Development of an Early Lung Cancer Diagnosis Method Based on a Neural Network
Abstract
1. Introduction
2. Materials and Methods
3. Results
- Class 1: Methods applicable across various localizations.
- Class 2: Methods applicable across fewer localizations.
- Class 3: Methods applicable exclusively to specific localizations.
| Class | Approaches |
|---|---|
| Class 1 | Neural network approach |
| Class 2 | Approach using factor analysis and physical research methods |
| Class 3 | Full formalization, associative rules, semantic networks, natural language analysis, and fuzzy logic approaches |
- Knowledge acquisition and analysis, identification, and conceptualization.
- Knowledge modeling and formalization.
- Development of a script-based question database and neural network training.
- Creation of an intelligent system prototype.
- Knowledge acquisition and analysis, identification, and conceptualization.
- 2.
- Knowledge modeling and formalization.
- 3.
- Development of a script-based question database and neural network training.
- 4.
- Creation of an intelligent system prototype.
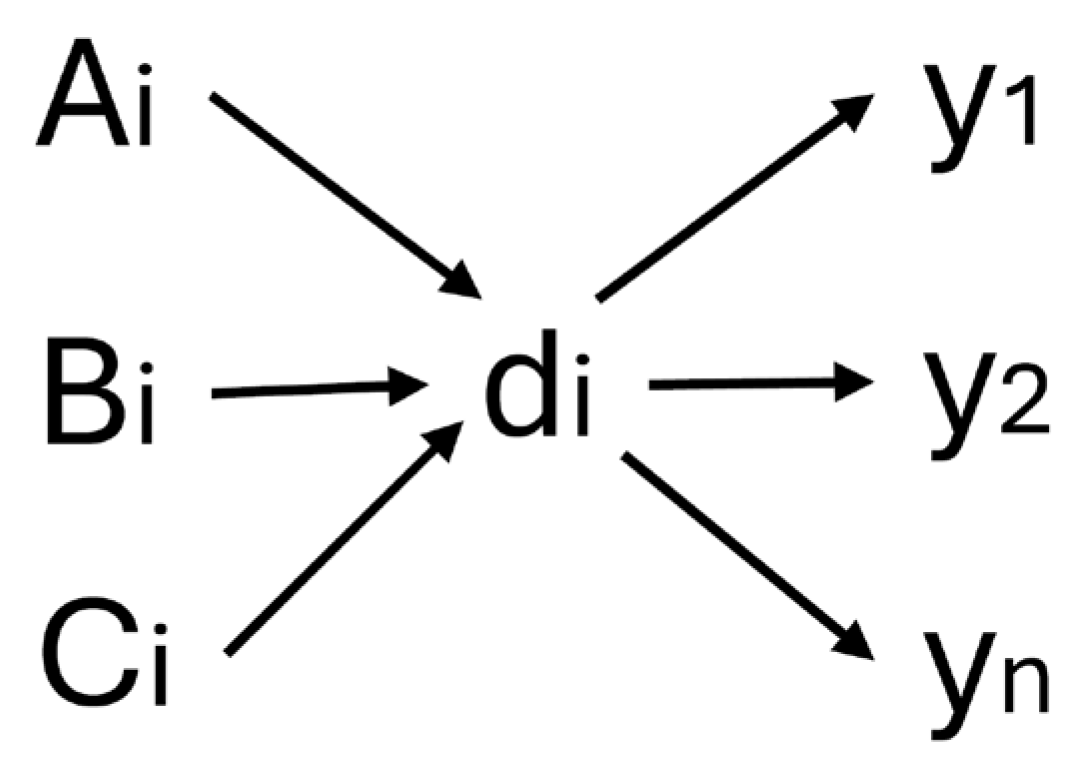
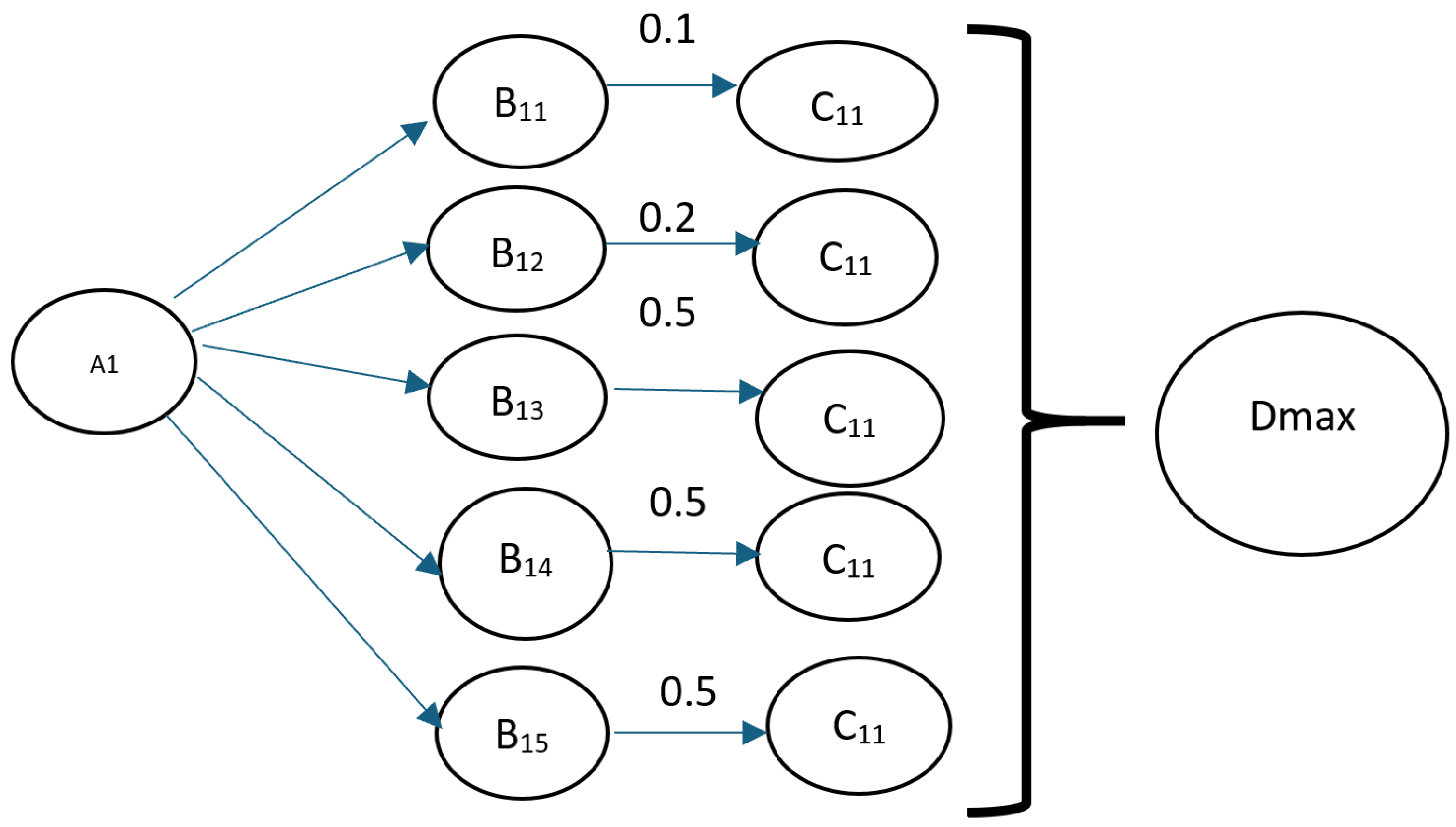
- A is the set of questions on the oncologic history.
- B is the set of answers on the oncologic history.
- C is the set of weights of answers on the oncologic history.
- E is the set of diagnoses.
- Robustness to Input Noise.
- 2.
- Simplicity of Implementation.
- -
- The first layer computes the correlation between the input vector and the reference patterns.
- -
- The second layer implements a winner-takes-all mechanism, selecting the closest matching pattern.
- 3.
- High Recognition Speed.
- 4.
- Efficiency with Small Datasets.
- 5.
- Applicability to Classification and Recognition Tasks.
- Pattern and character recognition.
- Signal and time-series analysis.
- Data integrity verification.
- Development of systems with limited computational resources.
- numeric_features = [
- “ Your age ”,
- “ Smoking experience ”,
- “ How many packs/pieces do you consume per day? ”,
- “ How many times a year do you get SARS?”
- X_train, X_test, y_train, y_test = train_test_split(
- X_train_full,
- y_train_full,
- test_size = 0.2,
- random_state = 42
- )
- categorical_features = [
- “ Your gender ”,
- “Oncoanamnesis of parents and close relatives? ”,
- “ Do you use cigarettes? ”
- ]
- -
- Insufficient amount of input data, as the survey was conducted among residents of regions affected by nuclear testing.
- -
- A low number of positive responses indicating cancer incidence. Since the majority of respondents were healthy, the percentage of positive cases was consequently low.
- Class Imbalance Adjustment:
- 2.
- Use of Additional Metrics:
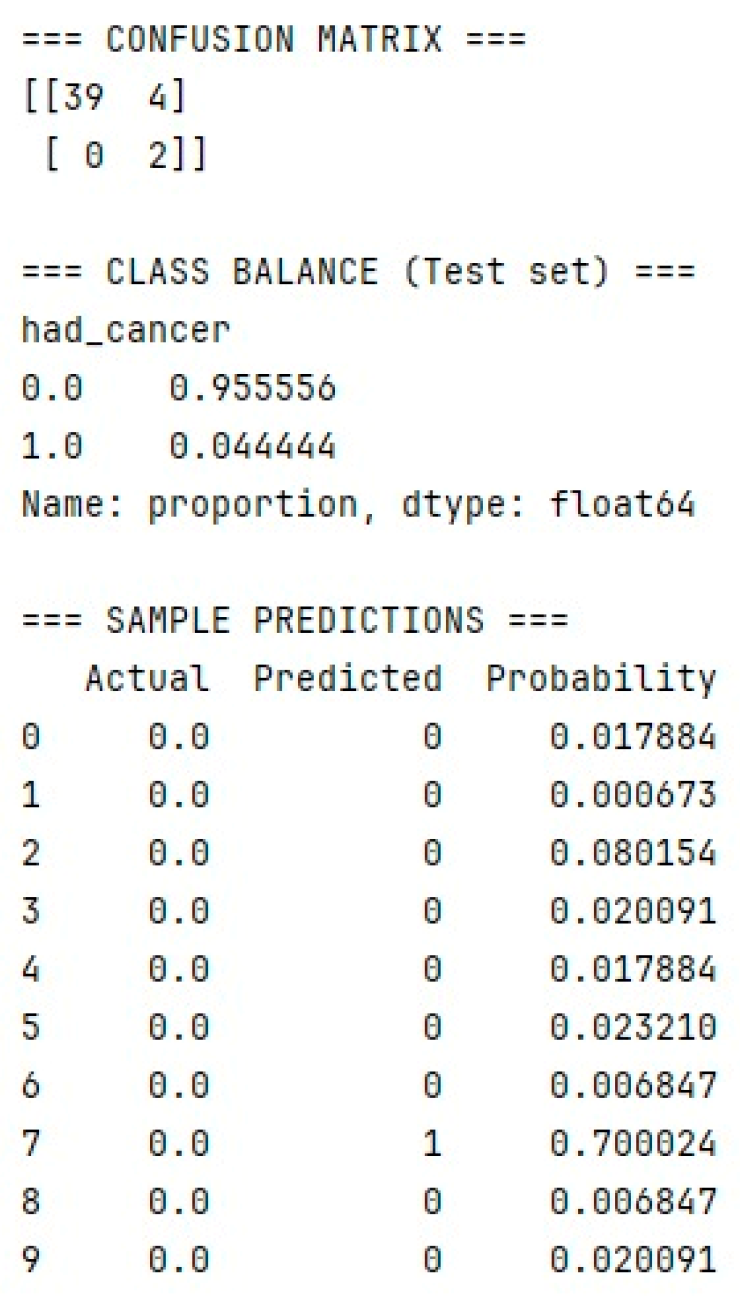
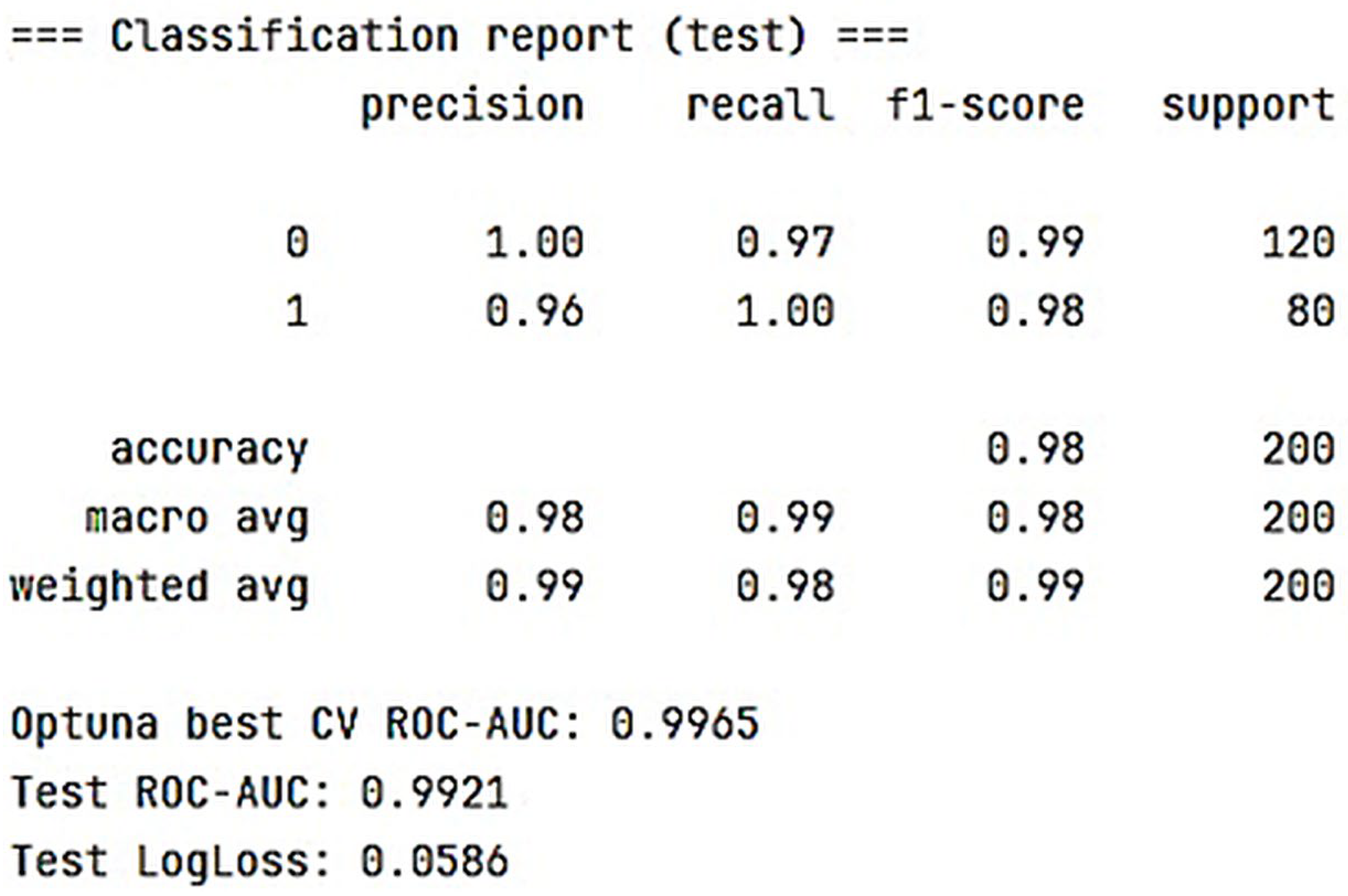
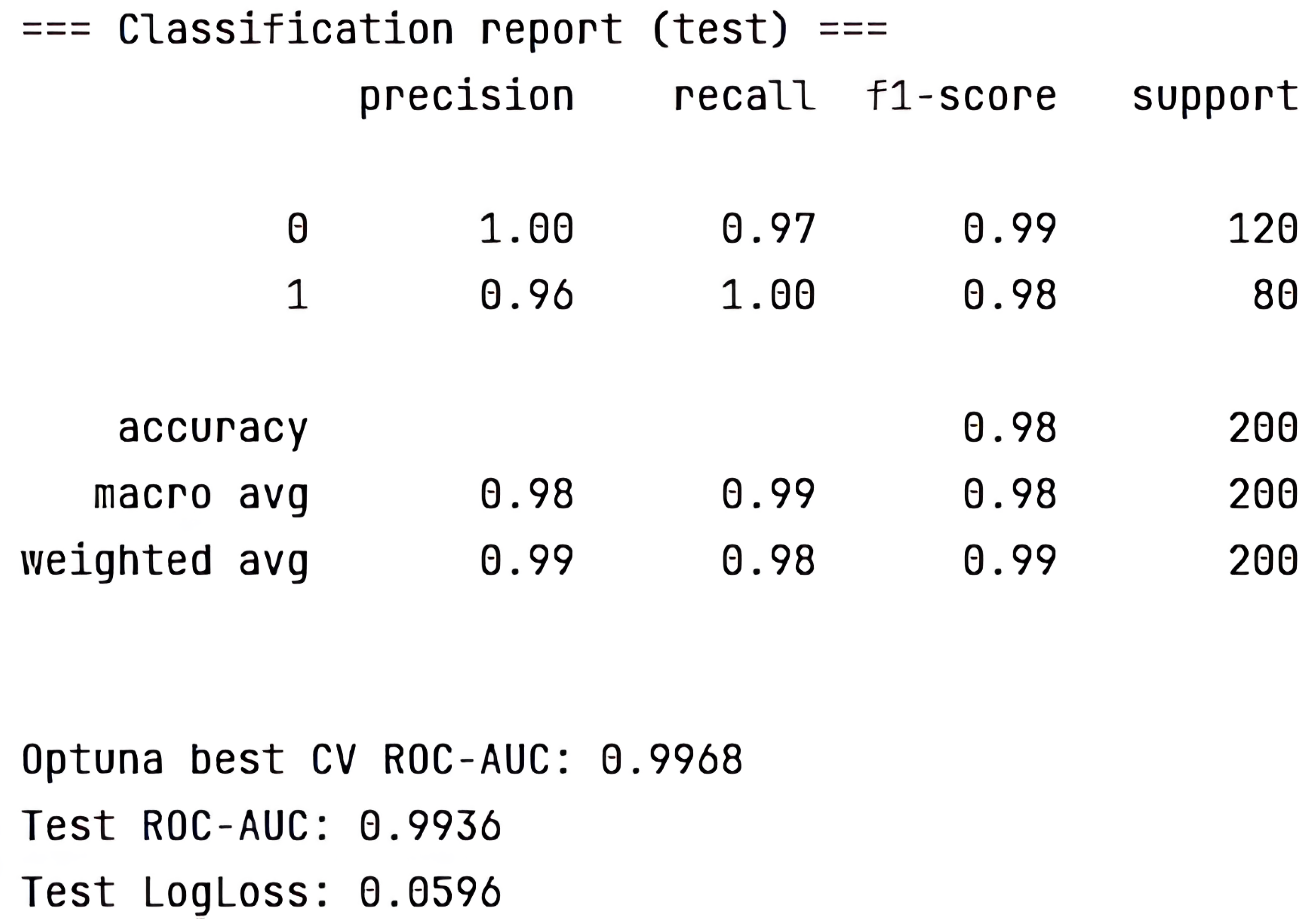
- Gradient boosting using LightGBM: Cross-Validation ROC-AUC (best from Optuna) = 0.9059; Test ROC-AUC = 1.0000.
- Multilayer perceptron model implemented in Keras: ROC-AUC (sklearn) = 0.9459.
- Hamming network model: ROC-AUC (sklearn) = 0.95.
- 3.
- Classification Threshold Adjustment:
- 4.
- Model Architecture Improvement:
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| DSS | decision support systems |
| AR | association rules |
| NN | neural networks |
| NLP | natural language processing |
| DDSS | diagnostic decision support systems |
| ACO | Ant Colony Optimization |
References
- Benusiglio, P.R.; Fallet, V.; Sanchis-Borja, M.; Coulet, F.; Cadranel, J. Lungcancer isalsoahereditary disease. Eur. Respir. Rev. 2021, 30, 210045. [Google Scholar] [CrossRef]
- Karabatak, M.; Ince, M.C. An expert system for detection of breast cancer based on association rules and neural network. Expert Syst. Appl. 2009, 36, 3465–3469. [Google Scholar] [CrossRef]
- Oyelade, O.N.; Obiniyi, A.A.; Junaidu, S.B.; Adewuyi, S.A. Patient symptoms elicitation process for breast cancer medical expert systems: A semantic web and natural language parsing approach. Future Comput. Inform. J. 2018, 3, 72–81. [Google Scholar] [CrossRef]
- Goldshtein, M.; Amin, G.; Rod, D. An NLP-Based Exploration of Variance in Student Writing and Syntax: Implications for Automated Writing Evaluation. Computers 2024, 13, 160. [Google Scholar] [CrossRef]
- Hassan, L.; Saleh, A.; Singh, V.K.; Puig, D.; Abdel-Nasser, M. Detecting Breast Tumors in Tomosynthesis Images Utilizing Deep Learning-Based Dynamic Ensemble Approach. Computers 2023, 12, 220. [Google Scholar] [CrossRef]
- Gavade, A.B.; Nerli, R.; Kanwal, N.; Gavade, P.A.; Pol, S.S.; Rizvi, S.T.H. Automated Diagnosis of Prostate Cancer Using mpMRI Images: A Deep Learning Approach for Clinical Decision Support. Computers 2023, 12, 152. [Google Scholar] [CrossRef]
- Kalra, G.; Rajoria, Y.K.; Boadh, R.; Rajendra, P.; Pandey, P.; Khatak, N.; Kumar, A. Study of fuzzy expert systems towards prediction and detection of fraud case in health care insurance. Mater. Today Proc. 2022, 56, 477–480. [Google Scholar] [CrossRef]
- Bakhtadze, N.N.; Belenkiy, V.M.; Pyatetsky, V.E.; Sakrutina, E.A.; Nikulina, I.V. Intelligent Screening for Diagnostic and Treatment of Cancer Diseases. Procedia Comput. Sci. 2017, 112, 1238–1245. [Google Scholar] [CrossRef]
- Keleş, A.; Keleş, A.; Yavuz, U. Expert system based on neuro-fuzzy rules for diagnosis breast cancer. Expert Syst. Appl. 2011, 38, 5719–5726. [Google Scholar] [CrossRef]
- Apasiya, E.; Salifu, A.; Agbedemnab, P. New Approaches to the Prognosis and Diagnosis of Breast Cancer Using Fuzzy Expert Systems. J. Comput. Commun. 2024, 12, 151–169. [Google Scholar] [CrossRef]
- Safdari, R.; Arpanahi, H.K.; Langarizadeh, M.; Ghazisaiedi, M.; Dargahi, H.; Zendehdel, K. Design a Fuzzy Rule-based Expert System to Aid Earlier Diagnosis of Gastric Cancer. Acta Inform. Medica 2018, 26, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Übeyli, E.D. Implementing automated diagnostic systems for breast cancer detection. Expert Syst. Appl. 2007, 33, 1054–1062. [Google Scholar] [CrossRef]
- Houssami, N.; Lee, C.I.; Buist, D.S.M.; Tao, D. Artificial intelligence for breast cancer screening: Opportunity or hype? Breast 2017, 36, 31–33. [Google Scholar] [CrossRef]
- Teramoto, A.; Kiriyama, Y.; Michiba, A.; Yazawa, N.; Tsukamoto, T.; Imaizumi, K.; Fujita, H. Automated Generation of Lung Cytological Images from Image Findings Using Text-to-Image Technology. Computers 2024, 13, 303. [Google Scholar] [CrossRef]
- Melyani, M.; Frasetyo, T.; Rahadjeng, I.; Mufid, Z.; Rafik, A.; Shaura, R.; Daniel, D.; Emita, I. Design Framework of Expert System Program in Otolaryngology Disease Diagnosis use Extreme Programming (XP) Method (Case Study in THB Bekasi Hospital). J. Technol. Inform. Eng. 2024, 3, 397. [Google Scholar] [CrossRef]
- Kunhimangalam, R.; Ovallath, S.; Joseph, P.K. A Novel Fuzzy Expert System for the Identification of Severity of Carpal Tunnel Syndrome. BioMed Res. Int. 2013, 2013, 846780. [Google Scholar] [CrossRef]
- Gashteroodkhani, O.; Majidi, M.; Etezadi-Amoli, M. A Fuzzy-based Control Scheme for Recapturing Waste Energy in Water Pressure Reducing Valves. In Proceedings of the IEEE Power and Energy Society General Meeting (PESGM), Portland, OR, USA, 5–10 August 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Wójcik, W.; Mezhiievska, I.; Pavlov, S.V.; Lewandowski, T.; Vlasenko, O.V.; Maslovskyi, V.; Volosovych, O.; Kobylianska, I.; Moskovchuk, O.; Ovcharuk, V.; et al. Medical Fuzzy-Expert System for Assessment of the Degree of Anatomical Lesion of Coronary Arteries. Int. J. Environ. Res. Public Health 2023, 20, 979. [Google Scholar] [CrossRef]
- Mazhar, T.; Nasir, Q.; Haq, I.; Kamal, M.M.; Ullah, I.; Kim, T.; Mohamed, H.G.; Alwadai, N. A Novel Expert System for the Diagnosis and Treatment of Heart Disease. Electronics 2022, 11, 3989. [Google Scholar] [CrossRef]
- Aguilera-Venegasa, G.; Roanes-Lozanob, E.; Rojo-Martínezc, G.; Galán-Garcíaa, J. A proposal of a mixed diagnostic system based on decision trees andprobabilistic experts rules. J. Comput. Appl. Math. 2023, 427, 115130. [Google Scholar] [CrossRef]
- Sarabi, S.; Han, Q.; Vries, B.; Georges, L. Methodology for development of an expert system to derive knowledge from existing nature-based solutions experiences. MethodsX 2023, 10, 101978. [Google Scholar] [CrossRef]
- Mustafa, E.; Saad, M.; Rizkallah, L. Building an enhanced case-based reasoning and rule-based systems for medical diagnosis. J. Eng. Appl. Sci. 2023, 70, 139. [Google Scholar] [CrossRef]
- Tariq, R. Make Your Own Neural Network; CreateSpace Independent Publishing Platform: North Charleston, SC, USA, 2016; p. 222. [Google Scholar]
- Chen, M.; Challita, U.; Saad, W.; Yin, C.; Debbah, M. Artificial Neural Networks-Based Machine Learning for Wireless Networks: A Tutorial. IEEE Commun. Surv. Tutor. 2019, 21, 3039–3071. [Google Scholar] [CrossRef]
- Molina, E.; Parraga-Alava, J. Redes Neuronales Artificiales en la Resolución de Problemas de Clasificación: Una Revisión Sistemática de la Literatura. ENFOQUE UTE 2024, 15, 1–10. [Google Scholar] [CrossRef]
- Vásquez-Coronel, J.A.; Mora, M.; Vilches, K. A Review of multilayer extreme learning machine neural networks. Artif. Intell. Rev. 2023, 56, 13691. [Google Scholar] [CrossRef]
- Syahidin, Y.; Ismail, A.; Siraj, F. Application of Artificial Neural Network Algorithms to Heart Disease Prediction Models with Python Programming. J. E-Komtek 2022, 6, 292–302. [Google Scholar] [CrossRef]
- Wang, Y.H.; Lin, G.Y. Design of Medical Diagnostic System Based on Artificial Intelligence. Int. J. Appl. Inf. Manag. 2023, 3, 170–176. [Google Scholar] [CrossRef]
- Tiwari, A.; Mishra, S.; Kuo, T.R. Current AI technologies in cancer diagnostics and treatment. Mol. Cancer 2025, 24, 159. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial intelligence assists precision medicine in cancer treatment. Front. Oncol. 2023, 12, 998222. [Google Scholar] [CrossRef]
- Adilgazyuly, S.; Bulegenov, T.; Mussakhanova, A.; Kenesh Dzhusupov, K.; Altybayeva Zh Medeulova, A.; Tokenova Zh Karymsakova, I.; Uruzbayeva, G.; Kussainova, A. Assessment of physicians’ perspectives on the quality and accessibility of rehabilitation services for lung cancer patients across all levels of healthcare delivery in the Republic of Kazakhstan. Cent. Asian J. Med. Hypotheses Ethics 2025, 6, 142–153. [Google Scholar] [CrossRef]








| Question | Answers | ||||
|---|---|---|---|---|---|
| Entry 1 | 1st answer | 2nd answer | 3rd answer | 4th answer | 5th answer |
| Age | 30–40 | 40–50 | 50–60 | 60 and above | 20–30 |
| 0.2 | 0.5 | 0.5 | 0.5 | ||
| Gender | Male | Female | |||
| 1 | 0.6 | ||||
| Do you smoke? | Yes | No | |||
| 1 | 0.5 | ||||
| Smoking experience | Up to 10 years | From 10 to 20 years | From 20 to 30 years | From 30 and above | I do not smoke |
| 0.7 | 0.9 | 1 | 1 | ||
| How many packs/pieces of cigarettes do you consume per day? | Up to 10 pieces | Up to 1 pack | 1 pack | From 1 pack and above | |
| 0.8 | 1 | 1 | 1 | ||
| How many times a year do you get sick with ARVI? | 1 time | 2 times | periodically | Was not sick | |
| 0.4 | 0.5 | 0.8 | 0.4 | ||
| Oncologic history of parents and close relatives | One of the parents | Both parents | Among close relatives on the maternal side | Among close relatives on the paternal side | No one has |
| 0.9 | 0.9 | 0.5 | 0.5 | ||
| Have you ever had hemoptysis? | Yes | No | |||
| 0.9 | 0.4 | ||||
| Do you ever have shortness of breath, a feeling of lack of air? | Periodically | Often | During physical exertion | ||
| 0.5 | 1 | 1 | |||
| Was there a change in the voice? | Yes | No | |||
| 0.8 | 0.2 | ||||
| Do you ever have weakness that is not related to anything? | Periodically | Often | No | ||
| 0.3 | 0.5 | 0.2 | |||
| Have you been coughing? | Not at all | A little | Not so little | Very much | |
| 0.2 | 0.2 | 0.3 | 0.8 | ||
| Have you had problems swallowing? | Not at all | A little | Not so little | Very much | |
| 0.3 | 0.3 | 0.4 | 1 | ||
| Have you had chest pain? | Not at all | A little | Not so little | Very much | |
| 0.5 | 0.6 | 1 | 1 | ||
| Have you had pain in your arm or shoulders? | Not at all | A little | Not so little | Very much | |
| 0.5 | 0.5 | 0.7 | 1 | ||
| Have you ever had a dry cough? | Not at all | A little | Not so little | Very much | |
| 0.4 | 0.6 | 0.8 | 0.8 | ||
| Have you experienced unrelated weight loss? | Not at all | A little | Not so little | Very much | |
| 0.5 | 0.5 | 1 | |||
| Have you experienced a loss of appetite? | Not at all | A little | Not so little | Very much | |
| 1 | 1 | 1 | 1 | ||
| Are you registered with a pulmonologist for other lung diseases? | Yes | No | |||
| 1 | 0.5 | ||||
| Birthplace | Region 1 | Region 2 | Region 3 | Region 4 | Other |
| 0.3 | 0.5 | 0.8 | 0.9 | 0.3 | |
| Place of residence | Region 1 | Region 2 | Region 3 | Region 4 | Other |
| 0.3 | 0.5 | 0.8 | 0.9 | 0.3 | |
| Question | Answers | ||||
|---|---|---|---|---|---|
| Entry 1 | 1st answer | 2nd answer | 3rd answer | 4th answer | 5th answer |
| Age | 30–40 | 40–50 | 50–60 | 60 and above | 20–30 |
| 12.3 | 18.3 | 18.7 | 11 | 19.6 | |
| Gender | Male | Female | |||
| 46.6 | 53.4 | ||||
| Do you smoke? | Yes | No | |||
| 24.7 | 75.3 | ||||
| Smoking experience | Up to 10 years | From 10 to 20 years | From 20 to 30 years | From 30 and above | I do not smoke |
| 9.4 | 5.7 | 6.6 | 1.9 | 76.4 | |
| How many packs/pieces of cigarettes do you consume per day? | Up to 10 pieces | Up to 1 pack | 1 pack | From 1 pack and above | |
| 25.4 | 41.3 | 9.5 | 23.8 | ||
| How many times a year do you get sick with ARVI? | 1 time | 2 times | periodically | Was not sick | |
| 26.5 | 22.8 | 19.2 | 31.5 | ||
| Oncologic history of parents and close relatives | One of the parents | Both parents | Among close relatives on the maternal side | Among close relatives on the paternal side | No one has |
| 15.1 | 4.1 | 5.5 | 7.8 | 67.6 | |
| Have you ever had hemoptysis? | Yes | No | |||
| 93.6 | 6.4 | ||||
| Do you ever have shortness of breath, a feeling of lack of air? | Periodically | Often | During physical exertion | ||
| 5.9 | 2.3 | 31.3 | 60.7 | ||
| Was there a change in the voice? | Yes | No | |||
| 18.7 | 81.3 | ||||
| Do you ever have weakness that is not related to anything? | Periodically | Often | No | ||
| 26.9 | 10.5 | 62.6 | |||
| Have you been coughing? | Not at all | A little | Not so little | Very much | |
| 31.1 | 53 | 8.2 | 7.8 | ||
| Have you had problems swallowing? | Not at all | A little | Not so little | Very much | |
| 63 | 26.9 | 7.3 | 2.7 | ||
| Have you had chest pain? | Not at all | A little | Not so little | Very much | |
| 60.3 | 31.5 | 5.5 | 2.7 | ||
| Have you had pain in your arm or shoulders? | Not at all | A little | Not so little | Very much | |
| 49.8 | 38.8 | 7.3 | 4.1 | ||
| Have you ever had a dry cough? | Not at all | A little | Not so little | Very much | |
| 40.2 | 48.9 | 6.4 | 4.6 | ||
| Have you experienced unrelated weight loss? | Not at all | A little | Not so little | Very much | |
| 72.1 | 19.6 | 8.2 | |||
| Have you experienced a loss of appetite? | Not at all | A little | Not so little | Very much | |
| 62.1 | 26 | 6.4 | 5.5 | ||
| Are you registered with a pulmonologist for other lung diseases? | Yes | No | |||
| 7.8 | 92.2 | ||||
| Birthplace | Region 1 | Region 2 | Region 3 | Region 4 | Other |
| 0.3 | 0.5 | 0.8 | 0.9 | 0.3 | |
| Place of residence | Region 1 | Region 2 | Region 3 | Region 4 | Other |
| 0.3 | 0.5 | 0.8 | 0.9 | 0.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karymsakova, I.; Kozhakhmetova, D.; Bekenova, D.; Ostroukh, D.; Bekbayeva, R.; Kydyralina, L.; Bugubayeva, A.; Kurushbayeva, D. Development of an Early Lung Cancer Diagnosis Method Based on a Neural Network. Computers 2025, 14, 397. https://doi.org/10.3390/computers14090397
Karymsakova I, Kozhakhmetova D, Bekenova D, Ostroukh D, Bekbayeva R, Kydyralina L, Bugubayeva A, Kurushbayeva D. Development of an Early Lung Cancer Diagnosis Method Based on a Neural Network. Computers. 2025; 14(9):397. https://doi.org/10.3390/computers14090397
Chicago/Turabian StyleKarymsakova, Indira, Dinara Kozhakhmetova, Dariga Bekenova, Danila Ostroukh, Roza Bekbayeva, Lazat Kydyralina, Alina Bugubayeva, and Dinara Kurushbayeva. 2025. "Development of an Early Lung Cancer Diagnosis Method Based on a Neural Network" Computers 14, no. 9: 397. https://doi.org/10.3390/computers14090397
APA StyleKarymsakova, I., Kozhakhmetova, D., Bekenova, D., Ostroukh, D., Bekbayeva, R., Kydyralina, L., Bugubayeva, A., & Kurushbayeva, D. (2025). Development of an Early Lung Cancer Diagnosis Method Based on a Neural Network. Computers, 14(9), 397. https://doi.org/10.3390/computers14090397






