Recent Advancement of the Sensors for Monitoring the Water Quality Parameters in Smart Fisheries Farming
Abstract
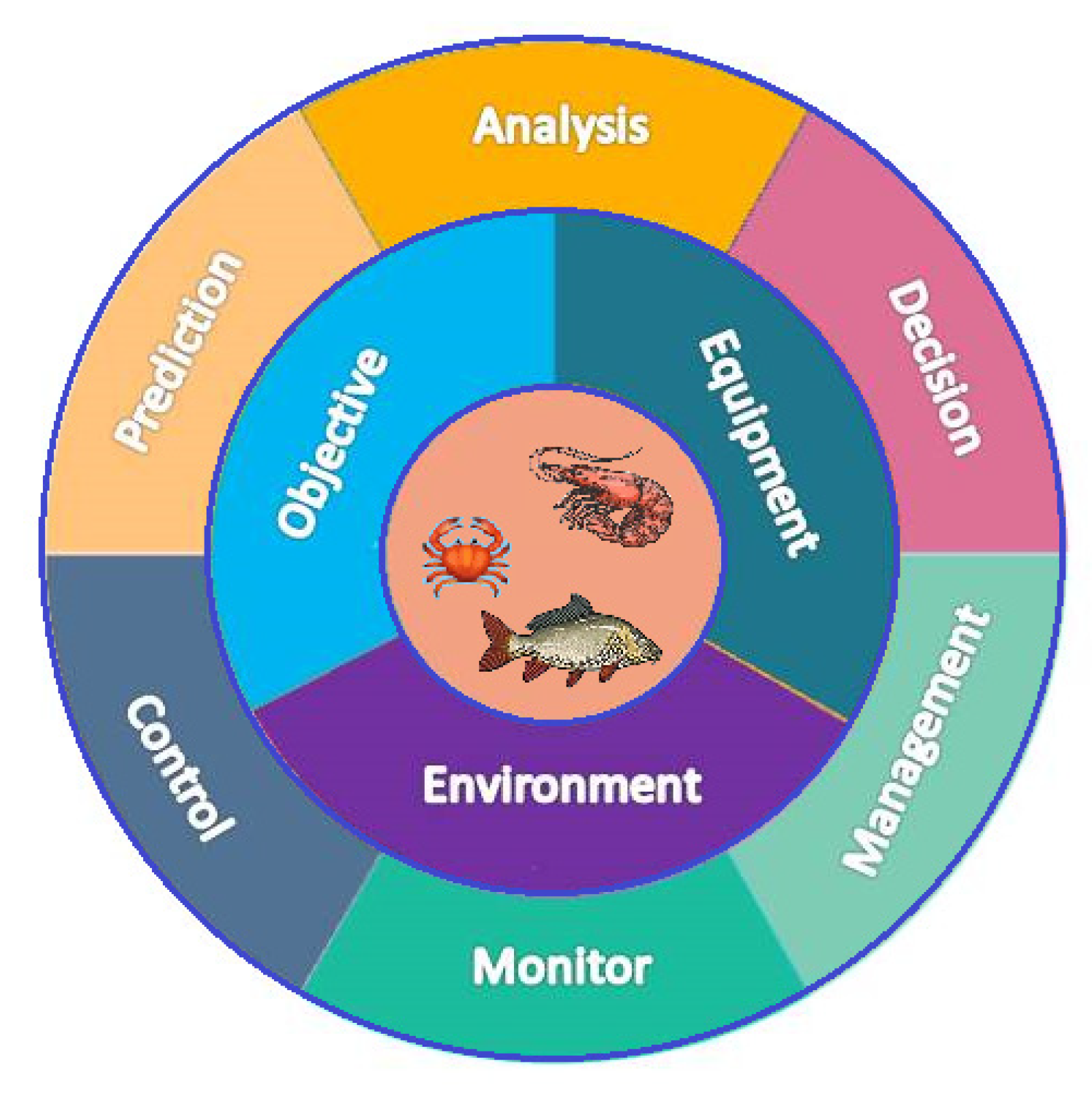
1. Introduction
2. Factors Deciding Water Quality and Related Sensors
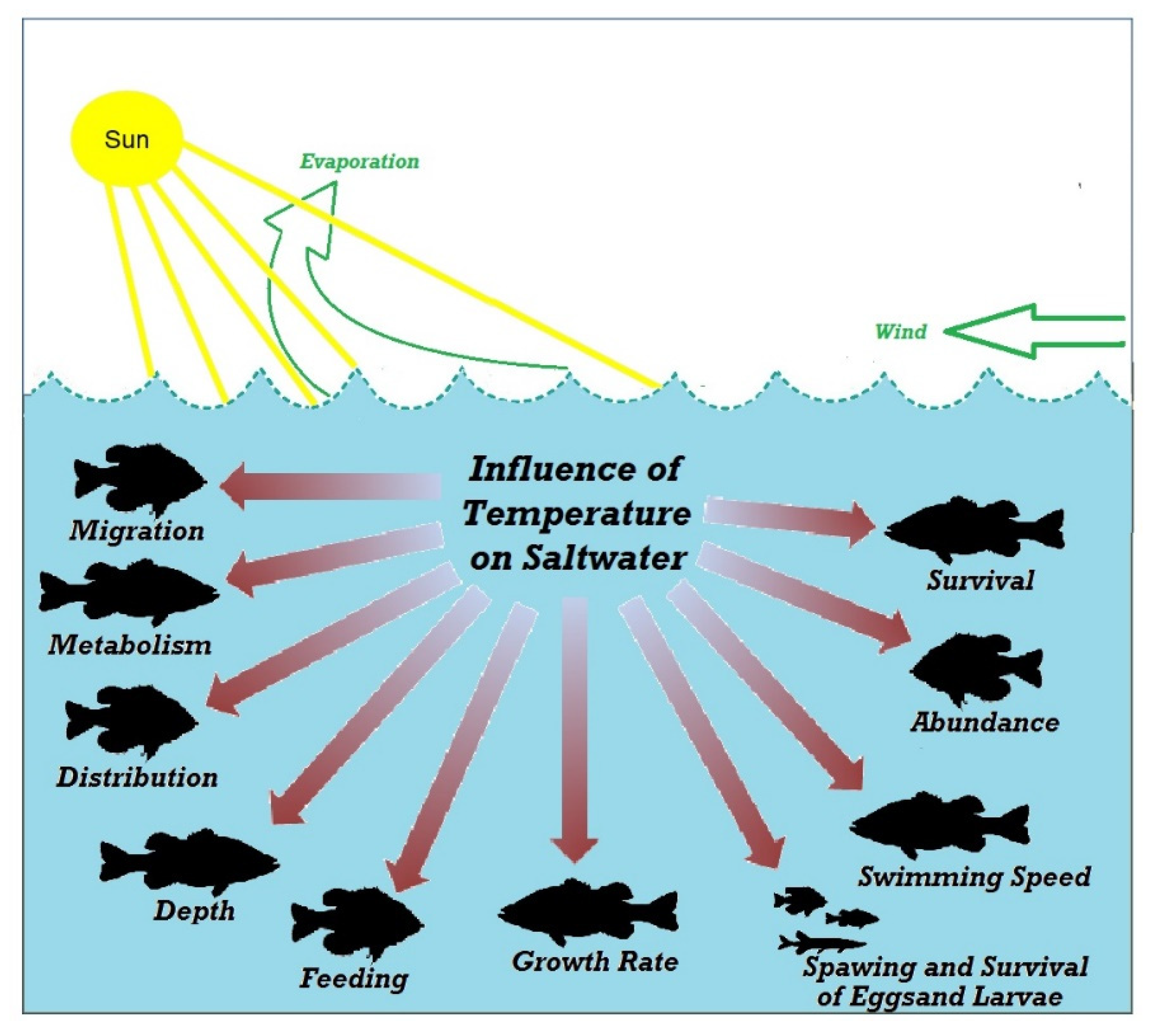
2.1. Water Temperature
2.2. pH
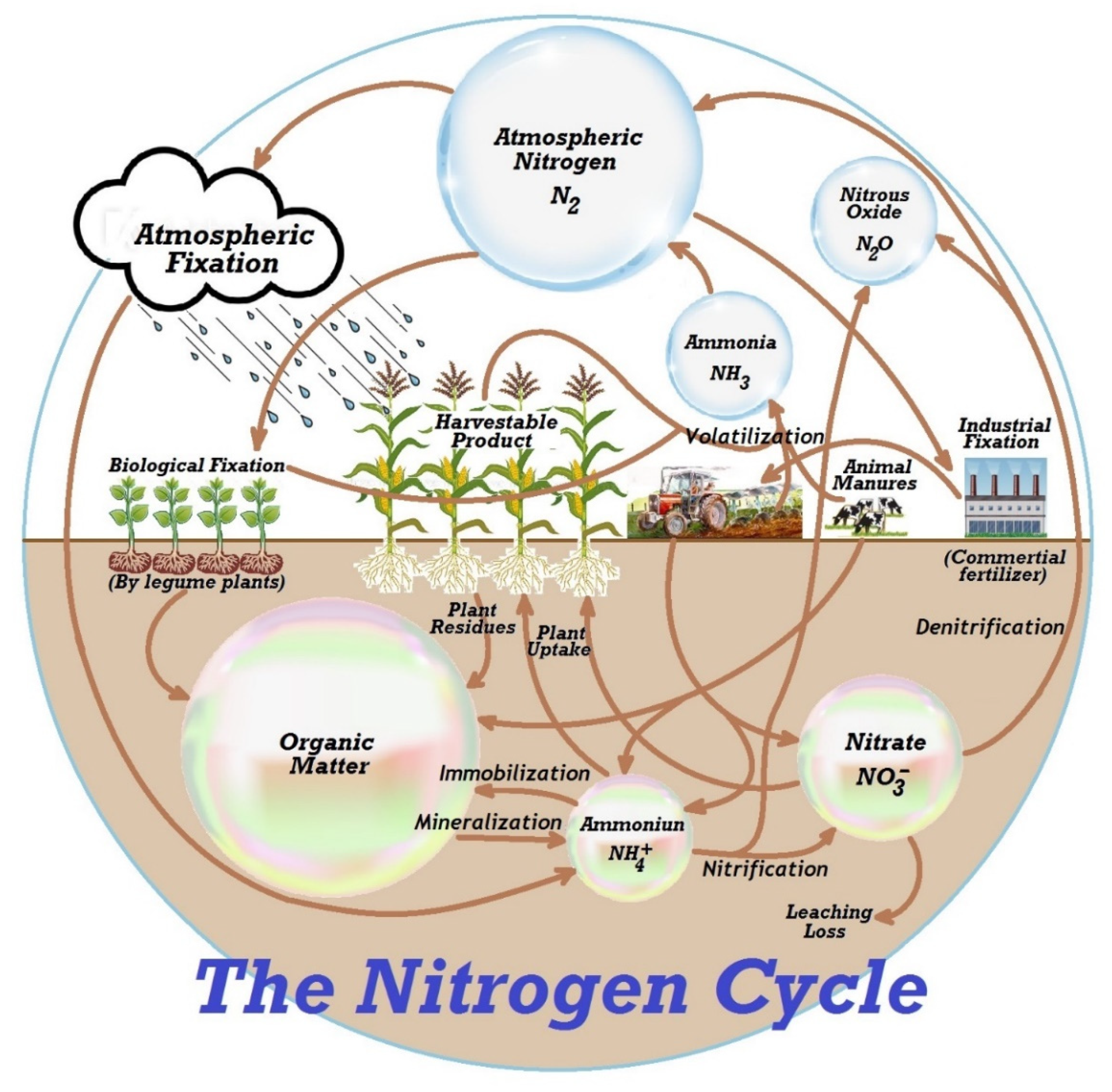
2.3. Nitrites and Nitrates
2.4. Phosphorous (P)
2.5. Calcium (Ca) and Magnesium (Mg)
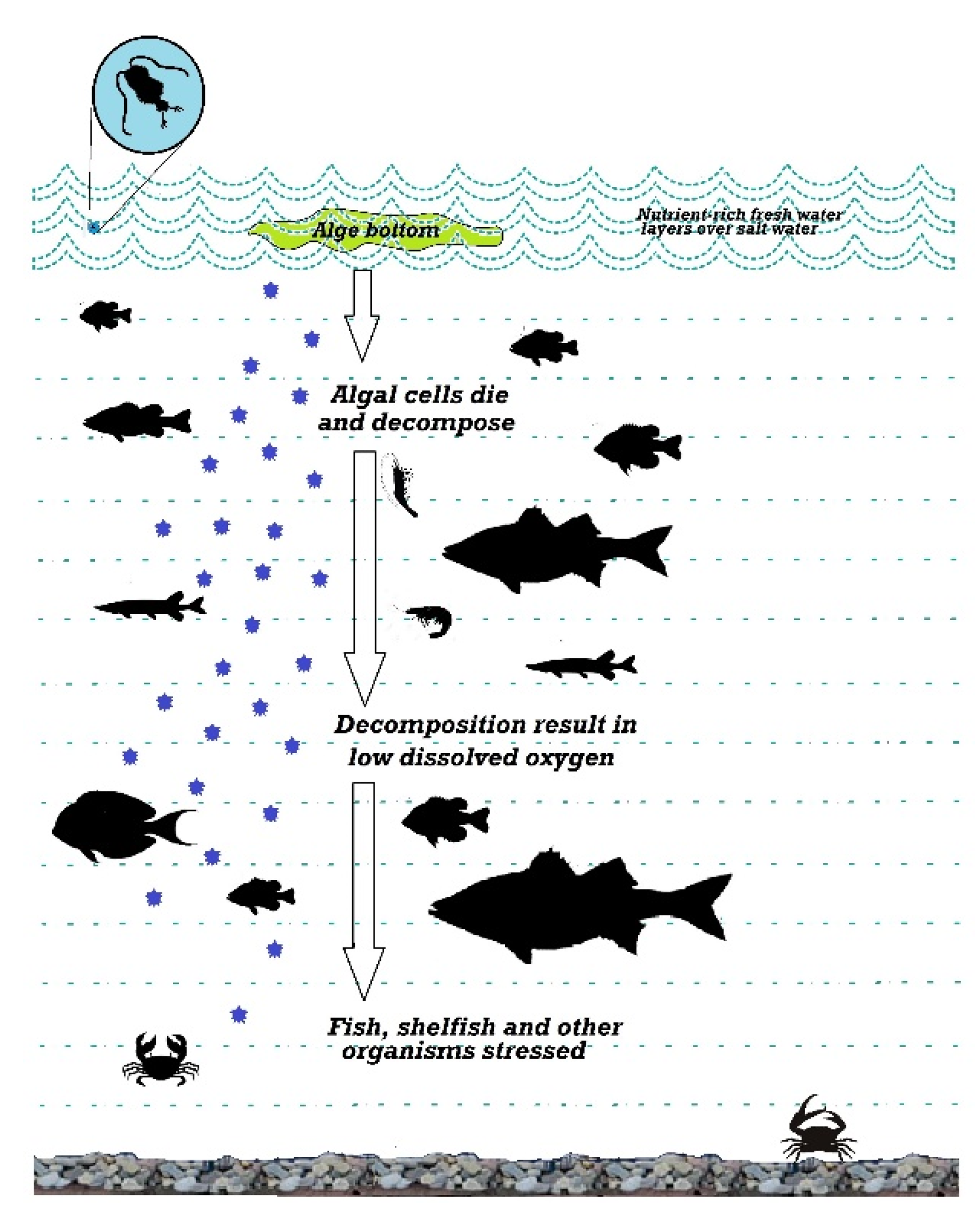
2.6. Dissolved Oxygen (DO)
3. Necessity of IoT-Based Farming and Related Research
3.1. K-Nearest Neighbour (KNN)
3.2. Random Forest (RF) Model
3.3. Decision Tree Regression
3.4. Polynomial Regression
3.5. Multiple Linear Regression
3.6. Principal Component Analysis (PCA)
4. A Proposed System
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aura, C.M.; Musa, S.; Yongo, E.; Okechi, J.K.; Njiru, J.M.; Ogari, Z.; Wanyama, R.; Charo-Karisa, H.; Mbugua, H.; Kidera, S.; et al. Integration of mapping and socio-economic status of cage culture: Towards balancing lake-use and culture fisheries in Lake Victoria, Kenya. Aquac. Res. 2017, 49, 532–545. [Google Scholar] [CrossRef]
- Curtis, J.; Stanley, B. Water quality and recreational angling demand in Ireland. J. Outdoor Recreat. Tour. 2016, 14, 27–34. [Google Scholar] [CrossRef]
- Giacomazzo, M.; Bertolo, A.; Brodeur, P.; Massicotte, P.; Goyette, J.O.; Magnan, P. Linking fisheries to land use: How an-thropogenic inputs from the watershed shape fish habitat quality. Sci. Total Environ. 2020, 717, 1–11. [Google Scholar] [CrossRef]
- MacNeil, M.A.; Mellin, C.; Matthews, S.; Wolff, N.H.; McClanahan, T.R.; Devlin, M.; Drovandi, C.; Mengersen, K.; Graham, N.A.J. Water quality mediates resilience on the Great Barrier Reef. Nat. Ecol. Evol. 2019, 3, 620–627. [Google Scholar] [CrossRef]
- Achim, W.; Robert, F.; Robert, H.; Nina, B. Smart farming is key to developing sustainable agriculture. Proc. Natl. Acad. Sci. USA 2017, 114, 6148–6150. [Google Scholar]
- Jungsu, P.; Keug, T.K.; Woo, H.L. Recent advances in information and communications technology (ICT) and sensor tech-nology for monitoring water quality. Water 2020, 510, 1–24. [Google Scholar]
- Dieisson, P.; Paulo, D.W.; Edson, T.; Caroline, P.S.F.; Vitor, F.D.C.; Giana, V.M. Scientific development of smart farming technologies and their application in Brazil. Inf. Process. Agric. 2018, 5, 21–32. [Google Scholar]
- Munroe, D.; Narváez, D.; Hennen, D.; Jacobson, L.; Mann, R.; Hofmann, E.; Powell, E.; Klinck, J. Fishing and bottom water temperature as drivers of change in maximum shell length in Atlantic surfclams (Spisula solidissima). Estuar. Coast. Shelf Sci. 2016, 170, 112–122. [Google Scholar] [CrossRef]
- Dadras, H.; Dzyuba, B.; Cosson, J.; Golpour, A.; Siddique, M.A.M.; Linhart, O. Effect of water temperature on the physiology of fish spermatozoon function: A brief review. Aquac. Res. 2017, 48, 729–740. [Google Scholar] [CrossRef]
- Jackson, F.; Hannah, D.M.; Fryer, R.; Millar, C.; Malcolm, I. Development of spatial regression models for predicting summer river temperatures from landscape characteristics: Implications for land and fisheries management. Hydrol. Process. 2017, 31, 1225–1238. [Google Scholar] [CrossRef]
- Graf, R.; Zhu, S.; Sivakumar, B. Forecasting river water temperature time series using a wavelet–neural network hybrid modelling approach. J. Hydrol. 2019, 578, 124115. [Google Scholar] [CrossRef]
- Bowerman, T.; Roumasset, A.; Keefer, M.L.; Sharpe, C.S.; Caudill, C.C. Prespawn mortality of female Chinook salmon in-creases with water temperature and percent hatchery origin. Trans. Am. Fish. Soc. 2018, 147, 31–42. [Google Scholar] [CrossRef]
- Teffer, A.K.; Bass, A.L.; Miller, K.M.; Patterson, D.A.; Juanes, F.; Hinch, S.G. Infections, fisheries capture, temperature, and host responses: Multistressor influences on survival and behaviour of adult Chinook salmon. Can. J. Fish. Aquat. Sci. 2018, 75, 2069–2083. [Google Scholar] [CrossRef]
- Kędra, M.; Wiejaczka, Ł. Climatic and dam-induced impacts on river water temperature: Assessment and management im-plications. Sci. Total Environ. 2018, 626, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.W. The future of fishes and fisheries in the changing oceans. J. Fish Biol. 2018, 92, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan-Uddin, H.; Bain, V.; Haque, M. Increased water temperature altered hemato-biochemical parameters and structure of peripheral erythrocytes in striped catfish Pangasianodon hypophthalmus. Fish. Physiol. Biochem. 2018, 44, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- The Thermal Optimum by David A. Ross. Available online: https://midcurrent.com/science/the-thermal-optimum/ (accessed on 28 January 2021).
- Zhaozhao, T.; Wenyan, W.; Jinliang, G. A wireless passive SAW delay line temperature and pressure sensor for monitoring water distribution system. In Proceedings of the IEEE Sensors Conference, New Delhi, India, 28–31 October 2018. [Google Scholar]
- Martin, A.; Peter, H.; Shima, T.; Simon, C.; Michael, J.W.; Heriberto, B.; Jose, G.; Louisa, V. Fibre optic temperature and humidity sensors for harsh wastewater environments. In Proceedings of the 19th International Conference on Sensing Technology (ICST), Sydney, Australia, 4–6 December 2017. [Google Scholar]
- Pushkar, S.; Sanghamitra, S. Arduino-based smart irrigation using water flow Sensor, soil moisture sensor, temperature sensor and esp8266 wifi module. In Proceedings of the IEEE Region 10 Humanitarian Technology Conference (R10-HTC), Agra, India, 21–23 December 2016. [Google Scholar]
- Muhammad, D.K.; Endro, A.; Sidik, P. Design and implementation of smart bath water heater using Arduino. In Proceedings of the 6th International Conference on Information and Communication Technology (ICoICT), Bandung, Indonesia, 3–5 May 2018. [Google Scholar]
- Waterproof DS18B20 Digital Temperature Sensor. Available online: https://core-electronics.com.au/waterproof-ds18b20-digital-temperature-sensor.html (accessed on 28 January 2021).
- Dong, Y.; Son, D.-H.; Dai, Q.; Lee, J.-H.; Won, C.-H.; Kim, J.-G.; Kang, S.-H.; Lee, J.-H.; Chen, D.; Lu, H.; et al. AlGaN/GaN heterostructure pH sensor with multi-sensing segments. Sens. Actuators B Chem. 2018, 260, 134–139. [Google Scholar] [CrossRef]
- Solovyev, M.M.; Izvekova, G.I.; Kashinskaya, E.N.; Gisbert, E. Dependence of pH values in the digestive tract of freshwater fishes on some abiotic and biotic factors. Hydrobiology 2017, 807, 67–85. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Cosson, J.; Bondarenko, O.; Linhart, O. Sperm motility in fishes: (III) diversity of regulatory signals from membrane to the axoneme. Theriogenology 2019, 136, 143–165. [Google Scholar] [CrossRef]
- Stevens, C.H.; Croft, D.P.; Paull, G.C.; Tyler, C.R. Stress and welfare in ornamental fishes: What can be learned from aqua-culture? J. Fish Biol. 2017, 91, 409–428. [Google Scholar] [CrossRef]
- Ahmed, I.; Reshi, Q.M.; Fazio, F. The influence of the endogenous and exogenous factors on hematological parameters in different fish species: A review. Aquac. Int. 2020, 28, 869–899. [Google Scholar] [CrossRef]
- Shartau, R.B.; Baker, D.W.; Brauner, C.J. White sturgeon (Acipenser transmontanus) acid–base regulation differs in response to different types of acidoses. J. Comp. Physiol. B 2017, 187, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Borvinskaya, E.; Gurkov, A.; Shchapova, E.; Baduev, B.; Shatilina, Z.; Sadovoy, A.; Meglinski, I.; Timofeyev, M. Parallel in vivo monitoring of pH in gill capillaries and muscles of fishes using microencapsulated biomarkers. Biol. Open 2017, 6, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, R.L.; Wiber, M.; Paul, S.; Angel, E.; Benson, A.; Charles, A.; Chouinard, O.; Edwards, D.; Foley, P.; Lane, D.; et al. Integrating diverse objectives for sustainable fisheries in Canada. Can. J. Fish. Aquat. Sci. 2019, 76, 480–496. [Google Scholar] [CrossRef]
- Shogo, H.; Hironao, O.; Seiichi, T.; Toshihiro, I. Valve-actuator-integrated reference electrode for an ultra-long-life rumen pH sensor. Sensors 2020, 20, 1249. [Google Scholar]
- Wen-Chi, L.; Klaus, B.; Charles, W.M.; Mark, A.B. Multifunctional water sensors for pH, ORP, and conductivity using only microfabricated platinum electrodes. Sensors 2017, 17, 1655. [Google Scholar]
- Tanomsak, W.; Sarawoot, B.; Songgrod, P. Wireless sensor network for monitoring of water quality for pond Tilapia. In Proceedings of the 12th International Conference on UbiMedia Computing (UbiMedia), Bali, Indonesia, 5–8 August 2019. [Google Scholar]
- Noor, A.A.; Lee, Y.H.; Faridah, S.; Mohd, H.M.Z.; Sharina, A.H. A colorimetric pH sensor based on clitoria sp. and brassica sp. for monitoring of food spoilage using chromametry. Sensors 2019, 19, 4813. [Google Scholar]
- Nedal, A.T.; Yunusa, U.; Elaref, R.; Ayman, A.; Faraj, A.A. A flexible optical pH sensor based on polysulfone membranes coated with pH-responsive polyaniline nanofiber. Sensors 2016, 16, 986. [Google Scholar]
- Wade, L.; Magdalena, W.; Kamal, A. RuO2 pH sensor with super-glue-inspired reference electrode. Sensors 2017, 17, 2036. [Google Scholar]
- Gerwing, T.G.; Plate, E. Effectiveness of nutrient enhancement as a remediation or compensation strategy of salmonid fisheries in culturally oligotrophic lakes and streams in temperate climates. Restor. Ecol. 2018, 27, 279–288. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, J.; Feng, J.; Liu, Q.; Nan, F.; Xie, S. Treatment of real aquaculture wastewater from a fishery utilizing phytoreme-diation with microalgae. J. Chem. Technol. Biotechnol. 2019, 94, 900–910. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquac. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Md, E.E.A.; Anindya, N.; Subhas, C.M.; Lucy, B. A temperature-compensated graphene sensor for nitrate monitoring in real-time application. Sens. Actuators A Phys. 2018, 269, 79–90. [Google Scholar]
- Md, E.E.A.; Subhas, C.M.; Lucy, B. Imprinted polymer coated impedimetric nitrate sensor for real-time water quality moni-toring. Sens. Actuators B Chem. 2018, 259, 753–761. [Google Scholar]
- Md, E.E.A.; Li, X.; Subhas, C.M.; Lucy, B. Temperature compensated smart nitrate-sensor for agricultural industry. IEEE Trans. Ind. Electron. 2017, 64, 7333–7341. [Google Scholar]
- Md, E.E.A.; Li, X.; Asif, I.Z.; Subhas, C.M.; Lucy, B. Practical nitrate sensor based on electrochemical impedance measurement. In Proceedings of the International Instrumentation and Measurement Technology Conference, Taipei, Taiwan, 23–26 May 2016. [Google Scholar]
- Md, E.E.A.; Subhas, C.M. Detection methodologies for pathogen and toxins: A review. Sensors 2017, 17, 1885. [Google Scholar]
- Li, S.-J.; Zhao, G.-Y.; Zhang, R.-X.; Hou, Y.-L.; Liu, L.; Pang, H. A sensitive and selective nitrite sensor based on a glassy carbon electrode modified with gold nanoparticles and sulfonated graphene. Microchim. Acta 2013, 180, 821–827. [Google Scholar] [CrossRef]
- Wanga, P.; Wanga, M.; Zhoua, F.; Yanga, G.; Qua, L.; Miaoa, X. Development of a paper-based, inexpensive, and disposable electrochemical sensing platform for nitrite detection. Electrochem. Comm. 2017, 81, 74–78. [Google Scholar] [CrossRef]
- Hameed, R.A.; Medany, S.S. Construction of core-shell structured nickel@platinum nanoparticles on graphene sheets for electrochemical determination of nitrite in drinking water samples. Microchem. J. 2019, 145, 354–366. [Google Scholar] [CrossRef]
- Chen, X.; Pu, H.; Fu, Z.; Sui, X.; Chang, J.; Chen, J.; Mao, S. Real-time and selective detection of nitrates in water using gra-phene-based field-effect transistor sensors. Environ. Sci. Nano 2018, 5, 1990–1999. [Google Scholar] [CrossRef]
- Amanulla, B.; Palanisamy, S.; Chen, S.M.; Chiu, T.W.; Velusamy, V.; Hall, J.M.; Chen, T.W.; Ramaraj, S.K. Selective col-orimetric detection of nitrite in water using chitosan stabilised gold nanoparticles decorated reduced graphene oxide. Nat. Sci. Reports 2017, 7, 1–9. [Google Scholar]
- Hameed, R.A.; Medany, S.S. Sensitive nitrite detection at core-shell structured Cu@Pt nanoparticles supported on graphene. Appl. Surf. Sci. 2018, 458, 252–263. [Google Scholar] [CrossRef]
- Choosang, J.; Numnuam, A.; Thavarungkul, P.; Kanatharana, P.; Radu, T.; Ullah, S.; Radu, A. Simultaneous Detection of Ammonium and Nitrate in Environmental Samples Using on Ion-Selective Electrode and Comparison with Portable Colorimetric Assays. Sensors 2018, 18, 3555. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, M.; Wang, X.; Yang, Q.; Wang, M.; Liu, G.; Yao, L. An All-Solid-State Nitrate Ion-Selective Electrode with Nanohybrids Composite Films for In-Situ Soil Nutrient Monitoring. Sensors 2020, 20, 2270. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.F.; Al-Faqheri, W.; Al-Halhouli, A. Low Cost Lab on Chip for the Colorimetric Detection of Nitrate in Mineral Water Products. Sensors 2017, 17, 2345. [Google Scholar] [CrossRef]
- Prabhu, A.J.; Schrama, J.W.; Kaushik, S.J. Quantifying dietary phosphorus requirement of fish—a meta-analytic approach. Aquac. Nutr. 2013, 19, 233–249. [Google Scholar] [CrossRef]
- Hongwei, C.; Linlu, Z.; Fabiao, Y.; Qiaoling, D. Detection of phosphorus species in water: Technology and strategies. Analyst 2019, 144, 7130–7148. [Google Scholar]
- Environmental Impact of Nitrogen and Phosphorus Fertilisers in High Rainfall Areas. Available online: https://www.agric.wa.gov.au/high–rainfall-pastures/environmental–impact–nitrogen–and-phosphorus-fertilisers-high-rainfall-areas (accessed on 28 January 2021).
- Unni, S.; Lena, R.; Sanu, K.A.; Kamila, M.; Krishnapillai, G.K.; Hanna, R.; Stefan, K.; Jerzy, R. Ultrasensitive electrochemical sensing of phosphate in water mediated by a dipicolylamine-zinc(II) complex. Sens. Actuators B Chem. 2020, 321, 1–8. [Google Scholar]
- Mehenur, S.; Jared, L.; Ghinwa, M.N.; Chen-Zhong, L. Smart-phone, paper-based fluorescent sensor for ultra-low inorganic phosphate detection in environmental samples. Microsyst. Nanoeng. 2019, 5, 1–10. [Google Scholar]
- Sumaiya, I.; Nasim, R.; Jeong, J.; Lee, K. Sensing Technology for Rapid Detection of Phosphorus in Water: A Review. J. Biosyst. Eng. 2016, 41, 138–144. [Google Scholar]
- Kiesar, S.B.; Umesh, T.N.; Jin-Young, Y.; Yousheng, W.; Tahmineh, M.; Yoon-Bong, H. Nozzle-jet-printed silver/graphene composite-based field-effect transistor sensor for phosphate ion detection. ACS Omega 2019, 4, 8373–8380. [Google Scholar]
- Zhou, G.; Jin, B.; Wang, Y.; Dong, Q.; Maity, A.; Chang, J.; Ren, R.; Pu, H.; Sui, X.; Mao, S.; et al. Ultrasensitive sensors based on aluminum oxide protected reduced graphene oxide for phosphate ion detection in real water. Mol. Syst. Des. Eng. 2020, 5, 936–942. [Google Scholar]
- Mao, S.; Pu, H.; Chang, J.; Sui, X.; Zhou, G.; Ren, R.; Chen, Y.; Chen, J. Ultrasensitive detection of ortho-phosphate ions with reduced graphene oxide/ferritin field-effect transistor sensors. Environ. Sci. Nano 2017, 4, 856–863. [Google Scholar]
- Zhu, L.; Zhou, X.; Shi, H. A potentiometric cobalt-based phosphate sensor based on screen-printing technology. Front. Environ. Sci. Eng. 2014, 8, 945–951. [Google Scholar]
- Pankaj, K.; Dong, M.K.; Myong, H.H.; Yoon-Bo, S. An all-solid-state monohydrogen phosphate sensor based on a macrocyclic ionophore. Talanta 2010, 82, 1107–1112. [Google Scholar]
- John, J.W.; Paul, L.B. Fabrication, calibration and evaluation of a phosphate ion-selective microelectrode. Environ. Pollut. 2010, 158, 3612–3617. [Google Scholar]
- Stefano, C.; Daria, T.; Giuseppe, P.; Danila, M.; Fabiana, A. Novel reagentless paper-based screen-printed electrochemical sensor to detect phosphate. Anal. Chim. Acta 2016, 919, 78–84. [Google Scholar]
- Manová, A.; Beinrohr, E. Determination of phosphate in water by flow coulometry. Acta Chim. Slovaca 2020, 13, 102–107. [Google Scholar] [CrossRef]
- Luo, S.; Wu, B.; Xiong, X.; Wang, J. Effects of Total Hardness and Calcium:Magnesium Ratio of Water during Early Stages of Rare Minnows (Gobiocypris rarus). Comp. Med. 2016, 66, 181–187. [Google Scholar]
- Calcium and Magnesium Use in Aquaculture. Available online: https://www.aquaculturealliance.org/advocate/calcium-and-magnesium-use-in-aquaculture/ (accessed on 22 February 2021).
- Butler, D.H.; Koivisto, S.; Brumfeld, V.; Shahack-Gross, R. Early Evidence for Northern Salmonid Fisheries Discovered using Novel Mineral Proxies. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Arias, D.; Rivas, M.; Guinez, R.; Cisternas, L.A. Modeling the calcium and magnesium removal from seawater by immobilised biomass of ureolytic bacteria Bacillus subtilis through response surface methodology and artificial neural networks. Desalinat. Water Treat. 2018, 118, 294–303. [Google Scholar] [CrossRef]
- Joquiño, C.M.; Sarmiento, J.M.; Estaña, L.M.; Nañola, J.; Pedro, A.A. Seasonal Change, Fishing Revenues, and Nutrient In-takes of Fishers’ Children in Davao Gulf, Philippines. Philipp. J. Sci. 2021, 150, 307–323. [Google Scholar]
- Zhang, H.; Meng, Y.; Wang, Y.; Hu, X.; Wang, Y.; Huang, Y.; Zhou, Q.; Li, A.; Wang, L.; Xing, X. Correlation analysis between pH, major organic acids, calcium and magnesium ions of stratified bottom-pit-mud from Chinese strong-flavor Baijiu pit. Shipin Kexue/Food Sci. 2020, 41, 90–97. [Google Scholar]
- Hicks, C.C.; Cohen, P.J.; Graham, N.A.J.; Nash, K.L.; Allison, E.H.; D’Lima, C.; Mills, D.J.; Roscher, M.; Thilsted, S.H.; Thorne-Lyman, A.L.; et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nat. Cell Biol. 2019, 574, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Mozharovskaya, P.N.; Lykova, Y.A.; Semenishchev, V.S. Systematisation of chemical pollutants in samples of river water in Yekaterinburg city and Sverdlovsk Region. In Proceedings of the AIP Conference, Ekaterinburg, Russia, 20–23 May 2019. [Google Scholar]
- Moshoeshoe, M.N.; Obuseng, V. Simultaneous determination of nitrate, nitrite and phosphate in environmental samples by high performance liquid chromatography with UV detection. S. Afr. J. Chem. 2018, 71, 79–85. [Google Scholar] [CrossRef]
- Drolc, A.; Vrtovšek, J. Nitrate and nitrite nitrogen determination in wastewater using on-line UV spectrometric method. Bioresour. Tech. 2010, 101, 4228–4233. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection methods of nitrate in water: A review. Sens. Actuators A Phys. 2018, 280, 210–221. [Google Scholar] [CrossRef]
- Azmi, A.; Azman, A.A.; Kaman, K.K.; Ibrahim, S.; Mukhopadhyay, S.C.; Nawawi, S.W.; Yunus, M.A.M. Performance of Coating Materials on Planar Electromagnetic Sensing Array to Detect Water Contamination. IEEE Sens. J. 2017, 17, 5244–5251. [Google Scholar] [CrossRef]
- Coppola, L.; Legendre, L.; Lefevre, D.; Prieur, L.; Taillandier, V.; Riquier, E.D. Seasonal and inter-annual variations of dissolved oxygen in the northwestern Mediterranean Sea (DYFAMED site). Prog. Oceanogr. 2018, 162, 187–201. [Google Scholar] [CrossRef]
- Boyd, C.E.; Torrans, E.L.; Tucker, C.S. Dissolved Oxygen and Aeration in Ictalurid Catfish Aquaculture. J. World Aquac. Soc. 2018, 49, 7–70. [Google Scholar] [CrossRef]
- Kralj, M.; Lipizer, M.; Čermelj, B.; Celio, M.; Fabbro, C.; Brunetti, F.; Francé, J.; Mozetič, P.; Giani, M. Hypoxia and dissolved oxygen trends in the northeastern Adriatic Sea (Gulf of Trieste). Deep. Sea Res. Part II Top. Stud. Oceanogr. 2019, 164, 74–88. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, L.; Wei, Y.; Li, D. A method for predicting dissolved oxygen in aquaculture water in an aquaponics system. Comput. Electron. Agric. 2018, 151, 384–391. [Google Scholar] [CrossRef]
- Ta, X.; Wei, Y. Research on a dissolved oxygen prediction method for recirculating aquaculture systems based on a convolution neural network. Comput. Electron. Agric. 2018, 145, 302–310. [Google Scholar] [CrossRef]
- Parra, L.; Lloret, G.; Lloret, J.; Rodilla, M. Physical Sensors for Precision Aquaculture: A Review. IEEE Sens. J. 2018, 18, 3915–3923. [Google Scholar] [CrossRef]
- Defe, G.A.; Antonio, A.Z.C. Multi-parameter Water Quality Monitoring Device for Grouper Aquaculture. In Proceedings of the 2018 IEEE 10th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment and Management (HNICEM), Baguio, Philippines, 29 November–2 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–5. [Google Scholar]
- Kramer, D.L. Dissolved oxygen and fish behavior. Environ. Boil. Fishes 1987, 18, 81–92. [Google Scholar] [CrossRef]
- Franklin, P. Dissolved oxygen criteria for freshwater fish in New Zealand: A revised approach. New Zealand J. Mar. Freshw. Res. 2013, 48, 112–126. [Google Scholar] [CrossRef]
- Honglin, Z.; Zhiguo, Z. Ratiometric sensor based on PtOEP-C6/Poly (St-TFEMA) film for automatic dissolved oxygen content detection. Sensors 2020, 20, 6175. [Google Scholar]
- Lola, G.O.; Alba, R.O.; Petra, J.C.; Mirella, D.L. Ceramic soil microbial fuel cells sensors for early detection of eutrophication. Proceedings 2020, 60, 1–12. [Google Scholar]
- Selvaraj, C.; Sumit, K.; Gert-Jan, W.E. Fabrication of a nitrogen and Boron-doped reduced graphene oxide membrane-less amperometric sensor for measurement of dissolved oxygen in a microbial fermentation. Chemosensors 2020, 44, 1–10. [Google Scholar]
- Zike, J.; Xinsheng, Y.; Shikui, Z.; Yingyan, H. Ratiometric dissolved oxygen sensors based on ruthenium complex doped with silver nanoparticles. Sensors 2017, 17, 548. [Google Scholar]
- Manuel, V.; Ryan, K.W.; Barbara, A.B.; Jose, E.C. Machine learning based predictions of dissolved oxygen in a small coastal embayment. J. Mar. Sci. Eng. 2020, 1007, 1–16. [Google Scholar]
- Rune, I.; Emil, M.; Jonas, H.; Ole, B.; Jakob, J. Optimisation of all-polymer optical fiber oxygen sensors with antenna dyes and improved solvent selection using hansen solubility parameters. Sensors 2021, 21, 5. [Google Scholar]
- Salvatore, G.L.; Maryam, B.; Kaushik, G.; Ashish, K.D.; Luca, L.; Nicola, D.; Giovanni, N. Development of a Novel Cu(II) Complex Modified Electrode and a Portable Electrochemical Analyzer for the Determination of Dissolved Oxygen (DO) in Water. Chemosensors 2016, 7, 1–10. [Google Scholar]
- Fowzia, A.; Sam, K.; Hasin, R.S.; Md, E.E.A.; Subhas, C.M. IoT enabled intelligent sensor node for smart city: Pedestrian counting and ambient monitoring. Sensors 2019, 19, 3374. [Google Scholar]
- Fowzia, A.; Sam, K.; Jordan, L.; Hasin, R.S.; Md, E.E.A.; Subhas, C.M. Design and development of an IoT enabled pedestrian counting and environmental monitoring system for a smart city. In Proceedings of the 13th International Conference on Sensing Technology (ICST), Sydney, Australia, 2–4 December 2019. [Google Scholar]
- Lina, R.; Anitha, M. Sensor data classification using machine learning algorithm. J. Stat. Manag. Syst. 2020, 23, 363–371. [Google Scholar]
- Demi, A.; Nico, S. Hydroponic nutrient control system based on internet of things. In Proceedings of the International Conference on Computer, Control, Informatics and Its Applications (IC3INA), Tangerang, Indonesia, 23–24 October 2019. [Google Scholar]
- Jaime, V.; Francesc, P.; Diego, A.T.; Maribel, A. A sensor data fusion system based on k-nearest neighbor pattern classification for structural health monitoring applications. Sensors 2017, 17, 417. [Google Scholar]
- Hristos, T.; Georgia, P.; Andreas, L. A brief review of random forests for water scientists and practitioners and their recent history in water resources. Water 2019, 11, 1–37. [Google Scholar]
- Devi, S.V.S.G. Random forest advice for water quality prediction in the regions of Kadapa district. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 1–3. [Google Scholar]
- Shajulin, B.; Nila, G.; Deepak, G.; Sreelakshmi, N. Real time water quality analysis framework using monitoring and prediction mechanisms. In Proceedings of the Conference on Information and Communication Technology (CICT), Jabalpur, India, 26–28 October 2018. [Google Scholar]
- Kangyang, C.; Hexia, C.; Chuanlong, Z.; Yichao, H.; Xiangyang, Q.; Ruqin, S.; Fengrui, L.; Min, Z.; Xinyi, Z.; Jinfeng, W.; et al. Comparative analysis of surface water quality prediction performance and identification of key water parameters using different machine learning models based on big data. Water Res. 2020, 171, 1–10. [Google Scholar]
- Consolata, G.; Jeniffer, J. A classification model for water quality analysis using decision tree. Eur. J. Comput. Sci. Inf. Technol. 2019, 7, 1–8. [Google Scholar]
- Hao, L.; Wen, S. Forecasting and evaluating water quality of Chao lake based on an improved decision tree method. Procedia Environ. Sci. 2010, 2, 970–979. [Google Scholar]
- Saghebian, S.M.; Sattari, M.T.; Rasoul, M.; Mahesh, P. Ground water quality classification by decision tree method in Ardebil region, Iran. Arab. J. Geosci. 2014, 7, 4767–4777. [Google Scholar] [CrossRef]
- Saptami, R.; Protik, C.B.; Md, A.H.; Md, R.I.; John, C. Polynomial regression of multiple sensing variables for high-performance smartphone colorimeter. OSA Contin. 2021, 4, 374–384. [Google Scholar]
- Huang, H.; Wang, Z.; Xia, F.; Shang, X.; Liu, Y.; Zhang, M.; Dahlgren, R.A.; Mei, K. Water quality trend and change-point analyses using integration of locally weighted polynomial regression and segmented regression. Environ. Sci. Pollut. Res. 2017, 24, 15827–15837. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.B.; Roohollah, N.; Ronny, B.; Alireza, G.; Behzad, G. Evolutionary polynomial regression approach to predict longitudinal dispersion coefficient in rivers. J. Water Supply Res. Technol. AQUA 2018, 67, 1–11. [Google Scholar]
- Wei-Chih, H.; Pao-Yuan, C.; Chia-Sui, W.; Jen-Chieh, H.; Wesley, H. Application of regression analysis to achieve a smart monitoring system for aquaculture. Information 2020, 387, 1–9. [Google Scholar]
- Krishnan, K.S.D.; Bhuvaneswari, P.T.V. Multiple linear regression based water quality parameter modeling to detect hexa-valent chromium in drinking water. In Proceedings of the International Conference on Wireless Communications, Signal Processing and Networking (WiSPNET), Chennai, India, 22–24 March 2017. [Google Scholar]
- Reham, E.K. Using regression analysis to estimate water quality constituents in Bahr El Baqar drain. J. Appl. Sci. Res. 2009, 5, 1067–1076. [Google Scholar]
- Wei-Bo, C.; Wen-Cheng, L. Water quality modeling in reservoirs using multivariate linear regression and two neural network models. Adv. Artif. Neural Syst. 2015, 521721, 1–12. [Google Scholar]
- Abbaa, S.I.; Hadia, S.J.; Abdullah, J. River water modelling prediction using multi-linear regression, artificial neural network, and adaptive neuro-fuzzy inference system techniques. In Proceedings of the 9th International Conference on Theory and Application of Soft Computing, Computing with Words and Perception (ICSCCW), Budapest, Hungary, 24–25 August 2017. [Google Scholar]
- Tao, X.F.; Huang, T.; Li, X.F.; Peng, D.P. Application of a PCA based water quality classification method in water quality assessment in the Tongjiyan Irrigation Area, China. In Proceedings of the International Conference on Energy and Environmental Protection (ICEEP), Shenzhen, China, 17–18 September 2016. [Google Scholar]
- Salim, A.B.; Gowhar, M.; Sayar, Y.; Ashok, K.P. Statistical assessment of water quality parameters for pollution source identification in sukhnag stream: An inflow stream of lake Wular (Ramsar Site), Kashmir Himalaya. J. Ecosyst. 2014, 898054, 1–18. [Google Scholar]
- Mahapatra, S.S.; Sahu, M.; Patel, R.K.; Panda, B.N. Prediction of Water Quality Using Principal Component Analysis. Water Qual. Expo. Health 2012, 4, 93–104. [Google Scholar] [CrossRef]
- Carlos, A.B.G.; Helenice, L.G.; Maria, C.S.; Anamália, F.S.; José, P.H.A.; Silvânio, S.L.C.; Geovanny, O.A.; Igor, S.S. Assessment of water quality using principal component analysis: A case study of the açude da Macela—Sergipe—Brazil. In Proceedings of the 16th International World Water Congress (IWRC), Cancum, Mexico, 29 May–3 June 2017. [Google Scholar]
- Emerson, N.; Nuno, C.; António, P. A Systematic Review of IoT Solutions for Smart Farming. Sensors 2020, 20, 2345. [Google Scholar]
- Md, E.E.A.; Najid, P.I.; Subhas, C.M.; Lucy, B. An internet-of-things enabled smart sensing system for nitrate monitoring. IEEE Internet Things J. 2018, 5, 4409–4417. [Google Scholar]
- SEAFLO 21 Series Diaphragm Pump 12V. Available online: https://12voltpumps.com.au/product/12v-21-series-diaphragm-pump-sfdp1-010-035-21-seaflo/ (accessed on 28 January 2021).
- Stepper Motor Controller Module for Arduino Projects. Available online: https://www.auselectronicsdirect.com.au/stepper-motor-controller-module-for-arduino-projec?gclid=Cj0KCQiA3smABhCjARIsAKtrg6LbnXTiUl9Rt4UCoPRYiUTLWGviqctaB_ilo-f3IJpFNeQ6Pp2DgwEaAtsDEALw_wcB (accessed on 28 January 2021).
- AD5933. Analogue Devices, 1 MSPS, 12-Bit Impedance Converter, Network Analyzer. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/AD5933.pdf (accessed on 28 January 2021).
- Arduino Uno Rev3. Available online: https://core-electronics.com.au/arduino-uno-r3.html (accessed on 28 January 2021).
- Lora Shield for Arduino—Long Range Transceiver. Available online: https://www.iot-store.com.au/products/lora-shield-for-arduino-long-range-transceiver (accessed on 28 January 2021).
- 12V 10W Solar Panel with Clips. Available online: https://www.jaycar.com.au/12v-10w-solar-panel-with-clips/p/ZM9051?gclid=Cj0KCQiA3smABhCjARIsAKtrg6JvnJioP4MJkkjeCcEs6E0V7_mhCHOc0f7f-R7TsYnqDVc_OIZX3yIaAsc2EALw_wcB (accessed on 28 January 2021).
- 12V 12Ah SLA Battery. Available online: https://www.jaycar.com.au/12v-12ah-sla-battery/p/SB2489 (accessed on 28 January 2021).
- 12/24V 10A Dual Battery PWM Solar Charge Controller with LED Indicator. Available online: https://www.jaycar.com.au/12-24v-10a-dual-battery-pwm-solar-charge-controller-with-led-indicator/p/MP3760 (accessed on 28 January 2021).
- Fowzia, A.; Anindya, N.; Md, E.E.A.; Hangrui, L.; Subhas, C.M. Electrochemical detection of calcium and magnesium in water bodies. Sens. Actuators A Phys. 2020, 305, 1–10. [Google Scholar]
- Anindya, N.; Md, E.E.A.; Shilun, F.; Subhas, C.M. IoT-based sensing system for phosphate detection using Graphite/PDMS sensors. Sens. Actuators A Phys. 2019, 286, 43–50. [Google Scholar]
- Anindya, N.; Omer, F.D.; Subhas, M.; Jurgen, K. pH sensing of printed flexible sensors. In Proceedings of the 12th Inter-national Conference on Sensing Technology (ICST), Limerick, Ireland, 4–6 December 2018. [Google Scholar]
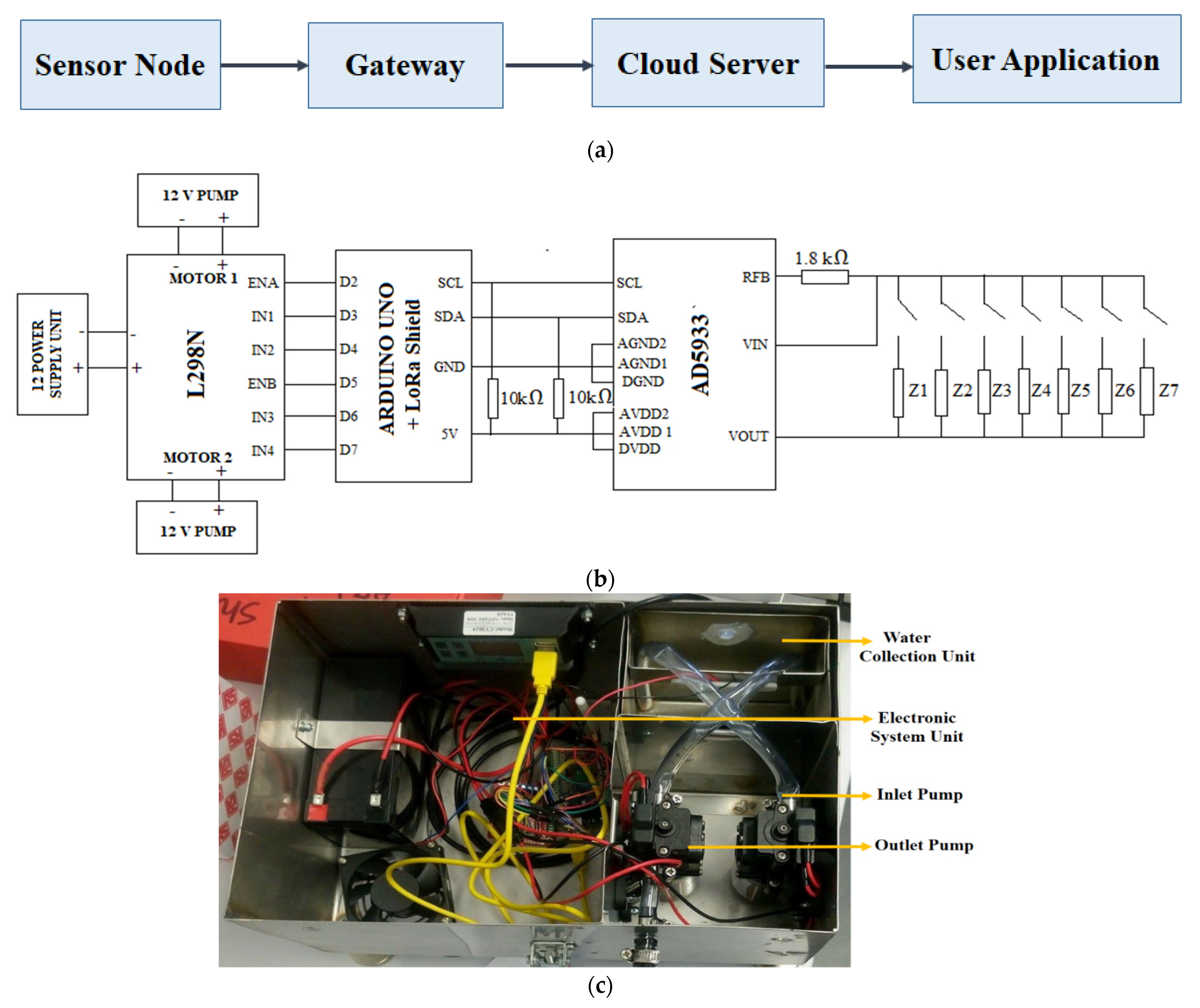
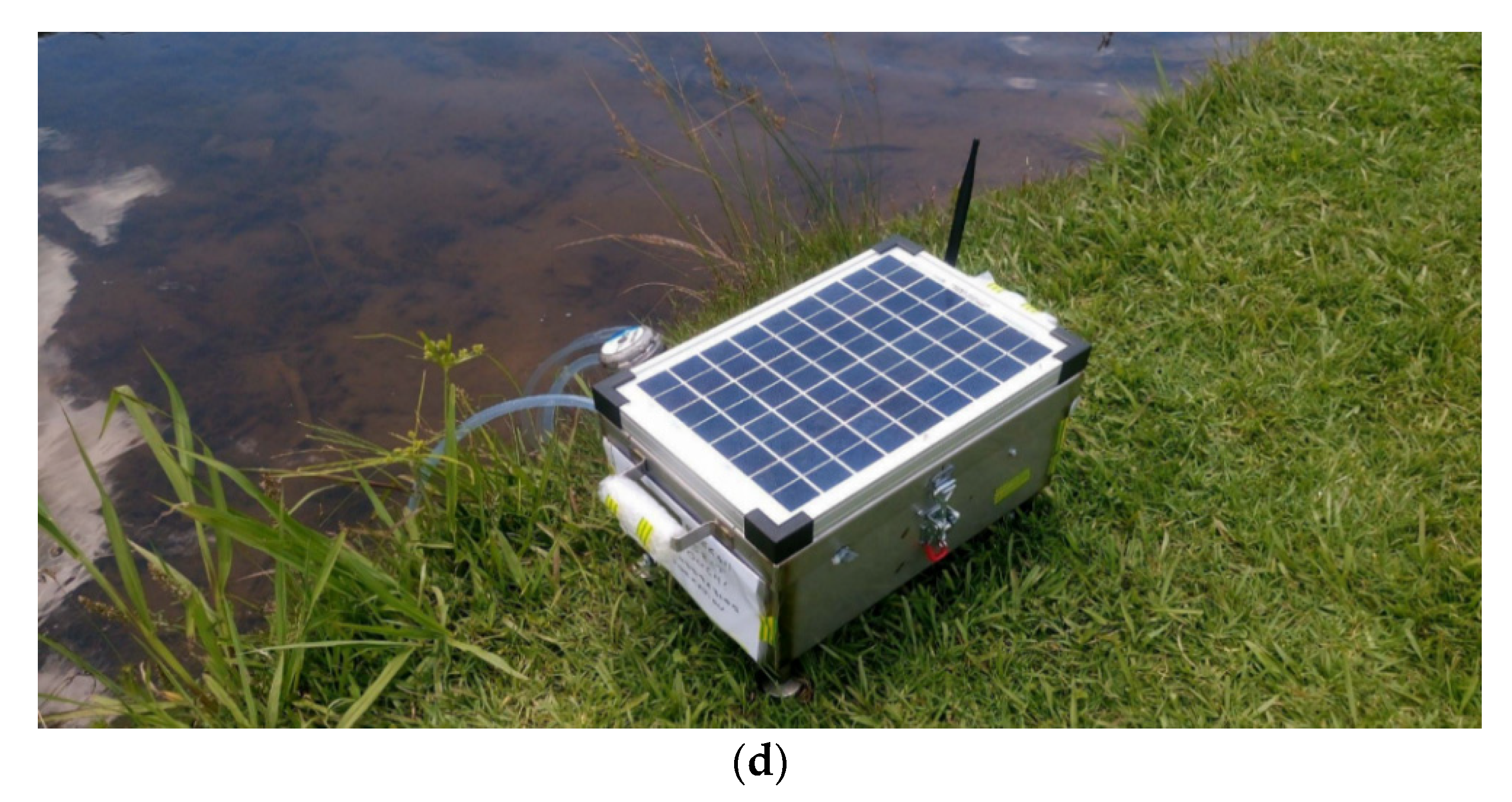
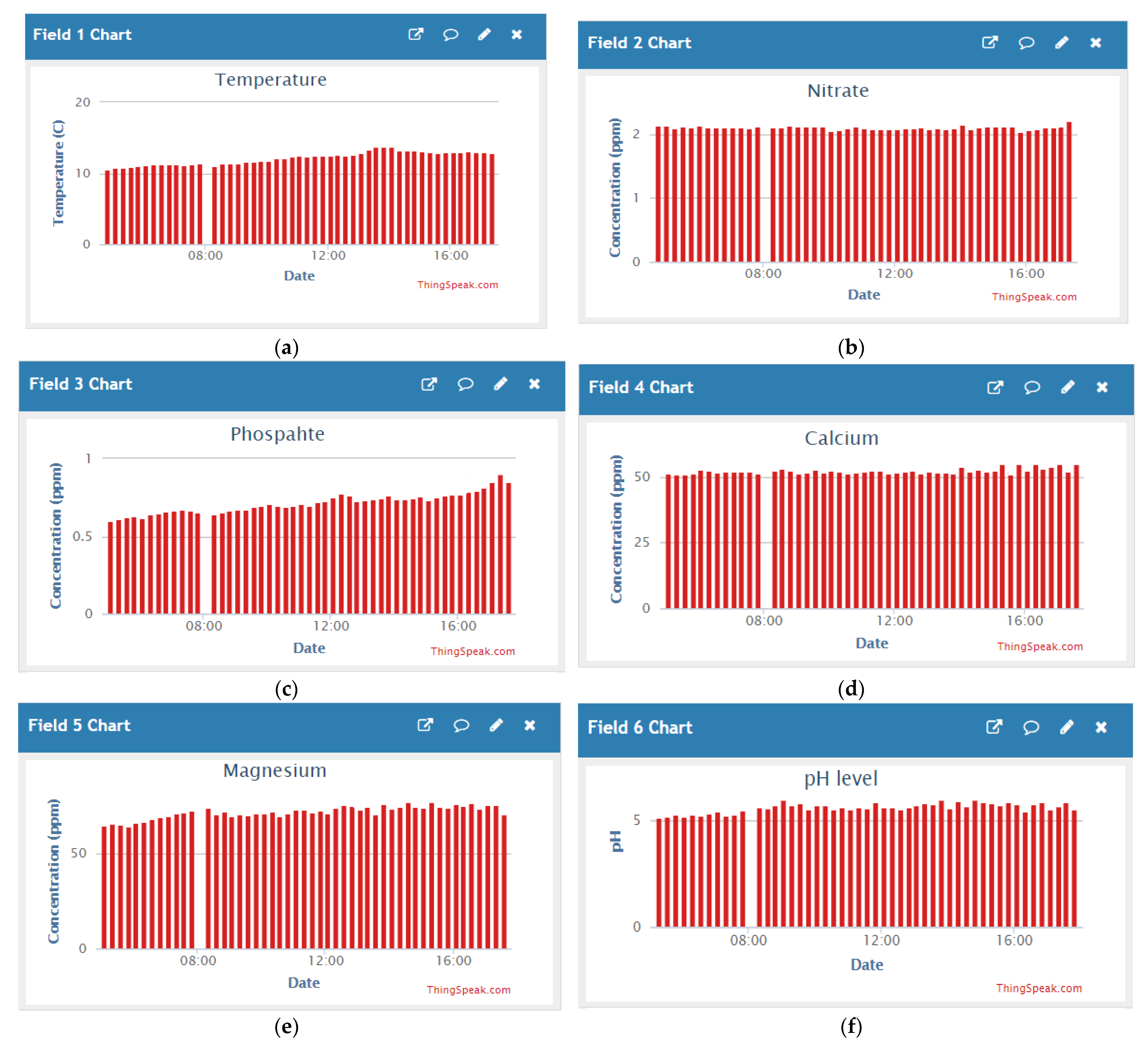

| Type of Fish | Optimum Temperature Range (°C) |
|---|---|
| Crappie | 22~24 |
| Smallmouth Bass | 18~21 |
| Largemouth Bass | 18~23 |
| Walleye | 18~21 |
| White Bass | 18~21 |
| Rock Bass | 21~23 |
| Channel Catfish | 28~31 |
| Rainbow Trout | 16~17 |
| Brown Trout | 13~19 |
| Yellow Perch | 20~22 |
| Lake Trout | 10~13 |
| Steelhead Trout | 13~16 |
| Chinook and Coho Salmon | 13~15 |
| Value of pH | Impact on Warm-Water Fish |
|---|---|
| Less than 4.0 | Reach to the point of acid death |
| 4.0 to 5.0 | Not productive |
| 6.5 to 9.0 | Fish production desirable range |
| 9.0 to 11.0 | Slow growth |
| Greater than 11.0 | Reach to an of alkaline death |
| Name of the Fish | Optimum pH Level |
|---|---|
| Zebra Danio | 6.5–7.0 |
| Tiger Barb | 6.0–6.5 |
| Silver Dollar | 6.0–7.0 |
| Plecostomus | 5.0–7.0 |
| Neon Tetra | 5.8–6.2 |
| Hatchet fish | 6.0–7.0 |
| Harlequin Rasbora | 6.0–6.5 |
| Goldfish | 7.0–7.5 |
| Clown Loach | 6.0–6.5 |
| Angelfish | 6.5–7.0 |
| Sensing Material | Detection Range | Detection Limit | Ref |
|---|---|---|---|
| Ag/AgCl | 4.01–6.86 | 4.01 | [31] |
| Platinum | 4–10 | 4 | [32] |
| SEN-10972 (commercial) | 1–14 | 1 | [33] |
| Colorimetric pH sensor | 1–10 | 1 | [34] |
| Polysulfone/Polyaniline | 4–12 | 4 | [35] |
| Ruthenium (IV) oxide (RuO2) | 1.5–12 | 1.51 | [36] |
| Parameter | Freshwater Community | Brackish | Discus | African Cichlids |
|---|---|---|---|---|
| Nitrate (ppm) | <50 | <30 | <10 | <50 |
| Nitrite (ppm) | 0.0~0.2 | 0.0 | 0.0 | 0.0 |
| Sensing Material | Detection Range | Detection Limit | Ref |
|---|---|---|---|
| AuNps/SG | 10~3960 | 0.2 | [45] |
| AuNps/Graphene sheet | 0.3~720 | 0.1 | [46] |
| Ni@Pt/Graphene sheet | 10~1500 | 10 | [47] |
| rGO/TEBAC | 0.2~200 | 0.2 | [48] |
| AuNPs/rGO | 0.1~20 | 0.1 | [49] |
| Cu@Pt/Graphene | 1~1000 | 1 | [50] |
| MMA-DMA copolymer | 0.5~10 | 0.55 | [51] |
| AuNPs/ERGO | 0.1~10 M | 0.1 | [52] |
| Colorimetric | 0.03~5.19 | 0.03 | [53] |
| Sensing Material | Detection Range | Detection Limit | Ref |
|---|---|---|---|
| Ag/rGO | 5~6000 | 1.20 | [60] |
| Al2O3/rGO | 1~10 | 1 | [61] |
| rGO/ferritin | 16.7~500 | 0.806 | [62] |
| Co/Pt/C/Ag-AgCl | 1~9500 | 0.3 | [63] |
| Poly vinyl chloride/Polyurethane | 0.096~960 | 0.096 | [64] |
| Cobalt/Glass | 1~3300 | 0.08 | [65] |
| Wax paper | 4~300 | 4 | [66] |
| Coulometric titration | 1.5~55 | 1.5 | [67] |
| Parameter | Coral Reefs | FOWLR Aquarium | Reef Aquarium |
|---|---|---|---|
| Calcium (ppm) | 380~420 | 350~450 | 350~450 |
| Magnesium (ppm) | 1300 | 1150~1350 | 1250~1350 |
| Sensing Material | Detection Range | Detection Limit | Ref |
|---|---|---|---|
| PtOEP-C6/Poly (St-TFEMA) | 0.1~39.3 | 0.1 | [89] |
| Ceramic Soil Microbial Fuel Cells | 1.0~10.0 | 1.0 | [90] |
| Nitrogen and Boron-Doped Reduced Graphene Oxide Membrane-Less | 1.5~10.0 | 1.5 | [91] |
| Ruthenium Complex Doped with Silver Nanoparticles | 1.0~15.0 | 1.0 | [92] |
| Seabird 63 DO | 1.0~10.0 | 1.0 | [93] |
| Polymer Optical Fibers (POF) | 1.0~10.0 | 1.0 | [94] |
| Cu (II) Complex Modified Electrode | 2.1~8.5 | 2.1 | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhter, F.; Siddiquei, H.R.; Alahi, M.E.E.; Mukhopadhyay, S.C. Recent Advancement of the Sensors for Monitoring the Water Quality Parameters in Smart Fisheries Farming. Computers 2021, 10, 26. https://doi.org/10.3390/computers10030026
Akhter F, Siddiquei HR, Alahi MEE, Mukhopadhyay SC. Recent Advancement of the Sensors for Monitoring the Water Quality Parameters in Smart Fisheries Farming. Computers. 2021; 10(3):26. https://doi.org/10.3390/computers10030026
Chicago/Turabian StyleAkhter, Fowzia, Hasin Reza Siddiquei, Md Eshrat E. Alahi, and Subhas C. Mukhopadhyay. 2021. "Recent Advancement of the Sensors for Monitoring the Water Quality Parameters in Smart Fisheries Farming" Computers 10, no. 3: 26. https://doi.org/10.3390/computers10030026
APA StyleAkhter, F., Siddiquei, H. R., Alahi, M. E. E., & Mukhopadhyay, S. C. (2021). Recent Advancement of the Sensors for Monitoring the Water Quality Parameters in Smart Fisheries Farming. Computers, 10(3), 26. https://doi.org/10.3390/computers10030026








