voluntary vaccination and mosquito bite prevention as two strategies that individuals can take to reduce their risk of contracting the disease. We show that due to the relatively low vaccination cost, vaccination is the preferred protection strategy. Moreover, the current vaccination coverage in Angola is in agreement with our theoretical predictions. Thus, the model suggests that a mandatory vaccination policy is needed to mitigate the threat of yellow fever outbreaks.
2. Mathematical Model
In this section, we build a mathematical model for voluntary protection against yellow fever. We first introduce a compartmental ODE model of yellow fever transmission. Then, we add the game-theoretic component that allows us to investigate individuals’ optimal decisions regarding vaccination and bite protection. Finally, to make quantitative predictions, we describe how we picked the values for the model parameters.
2.1. Compartmental Model
We adapt the model from [18]. We distinguish the human population (subscript H) and mosquito/vector population (subscript V).
The human population is subdivided into susceptible (), exposed (), symptomatic infectious (), asymptomatic infectious (), toxic fever () and recovered (). The vector population is divided into susceptible (), exposed () and infectious ().
The human individuals are born at rate . A fraction of the individuals is vaccinated, becomes permanently protected against yellow fever (YF) and enters the compartment . The remaining fraction, , remains susceptible and enters a compartment . The susceptible individuals become exposed after a bite by an infectious mosquito. The per capita rate is given by , where is the level of bite protection in the population, a is the mosquito biting rate (without any protection), b is the probability of a transmission of yellow fever virus (YFV) from a mosquito to a human, and is the number of infected mosquitoes per human. The most common interpretation of is the level of repellent usage, but one can also consider other measures, such as avoiding being outside when mosquitoes are active or avoiding traveling to parts of the town where YF is more prevalent. After an incubation period , the exposed individuals move either to a symptomatic stage with a probability or to the asymptomatic stage, , with probability . Either one of those stages lasts for a period . The asymptomatic individuals fully recover and become permanently immune to YFV and enter . The symptomatic individuals enter a toxic stage that lasts for a period . For simplicity, we assume that all cases fully recover and enter . All individuals can die at the natural mortality rate .
The vectors are born at rate as susceptible. They become exposed after they bite a symptomatic or asymptomatic individual. The force of infection is given as , where c is the probability of a transmission of YFV from symptomatic cases, and is the transmission probability from asymptomatic cases. The incubation period is after which mosquitoes become infectious. All vectors die at the natural mortality rate .
We also assume that there are m mosquitoes per human, i.e., the total number of mosquitoes, , is given by .
The schematic diagram of the model is shown in Figure 1 and the model parameters are summarized in Table 1. The model yields the following differential equations:
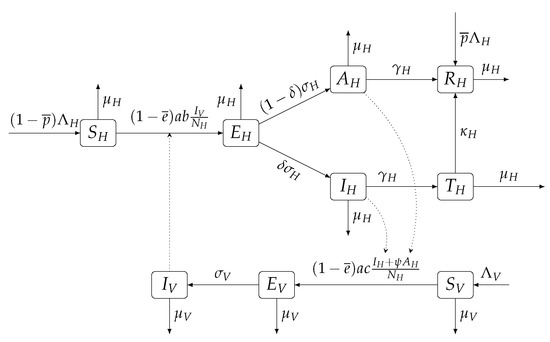
Figure 1.
Scheme of the compartmental ODE model for yellow fever transmission. Dotted lines represent the influence of a compartment over the transmission rates.

Table 1.
Model parameters. The per capita rates are per day. The times are in days.
2.2. Game-Theoretic Component
We add a game-theoretic component to study individual vaccination and bite prevention strategies and introduce the following game inspired by the framework introduced in [23].
The players of the game are susceptible individuals. The individuals can (a) choose to vaccinate against YF or not, and (b) choose to protect themselves against mosquito bites. Their strategy is given by a pair where specifies if they vaccinate () or not (), and specifies the bite prevention (with being no prevention and being a complete prevention). We will also consider cases when individuals can choose only to vaccinate or only to prevent bites.
There is a cost associated with vaccination and bite prevention. We assume the vaccine costs and, for simplicity, the bite prevention costs . We assume that the cost of contracting YF and proceeding to the symptomatic and toxic stage is , while the cost of asymptomatic stage is assumed to be 0.
The solution of the game, called the Nash equilibrium, is the population-level value at which no individual can increase their own benefits by deviating from the population strategy.
The individual’s benefit depends on the individual’s strategy but also on the prevalence of YF in the population, i.e., on the strategies of other players. Following [23], we assume that all individuals are provided with the same information and that they all use the information in the same and rational way to assess costs and risks.
2.3. Model Calibration
Most of the specific model parameter values were taken from [18], and the original sources are shown in Table 1. The birth and death rates in Angola were found in [35,36].
We estimated the relative infectivity of asymptomatic cases as as opposed to used in [18]. This value brought the basic reproduction number in line with other models as discussed in the model validation section.
The cost of the vaccine was estimated as $2 [5], while the cost of yellow fever disease was estimated as $30 [42]. We could not find reliable estimates for the cost of bite protection, but since the protection must be ongoing rather than one time, we used $3 to indicate a slightly bigger cost than the cost of the vaccine.
3. Analysis of the ODE System
Because we assume no disease-induced death, the population size remains constant at and . There are two equilibria of the dynamics.
3.1. Disease-Free Equilibrium
The disease-free equilibrium is given by , , and . Similarly as in [18], the effective reproduction number is
where
is the basic reproduction number in the population without vaccination () and without any bite prevention ().
The disease-free equilibrium is locally asymptotically stable if and unstable if [43]. It follows that the population will reach the disease-free equilibrium if where
or if where
This is illustrated in Figure 2.
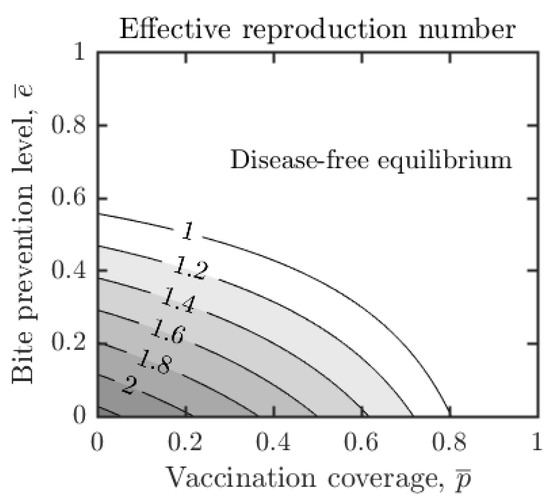
Figure 2.
Dependence of the effective reproduction number, , on vaccination coverage and bite protection levels in the population. Other parameter values as in Table 1. The number of secondary infections can be estimated by . Note that , i.e., one can contain outbreaks whenever , regardless of the value of . If the bite prevention is higher, the vaccination coverage needed for decreases. If , the outbreaks can be contained even without the vaccination.
3.2. Endemic Equilibrium
The endemic equilibrium is stable if . To highlight the dependence on and , we express as
Furthermore,
Note that by (14), is decreasing in and .
4. Results
Our overall aim is to understand what the individuals would choose if they can decide about about the optimal use of vaccination and bite prevention simultaneously. To achieve this, we will first consider a game when the individuals only decide whether to vaccinate or not, assuming the bite prevention level is fixed. Then, we will consider the game when the individuals decide on the bite protection, assuming the vaccination level is fixed. We will then combine these two results and derive optimal actions when the individuals can use both preventive options at the same time. We validate the model by comparing our reproduction number to previous studies. We also compare the predicted vaccination coverage at Nash equilibrium with the actual coverage in Angola. We conclude this section by sensitivity analysis to see how different parameters influence the predictions of our model.
4.1. Optimal Vaccination Decisions
Let us first assume that individuals can only decide whether or not to vaccinate; all other model parameters including are fixed.
In the disease-free equilibrium, the optimal decision is not to vaccinate. Therefore, for the rest of the section, we assume that the population is, or at least can be, in the endemic equilibrium, i.e., .
For a moment, assume that the rest of the population uses a strategy and the focal individual is still making a choice whether to vaccinate or not. When the focal individual decides to vaccinate, they will pay the cost . If they do not vaccinate, they will remain susceptible. In this case, they can become exposed and eventually proceed to the symptomatic stage with probability
where is given by (14). Thus, the expected cost of not getting vaccinated is given by . Note that, is decreasing in . Thus, is decreasing in . Moreover, when , . Thus, as in [23], the Nash equilibrium is unique and given by
In particular, regardless of . Additionally, is a convergent stable Nash equilibrium (CSNE). If , then the best response is to vaccinate. Conversely, when , then the best response is to not vaccinate. In either case, the vaccination coverage in the population will tend toward .
Furthermore, as increases, does not increase and so the risk of infection is non-increasing in . Consequently, the equilibrium value of is non-increasing in . This is shown in Figure 3a.
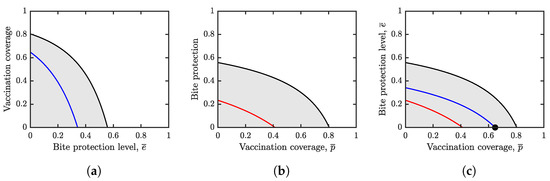
Figure 3.
Herd immunity values (black) and Nash equilibria values (blue in (a), red in (b) and the black dot in (c)). Unless varied, the parameters are as specified in Table 1. The white regions represent a parameter combination for which and the outbreaks can be contained. The gray regions represent a parameter combination for which . (a) The optimal voluntary vaccination coverage, , (blue) and the vaccination coverage needed for herd immunity, (black), as they depend on . Here we assume that is fixed and individuals can only choose whether to vaccinate or not. Both and are decreasing functions of and . (b) The optimal voluntary bite protection level, , (red) and the bite protection level needed to achieve herd immunity, , (black) as they depend on . Here we assume that individuals can only choose how much they want to prevent bites while the vaccination coverage is fixed. Both and are decreasing functions of and . (c) Nash equilibrium of the general game (black dot) when individuals can choose vaccination and bite prevention. The Nash equilibrium is at the intersection of the curves from (a,b).
When , there is no disease in the population and thus . Similarly, when is fixed and is close enough to 1, . Thus, there are only two possibilities as illustrated in Figure 3, depending on the relation of to
where is an equilibrium value of when . We have
- (1)
- If , then vaccination is too expensive and the Nash equilibrium is to not vaccinate, even if , i.e., to not vaccinate for any and thus for all .
- (2)
- When , then and , i.e., the individuals should vaccinate (but not always) when and not vaccinate at all when .
This is illustrated in Figure 4a.
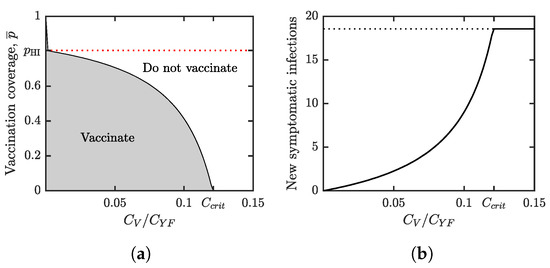
Figure 4.
(a) Parameter regions where voluntary vaccination is beneficial (gray) and not beneficial (white). The boundary between the two regions represents the optimal voluntary vaccination coverage, . The red dotted line is the vaccination coverage needed for herd immunity, . As increases, decreases while remains constant. When reaches the critical level given in (25), becomes 0. At that point, the relatively high vaccine cost makes “do not vaccinate” an optimal strategy from the individuals’ perspective. (b) As increases, the difference between and grows. This means that the number of price of anarchy increases. When reaches the critical level , becomes 0 and the price is maximal (shown by the dotted line). For our scenario, we evaluated the price of anarchy as the number of new symptomatic yellow fever infections per year per population when individuals use the Nash equilibrium level of protection. In both figures, and the values of all other parameters are as in Table 1.
Figure 4b shows the incidence rate of new YF infections in the population where individuals use the voluntary vaccination at the optimal (from the self-interest point of view) levels.
4.2. Optimal Bite Prevention
Here, we consider a game in which the individuals can only decide how much they prevent the vector bites. All other model parameters including are fixed.
Assume that the rest of the population is using , while the focal individual uses . The risk of infection for the focal individual is given by
The individual will choose that minimizes the overall costs, i.e., minimizes the function . We have
and thus . Hence, the minimum of h must occur either at or . The condition is equivalent to
Thus, the optimal choice for the focal individual is
Additionally, as increases, the right-hand side of (28) decreases. Thus is a non-increasing function of . Hence, as in the previous section, NE is unique and given by
Additionally, the right-hand side of (28) is decreasing in and thus and consequently is a non-increasing function of . This is shown in Figure 3b.
Similarly to the analysis in the previous section, the is CSNE.
The outcomes depend on the relationship between and the critical value defined in (25).
- (1)
- If , then for all , i.e., the cost of bite prevention is too high no matter what is the vaccination coverage in the population.
- (2)
- If , then and , i.e., the individuals should somewhat prevent vector bites (but never fully) if the vaccination coverage is relatively low, but do not prevent them at all once the vaccination coverage is above a certain threshold.
This is illustrated in Figure 5a.
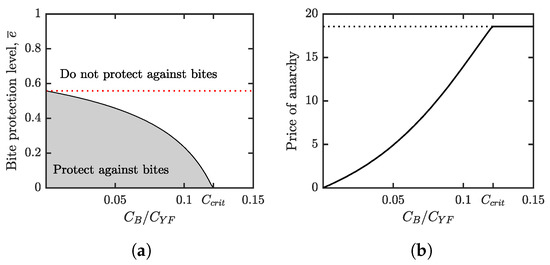
Figure 5.
(a) Parameter regions where voluntary bite protection is beneficial (gray) and not beneficial (white). The boundary between the two regions represents the optimal voluntary bite protection level, . The red dotted line is the bite protection level needed for YF elimination, . As increases, decreases while remains constant. When reaches the critical level , becomes 0, i.e., at that point, the relatively high cost of bite protection makes “do not protect against bites” an optimal strategy from the individuals’ perspective. (b) The price of anarchy is again evaluated as the number of new symptomatic yellow fever infections per year per population when individuals use the Nash equilibrium level of protection. As increases, the difference between and grows. This means that the number of price of anarchy increases. When reaches the critical level , becomes 0 and the price is maximal (shown by the dotted line). In both figures, and the values of all other parameters are as in Table 1.
Figure 5b shows the incidence rate of new YF infections in the population where individuals use the voluntary vaccination at the optimal (from the self-interest point of view) levels.
4.3. Optimal Vaccination and Bite Prevention
Here, we assume that individuals decide whether to vaccinate as well as whether to prevent the bites. The strategy is thus given by a pair and the NE must satisfy that is a NE of the game when is fixed (as discussed in Section 4.1) and is a NE of the game when is fixed (as discussed in Section 4.2). As demonstrated in Figure 3c, only the equilibrium is possible, although there is a relatively narrow region of values for which up to three NEs are possible. A similar situation is discussed in more detail in [44].
4.4. Validation
For the parameter values given in Table 1, Formula (11) yields , i.e., a single infectious case results in secondary infections [43]. This is reasonably close to previous modeling studies estimating the number of secondary infections around 5 or more [18,21,22].
Our model also predicts that one needs about 80% of people to be vaccinated for , which is in agreement with [10].
Finally, the game theoretical analysis predicts the NE to be around 65%. This is in a reasonable agreement with about 68% in Angola overall [45]. Additionally, following the 2016 YF outbreak, the vaccination coverage in Luanda province shot up from 57.9% to 92.9% in 2017 and has been on a slow but steady decline to 89.1% in 2022, while the overall vaccine coverage in Angola has been slowly rising. This may demonstrate the voluntary choice of new individuals in Luanda province to not get vaccinated, given the already high vaccination coverage there.
4.5. Sensitivity Analysis
We performed a sensitivity analysis based on [46]. The sensitivity index of on parameter x is calculated as . The sensitivity index means that 1% increase in a parameter value x will result in a 0.5% decrease in . The calculations of the sensitivity of are analogous. The results are shown in Figure 6.
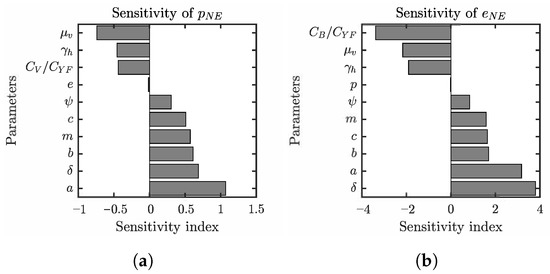
Figure 6.
Summaries of the sensitivity analysis of (a) and (b) based on [46]. Only parameters for which the absolute value of the sensitivity index is greater than 0.005 are shown. The sensitivity index of on a parameters x calculated as . The sensitivity index means that 1% increase in a parameter value x will result in a 0.5% decrease in . The values of parameters that are not varied are as specified in Table 1.
We can see that the sensitivities of and on various parameter values are similar, although the sensitivity indices of are roughly three to four times as much as those for . The NE values increase most with the increase in the mosquito biting rate a, but are also quite sensitive to the transmission probabilities b and c, and the number of mosquitoes per human. On the other hand, the equilibrium values decrease with the increased mortality of mosquitoes, and the shortening of the human incubation period. In all of these instances, the more likely it is to contract the disease, the higher the value of NE will be.
Similarly, decreasing the cost of vaccination or the bite protection (relative to the cost of the disease) also increases the NE values and lowers the incidence rates at this voluntary equilibrium.
5. Conclusions and Discussion
In this paper, we adapted the compartment model of yellow fever transmission developed by [18]. We added the game-theoretic component and used the model to study the voluntary prevention strategies by vaccination or bite protection. We calibrated the model based on the values from the literature and mostly in agreement with the parameter values used in [18]. We validated the model prediction based on the current vaccination coverage and trends in Angola.
The model shows that vaccination, as opposed to bite protection, should be a preferred way to protect against the disease. In agreement with previous studies [10], our model predicts that one needs about 80% vaccination coverage to contain the outbreaks of yellow fever in urban areas. This is in agreement with previous models of YF without the game theoretical component. Our model indicates that from the individual perspective, the optimal vaccination coverage is about 65%, which is in a reasonable agreement with the actual coverage in Angola. Thus, we show that voluntary vaccination alone is not enough to eliminate yellow fever from endemic areas and to mitigate the risks of outbreaks. These predictions are in line with similar studies on other vector borne diseases [47,48,49] or diseases when the cost of protection is relatively high, such as typhoid [50].
Studies on the prevention of other vector-borne diseases such as chagas disease [51] suggest that individuals behave rationally and that the actual level of protective action depends on the cost of the protection. Thus, from the policy making perspective, the vaccine should be made available for the population at as little cost as possible. This will align the selfish optimal voluntary vaccination coverage with the societal optimum of herd-immunity coverage.
Our model has several limitations. We assumed a homogeneous, well-mixed population and, as a result, we obtained a single Nash equilbrium. To model real-world populations, complex networks provide a better platform [24,52]. Heterogeneity in the population yields heterogeneity in vaccinating actions [53]. The individuals with many contacts may have higher inclination to voluntarily vaccinate than individuals with fewer contacts, and this can largely inhibit the outbreaks [54]. At the same time, we note that YF is transmitted by mosquitoes rather than directly from a person to another person. Thus the usual social network methodology may not apply in a straightforward way. The use of the multi-agent-simulation (MAS) methodology [55,56,57,58,59,60,61] would possibly allow for much higher flexibility and realism in the modeling approach, taking into account both geographical and social heterogeneity. This approach could also capture the qualitative difference between vaccination, which is a one-time decision, and bite protection, which is a repeated action.
Our model can be extended in several ways. Vector control is another preventative measure that could be considered. The control is closely linked to the social structure and living conditions. We focused on urban transmission and thus ignored the sylvanic cycle with non-human primates acting as alternative hosts for yellow fever. Incorporating the primates into the model is, thus, the next natural step which will account for the fact that as cities grow, they become increasingly connected to areas with YF potential. Additionally, the model should account for intensified population movement to the cities from rural areas [10].
Author Contributions
Conceptualization, L.J.C.A., H.O., J.R. and D.T.; Formal analysis, J.A.S.C., B.M.J., H.J.K., J.R.U.M., L.J.C.A., H.O., J.R. and D.T.; Funding acquisition, L.J.C.A., H.O. and D.T.; Investigation, J.A.S.C., B.M.J. and J.R.U.M.; Methodology, L.J.C.A., H.O., J.R. and D.T.; Project administration, L.J.C.A. and H.O.; Software, J.R.; Supervision, L.J.C.A. and H.O.; Visualization, D.T.; Writing—original draft, J.A.S.C., B.M.J., H.J.K., J.R.U.M., L.J.C.A., H.O., J.R. and D.T.; Writing—review & editing, J.R. and D.T. All authors have read and agreed to the published version of the manuscript.
Funding
Aaron S. Caasi, Brian Joseph, Heera J. Kodiyamplakkal, and Jaelene Renae U. Manibusan worked on the research as part of the NREUP, a program of the Mathematical Association of America, funded by the National Science Foundation grant DMS1652506. Their work was supervised by research mentors Hyunju Oh and Leslie J. Camacho Aquino. Dewey Taylor was supported by the National Science Foundation grant DMS1950015. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors would like to thank anonymous reviewers and the editor for their positive comments.
Conflicts of Interest
J.R. is a member of the editorial board of Games. The remaining authors have declared no competing interests.
References
- PAHO/WHO. Yellow Fever. 2022. Available online: https://www.paho.org/en/topics/yellow-fever (accessed on 5 June 2022).
- Monath, T.P.; Vasconcelos, P.F. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Yellow fever: An update. Lancet Infect. Dis. 2001, 1, 11–20. [Google Scholar] [CrossRef]
- WHO. Yellow Fever Fact Sheet. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/yellow-fever (accessed on 5 June 2022).
- WHO. Yellow Fever, Q&A. 2017. Available online: https://www.who.int/news-room/questions-and-answers/item/yellow-fever (accessed on 5 June 2022).
- Robert, M.A.; Stewart-Ibarra, A.M.; Estallo, E.L. Climate change and viral emergence: Evidence from Aedes-borne arboviruses. Curr. Opin. Virol. 2020, 40, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.A.; Christofferson, R.C.; Weber, P.D.; Wearing, H.J. Temperature impacts on dengue emergence in the United States: Investigating the role of seasonality and climate change. Epidemics 2019, 28, 100344. [Google Scholar] [CrossRef]
- Robert, M.A.; Christofferson, R.C.; Silva, N.J.; Vasquez, C.; Mores, C.N.; Wearing, H.J. Modeling mosquito-borne disease spread in US urbanized areas: The case of dengue in Miami. PLoS ONE 2016, 11, e0161365. [Google Scholar] [CrossRef]
- Barrett, A.D. Yellow fever in Angola and beyond—the problem of vaccine supply and demand. N. Engl. J. Med. 2016, 375, 301–303. [Google Scholar] [CrossRef]
- WHO. A global strategy to Eliminate Yellow Fever Epidemics (EYE) 2017–2026. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/272408/9789241513661-eng.pdf (accessed on 5 June 2022).
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Behrend, M.R.; Basáñez, M.G.; Hamley, J.I.; Porco, T.C.; Stolk, W.A.; Walker, M.; de Vlas, S.J.; Consortium, N.M. Modelling for policy: The five principles of the Neglected Tropical Diseases Modelling Consortium. PLoS Negl. Trop. Dis. 2020, 14, e0008033. [Google Scholar] [CrossRef]
- Raimundo, S.M.; Amaku, M.; Massad, E. Equilibrium analysis of a yellow fever dynamical model with vaccination. Comput. Math. Methods Med. 2015, 2015, 482091. [Google Scholar]
- Kung’aro, M.; Luboobi, L.S.; Shahada, F. Modelling and stability analysis of SVEIRS yellow fever two host model. Gulf J. Math. 2015, 3, 106–129. [Google Scholar]
- Yusuf, T.T.; Daniel, D.O. Mathematical modeling of yellow fever transmission dynamics with multiple control measures. Asian Res. J. Math. 2019, 13, 1–15. [Google Scholar] [CrossRef]
- Danbaba, U.; Garba, S. Stability analysis and optimal control for yellow fever model with vertical transmission. Int. J. Appl. Comput. Math. 2020, 6, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, S.M.; Yang, H.M.; Massad, E. Modeling vaccine preventable vector-borne infections: Yellow fever as a case study. J. Biol. Syst. 2016, 24, 193–216. [Google Scholar] [CrossRef]
- Zhao, S.; Stone, L.; Gao, D.; He, D. Modelling the large-scale yellow fever outbreak in Luanda, Angola, and the impact of vaccination. PLoS Negl. Trop. Dis. 2018, 12, e0006158. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Massad, E. Estimating the number of unvaccinated Chinese workers against yellow fever in Angola. BMC Infect. Dis. 2018, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Musa, S.S.; Hebert, J.T.; Cao, P.; Ran, J.; Meng, J.; He, D.; Qin, J. Modelling the effective reproduction number of vector-borne diseases: The yellow fever outbreak in Luanda, Angola 2015–2016 as an example. PeerJ 2020, 8, e8601. [Google Scholar] [CrossRef]
- Wu, J.T.; Peak, C.M.; Leung, G.M.; Lipsitch, M. Fractional dosing of yellow fever vaccine to extend supply: A modelling study. Lancet 2016, 388, 2904–2911. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Faria, N.R.; Reiner, R.C., Jr.; Golding, N.; Nikolay, B.; Stasse, S.; Johansson, M.A.; Salje, H.; Faye, O.; Wint, G.W. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–2016: A modelling study. Lancet Infect. Dis. 2017, 17, 330–338. [Google Scholar] [CrossRef]
- Bauch, C.T.; Earn, D.J. Vaccination and the theory of games. Proc. Natl. Acad. Sci. USA 2004, 101, 13391–13394. [Google Scholar] [CrossRef]
- Wang, Z.; Bauch, C.T.; Bhattacharyya, S.; d’Onofrio, A.; Manfredi, P.; Perc, M.; Perra, N.; Salathé, M.; Zhao, D. Statistical physics of vaccination. Phys. Rep. 2016, 664, 1–113. [Google Scholar] [CrossRef]
- Verelst, F.; Willem, L.; Beutels, P. Behavioural change models for infectious disease transmission: A systematic review (2010–2015). J. R. Soc. Interface 2016, 13, 20160820. [Google Scholar] [CrossRef]
- Chang, S.L.; Piraveenan, M.; Pattison, P.; Prokopenko, M. Game theoretic modelling of infectious disease dynamics and intervention methods: A review. J. Biol. Dyn. 2020, 14, 57–89. [Google Scholar] [CrossRef] [PubMed]
- Brettin, A.; Rossi-Goldthorpe, R.; Weishaar, K.; Erovenko, I.V. Ebola could be eradicated through voluntary vaccination. R. Soc. Open Sci. 2018, 5, 171591. [Google Scholar] [CrossRef] [PubMed]
- Agusto, F.B.; Erovenko, I.V.; Fulk, A.; Abu-Saymeh, Q.; Romero-Alvarez, D.; Ponce, J.; Sindi, S.; Ortega, O.; Saint Onge, J.M.; Peterson, A.T. To isolate or not to isolate: The impact of changing behavior on COVID-19 transmission. BMC Public Health 2022, 22, 1–20. [Google Scholar] [CrossRef]
- Choi, W.; Shim, E. Optimal strategies for social distancing and testing to control COVID-19. J. Theor. Biol. 2021, 512, 110568. [Google Scholar] [CrossRef] [PubMed]
- Piraveenan, M.; Sawleshwarkar, S.; Walsh, M.; Zablotska, I.; Bhattacharyya, S.; Farooqui, H.H.; Bhatnagar, T.; Karan, A.; Murhekar, M.; Zodpey, S. Optimal governance and implementation of vaccination programmes to contain the COVID-19 pandemic. R. Soc. Open Sci. 2021, 8, 210429. [Google Scholar] [CrossRef] [PubMed]
- Bankuru, S.V.; Kossol, S.; Hou, W.; Mahmoudi, P.; Rychtář, J.; Taylor, D. A game-theoretic model of Monkeypox to assess vaccination strategies. PeerJ 2020, 8, e9272. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.R.M.; Foster, A.O.; Feagins, D.A.; Rowell, J.T.; Erovenko, I.V. Optimal voluntary and mandatory insect repellent usage and emigration strategies to control the chikungunya outbreak on Reunion Island. PeerJ 2020, 8, e10151. [Google Scholar] [CrossRef]
- Scheckelhoff, K.; Ejaz, A.; Erovenko, I.V.; Rychtář, J.; Taylor, D. Optimal Voluntary Vaccination of Adults and Adolescents Can Help Eradicate Hepatitis B in China. Games 2021, 12, 82. [Google Scholar] [CrossRef]
- Kobe, J.; Pritchard, N.; Short, Z.; Erovenko, I.V.; Rychtář, J.; Rowell, J.T. A game-theoretic model of cholera with optimal personal protection strategies. Bull. Math. Biol. 2018, 80, 2580–2599. [Google Scholar] [CrossRef]
- World Bank. Birth Rate, Angola. 2020. Available online: https://data.worldbank.org/indicator/SP.DYN.CBRT.IN?locations=AO (accessed on 5 June 2022).
- World Bank. Life Expectancy, Angola. 2020. Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=AO (accessed on 5 June 2022).
- Andraud, M.; Hens, N.; Marais, C.; Beutels, P. Dynamic epidemiological models for dengue transmission: A systematic review of structural approaches. PLoS ONE 2012, 7, e49085. [Google Scholar] [CrossRef]
- Chikaki, E.; Ishikawa, H. A dengue transmission model in Thailand considering sequential infections with all four serotypes. J. Infect. Dev. Ctries. 2009, 3, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Arana-Vizcarrondo, N.; Biggerstaff, B.J.; Staples, J.E. Incubation periods of yellow fever virus. Am. J. Trop. Med. Hyg. 2010, 83, 183. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Treatment of yellow fever. Antivir. Res. 2008, 78, 116–124. [Google Scholar] [CrossRef] [PubMed]
- CDC. Yellow Fever, Q&A. 2019. Available online: https://www.cdc.gov/yellowfever/ (accessed on 5 June 2022).
- Ankrah, D. PIN41 Cost-Effectiveness of Vaccination Against Yellow Fever in Ghana. Value Health 2012, 15, A244. [Google Scholar] [CrossRef][Green Version]
- Van den Driessche, P.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Campo, V.N.; Palacios, J.L.D.; Nagahashi, H.; Oh, H.; Rychtář, J.; Taylor, D. A game-theoretic model of rabies in domestic dogs with multiple voluntary preventive measures. J. Math. Biol. 2022, submitted.
- Imperial College, London. Yellow Fever Immunization Coverage Across Africa. 2022. Available online: https://polici.shinyapps.io/yellow_fever_africa/ (accessed on 5 June 2022).
- Arriola, L.; Hyman, J.M. Sensitivity analysis for uncertainty quantification in mathematical models. In Mathematical and Statistical Estimation Approaches in Epidemiology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 195–247. [Google Scholar]
- Dorsett, C.; Oh, H.; Paulemond, M.L.; Rychtář, J. Optimal repellent usage to combat dengue fever. Bull. Math. Biol. 2016, 78, 916–922. [Google Scholar] [CrossRef]
- Fortunato, A.K.; Glasser, C.P.; Watson, J.A.; Lu, Y.; Rychtář, J.; Taylor, D. Mathematical modelling of the use of insecticide-treated nets for elimination of visceral leishmaniasis in Bihar, India. R. Soc. Open Sci. 2021, 8, 201960. [Google Scholar] [CrossRef]
- Angina, J.; Bachhu, A.; Talati, E.; Talati, R.; Rychtář, J.; Taylor, D. Game-theoretical model of the voluntary use of insect repellents to prevent Zika fever. Dyn. Games Appl. 2022, 12, 133–146. [Google Scholar] [CrossRef]
- Acosta-Alonzo, C.B.; Erovenko, I.V.; Lancaster, A.; Oh, H.; Rychtář, J.; Taylor, D. High endemic levels of typhoid fever in rural areas of Ghana may stem from optimal voluntary vaccination behaviour. Proc. R. Soc. A 2020, 476, 20200354. [Google Scholar] [CrossRef]
- Han, C.Y.; Issa, H.; Rychtář, J.; Taylor, D.; Umana, N. A voluntary use of insecticide treated nets can stop the vector transmission of Chagas disease. PLoS Negl. Trop. Dis. 2020, 14, e0008833. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Fu, Z.; Wang, B. Epidemic dynamics on complex networks. Prog. Nat. Sci. 2006, 16, 452–457. [Google Scholar]
- Fu, F.; Rosenbloom, D.I.; Wang, L.; Nowak, M.A. Imitation dynamics of vaccination behaviour on social networks. Proc. R. Soc. B Biol. Sci. 2011, 278, 42–49. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Zhou, C.; Small, M.; Wang, B. Hub nodes inhibit the outbreak of epidemic under voluntary vaccination. New J. Phys. 2010, 12, 023015. [Google Scholar] [CrossRef]
- Iwamura, Y.; Tanimoto, J. Realistic decision-making processes in a vaccination game. Phys. A Stat. Mech. Appl. 2018, 494, 236–241. [Google Scholar] [CrossRef]
- Kabir, K.A.; Jusup, M.; Tanimoto, J. Behavioral incentives in a vaccination-dilemma setting with optional treatment. Phys. Rev. E 2019, 100, 062402. [Google Scholar] [CrossRef]
- Kabir, K.A.; Tanimoto, J. Modelling and analysing the coexistence of dual dilemmas in the proactive vaccination game and retroactive treatment game in epidemic viral dynamics. Proc. R. Soc. A 2019, 475, 20190484. [Google Scholar] [CrossRef]
- Kuga, K.; Tanimoto, J.; Jusup, M. To vaccinate or not to vaccinate: A comprehensive study of vaccination-subsidizing policies with multi-agent simulations and mean-field modeling. J. Theor. Biol. 2019, 469, 107–126. [Google Scholar] [CrossRef]
- Arefin, M.R.; Masaki, T.; Kabir, K.A.; Tanimoto, J. Interplay between cost and effectiveness in influenza vaccine uptake: A vaccination game approach. Proc. R. Soc. A 2019, 475, 20190608. [Google Scholar] [CrossRef]
- Arefin, M.R.; Kabir, K.A.; Tanimoto, J. A mean-field vaccination game scheme to analyze the effect of a single vaccination strategy on a two-strain epidemic spreading. J. Stat. Mech. Theory Exp. 2020, 2020, 033501. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Xia, C. Role of vaccine efficacy in the vaccination behavior under myopic update rule on complex networks. Chaos Solitons Fractals 2020, 130, 109425. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).